Abstract
In Hawai‘i, lung cancer is among the top cancers diagnosed and a leading cause of death. Despite current understanding and modern surgery, radiology, and chemotherapy techniques, the survival of those suffering from lung cancer remains low. Current anticancer drugs have poor tumor tissue selectivity and toxicity issues that contribute to their overall low efficacy, detrimental effects to normal tissues, and drug resistance. A potential way of mitigating cancer is through RNA interference (RNAi) by the delivery of small interfering RNA (siRNA) to target select proteins or genes involved in cancer progression, known as oncoproteins or oncogenes, respectively. However, the clinical utility of delivering unformulated siRNA has been hindered due to poor cell penetration, nonspecific effects, rapid degradation, and short half-life. As an alternate for conventional chemotherapy, nanoparticles (AKA nanocarriers) may be designed to localize within the tumor environment and increase targeted cell internalization, thus reducing systemic adverse effects and increasing efficacy. Nanoparticles play important roles in drug delivery and have been widely studied for cancer therapy and diagnostics, termed collectively as theranostics. Nanoparticles composed of natural and artificial polymers, proteins, lipids, metals, and carbon-based materials have been developed for the delivery of siRNA. Cancer targeting has been improved by nanoparticle surface modification or conjugation with biomolecules that are attracted to or stimulate therapeutic agent release within cancer tissues or cells. In this mini-review article, we present recent progress in nanocarrier-mediated siRNA delivery systems that include lipid, polymer, metallic and carbon-based nanoparticles for lung cancer therapy.
Introduction
Cancer is a leading cause of death in Hawai‘i and is characterized as a set of diseases that involve abnormal cell growth that may initiate in one location and then metastasize, or invade other tissues in the body.1 Despite the recent advances in diagnosis and treatment, lung cancer is among the most common cancers diagnosed on the islands only following prostate and breast cancers in both men and women, respectively.1,2 The Native Hawaiian and Pacific Islander populations that originate from Hawai‘i, Guam, Samoa, or other Pacific islands are among the fastest-growing populations in the United States (U.S.) and have increased cancer rates than Asian Americans.3 Due to different rates of smoking habits, lung cancer rates in Samoan men are about 30% higher than those in Native Hawaiian men.3 Worldwide, in 2016 there were approximately 58,000 new cancer cases and nearly 17,000 cancer deaths among Asian Americans, Native Hawaiians, and Pacific Islanders.3 In Hawai‘i, about 6,500 Hawai‘i residents are diagnosed with invasive cancer and more than 2,000 die from the disease each year.4
Overall nationally, an estimated 221,200 lung cancer cases were detected and diagnosed in 2015 that made up approximately 13% of all cancer diagnoses.5 The lung cancer 5-year survival rate is 54% when the disease is still localized within the lungs, however only 15% of cases are diagnosed at early stages.6 For metastasized tumors, the 5-year survival rate is 4%.6 The overall lung cancer survival rate is much lower than other leading cancer causes.6 Non-small cell lung cancer (NSCLC) occurs when malignant cells form in the tissues of the lung and can be classified as adenocarcinoma, carcinoid tumor, large cell carcinoma, pleomorphic, salivary gland carcinoma, squamous cell carcinoma, and unclassified carcinoma.2 NSCLC accounts for 85% of the total cases of lung cancer, where 75% of these cases at diagnosis are metastatic.7 Small-cell carcinoma, also known as oat-cell carcinoma, is an aggressive cancer that begins in the bronchi and rapidly spreads, or metastasizes, throughout the body. Small-cell carcinoma occurs most often in smokers, almost exclusively, and represents approximately 15% of lung cancers in the U.S.8,9
Standard lung cancer treatment entails combinations of surgery, chemotherapy, and radiation therapy. Although early detection and treatment make a significant difference in life expectancy, many lung cancer patients are diagnosed with the advanced or metastatic disease.10 Surgical resection may be considered the most effective strategy to cure NSCLC; however, surgery is not possible in every case.11 Current NSCLC anticancer drugs have poor tumor tissue selectivity and toxicity issues that contribute to their overall low efficacy and detrimental effects to normal tissues.12 Chemotherapy options often have issues with multidrug resistance due to overexpression of drug-resistance genes that drive mechanisms to stop cancer cell death and increase the expression of cellular pumps that remove the anti-cancer drugs from the cells. Multidrug resistance leads to higher chemotherapy doses that in turn, lead to more risk for severe adverse effects. New treatment options for lung cancer include the drugs afatinib (Gilotrif), ramucirumab (Cyramza), and bevacizumab (Avastin).13 Afatinib, approved by the U.S. Food and Drug Administration (FDA) in 2013, targets the epidermal growth factor receptor (EGFR) and receptor tyrosine-protein kinase erbB-2, AKA Her2/neu, via irreversible covalent inhibition to inhibit metastasis and tumor growth in EGFR mutant positive NCSLC patients.14–16 Mutations in the expression or activity of receptor tyrosine kinases EGFR and ErbB-2 causes cancer.17 Ramucirumab, FDA approved in 2014, is a monoclonal antibody that acts as an angiogenesis inhibitor by binding the vascular endothelial growth factor 2 (VEGFR2).18 However, conventional delivery of these therapies leads to the potential intolerable side effects and risk of recurrence due to resistance.
More effective pharmacological interventions for cancer therapy are necessary because surgery and radiotherapy are not viable options in some patients and chemotherapy results in low response rates with detrimental adverse effects. A well-designed delivery system that can deliver anticancer therapeutics specifically to cancerous cells should be developed to avoid adverse effects and to increase efficacy.
Cancer Targeting siRNA Therapeutics
Several methods have been proposed to control protein markers that are overexpressed in cancer.19 These methods include small molecule inhibitors or antibody biologics. A method for downregulating protein expression that targets the mRNA level, before protein transcription even begins, is RNA interference (RNAi). RNAi is a naturally occurring protein regulating process that has a high degree of specificity and the potential to silence mRNA and associated protein expression.20 A method for eliciting RNAi is mediated through the delivery of small-interfering RNAs (siRNAs) to the cell cytoplasm of target cells. Therapeutic siRNAs are synthetic double-stranded RNA of 21–23 base pairs that can be designed to suppress target mRNA sequences, in a process known as post-transcriptional gene silencing. To exert the therapeutic effect, the siRNA must travel to the cell cytoplasm to be attached to the multi-protein RNA-induced silencing complex (RISC).21 Intracellular siRNA, specifically targeted to a particular mRNA for degradation, undergoes RNAi processing in the cell to induce short-term silencing of protein coding mRNA.22 The siRNAs, as a class of therapeutic agents, are capable of efficient knockdown of targeted oncoproteins and oncogenes, as well as those proteins that play a role in multidrug resistance, and therefore have great potential for the treatment of lung cancer or other diseases.23
Nanotechnology in Drug Delivery
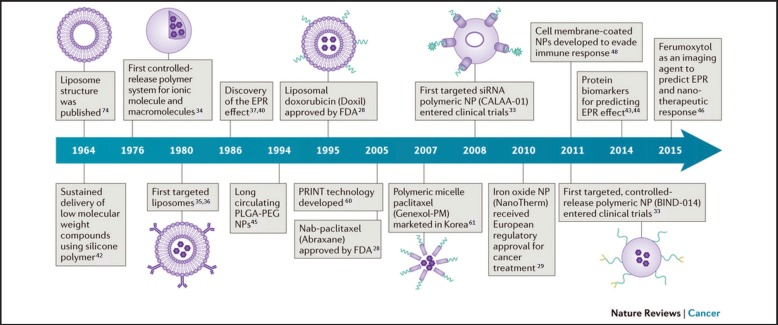
Nanotechnology has greatly impacted the field of medicine which in part has catapulted preclinical siRNA delivery systems into a new era. Drug half-life, retention, and targeting efficiency can be increased along with a subsequent reduction in adverse effects by incorporating nanotechnology-based therapeutic delivery systems. A brief history of the breakthroughs in cancer nanomedicine, or the therapeutic application of nanoparticle drug delivery systems, is shown in Figure 1.24 A few chemotherapeutic nanoparticle formulations have been approved by the FDA, namely liposomal doxorubicin (Doxil) and Nab-paclitaxel (Abraxane).25–29 Bind Biosciences, Inc. demonstrated nanoparticles containing a combination of chemotherapeutic and prostate-specific membrane antigens (PSMA) that outperform either drug alone at diminishing lesions of the lung and tonsillar regions and that the formulation greatly lowered the required dose.30,31 Calando Pharmaceuticals established the foremost clinical evidence of RNAi using a polymeric nanoparticle delivery system known as CALAA-01.32,33
Figure 1.
A timeline showing the history of major cancer nanomedicine breakthroughs.
Figure adapted with permission from Nature Publishing Group: Nature Reviews Cancer, 2017.24
As opposed to conventional chemotherapy delivery where the drug is administered systemically by i.v. injection and shows various drug concentrations in all tissues of the body, lung cancer targeting strategies using nanocarriers may achieve controlled release and are classified as having either passive or active targeting directly to cancer tissue.34–36 Passive cancer targeting is possible through the enhanced permeation and retention (EPR) effect that has been exploited in many cancer drug delivery systems since its identification in the late 1980's.37–40 Nanocarrier circulation times and accumulation levels in tumor tissues are influenced by the properties and characteristics of the nanocarriers, such as the polymer or lipid, surface molecules, particle size, or surface charge.41–46
Strategies to improve delivery to tumor sites by including microenvironment homing mechanisms including tumor penetrating peptide and stimuli responsive functional surface nanocarrier modifications have been explored.47 In addition to conjugating the polymer polyethylene glycol (PEG) to the surface of the nanoparticles, known as PEGylation, cell membrane-coated nanoparticles have been developed to evade immune responses by tricking the immune system.48 Active targeting involves molecular targeting agents, such as surface bound ligands, to specifically target the biomarkers or receptors on cancer tissues or cells that may provoke uptake of nanocarriers.49–51
Nanocarrier-based siRNA Delivery Systems for Lung Cancer
Naked siRNA is prone to degradation, has a shorter plasma half-life, rapid renal clearance, and limited permeability across cell membranes, making clinical efficacy unlikely.52,53 A variety of nanocarrier systems in early cancer therapeutic clinical studies have shown enhanced efficacy and reduced side effects.30,54 Cationic lipid based systems have emerged as the most attractive for siRNA delivery. However the use is limited due to poor transfection efficiency and toxicity in vivo.55 Natural polymer based delivery systems are biocompatible and biodegradable with high physiological tolerance and low immunogenicity.56,57 Rigid nanoparticles are composed of inorganic metals or of carbon-based materials.58 Here, we focus on siRNA nanocarriers that have undergone laboratory experimentation published recently.
Examples of Polymer-based siRNA Nanocarriers
Polymers used for siRNA nanocarriers include naturally occurring ones, such as chitosan, or synthetics ones, such as polyethylenimine (PEI). Polymeric nanocarriers have received much attention in the area of siRNA delivery because of their biocompatibility and versatile modifiability.32,54,59–61
Yan, et al, developed a combinatorial functional polyester library to identify formulations that have highly efficient delivery of siRNA to A549 (ATCC® CCL-185™) human epithelial lung carcinoma for potential lung delivery of siRNA.62 They found that two types of polyplex nanoparticles that contained PEG 2000 DMG modified lipid or Pluronic F-127 nonionic surfactant on their surface increased their serum stability and decreased their surface charge. This study showed that inhalation of the surface modified polyplex nanoparticles had resulted in significantly more nanoparticles localized within the lungs and significant gene downregulation in the A549 orthotropic lung tumors than when delivered by i.v. administration.
Another study that evaluated nanocarriers in an A549 lung cancer cell line was conducted by Seifi-Najmi, et al, who demonstrated the preparation and characterization of varying combinations of doxorubicin, High Mobility Group At-Hook 2 (HMGA2) siRNA, or combination siRNA/doxorubicin entrapped within carboxymethyl dextran trimethyl chitosan nanocarriers.63 Nanoparticles loaded with doxorubicin combined with HMGA2 siRNA outperformed the other formulations in treated A549 cell assays including lessened cancer cell viability, alteration of pro-cancer markers, induction of apoptosis, and inhibition of migration.
Cisplatin is an anti-cancer chemotherapy drug, also known as a cytotoxic or antineoplastic drug, that interferes with DNA replication by crosslinking DNA, thus preventing cell division by mitosis. However, cellular resistance to cisplatin therapy is commonly observed. A mediator of cisplatin sensitivity in human cancer cells at the mitotic checkpoint is the mitotic arrest deficient-2 (Mad2) protein.64 Nascimento, et al, designed chitosan polysaccharide (sugar polymer) nanoparticles containing surface conjugated EGFR targeting ligand that entrapped Mad2 siRNA in combination with cisplatin and determined them to be safe and efficacious in cisplatin resistance and sensitive NSCLC cell culture models.65,66 Their rationale was if the RNAi that downregulated the essential mitotic checkpoint gene Mad2, the cells would become more sensitive to cisplatin based chemotherapeutics and therefore lead to increased cellular death. EGFR targeted nanoparticles presented a steady and favored tumor targeting capability with fast blood plasma clearance to penetrate and localize within the tumor tissue for up to 4 days.67 These targeted nanoparticles showed a six-fold advanced tumor targeting ability when related to non-targeted chitosan nanoparticles.67
Another siRNA and chemotherapeutic combination study was conducted by Xu, et al, who designed a pH-responsive PEI nanoparticle containing doxorubicin and Survivin siRNA for potential inhalation therapies for metastatic lung cancers.68 Survivin is a protein that inhibits caspase activation, which causes negative regulation of programmed cell death, or apoptosis; thus it belongs to the family of proteins that inhibit apoptosis.69 Therefore, siRNA directed towards survivin expression will remove this negative regulation, allowing for normal cell death of cancerous cells. Doxorubicin release from these PEI nanocarriers was pH dependent, where the release was higher in acidic tumor microenvironments. The doxorubicin and Survivin siRNA was delivered in vitro and was shown to impart cellular death in B16F10 cancer cell culture lines. Using cancer mouse models that had B16F10 tumors, locally delivered siRNA loaded doxorubicin coupled PEI nanoparticles by inhalation resulted in the accumulation of doxorubicin and Survivin siRNA within the lung tissue and airways, where a substantial quantity of doxorubicin and siRNA were found in tumor tissues, with a low amount of doxorubicin and siRNA observed within normal lung tissues. Furthermore, the Survivin siRNA doxorubicin conjugated PEI nanoparticles presented superior antitumor effectiveness when compared to individual doxorubicin or Survivin siRNA delivery.
Srikar, et al, produced tri-block nanoparticles that were composed of enzymatically degradable gelatin nanoparticles with cetuximab-siRNA molecules conjugated to its surface and entrapped with the tyrosine kinase inhibitor, gefitinib.70 Delivery of siRNA to chemotherapeutic resistant KRAS mutated NSCLC cells via a targeted strategy was believed to foster cell sensitization to tyrosine kinase inhibitors, leading to increased cellular death. This study demonstrated targeted proto-oncogenes with nanoparticle therapies.
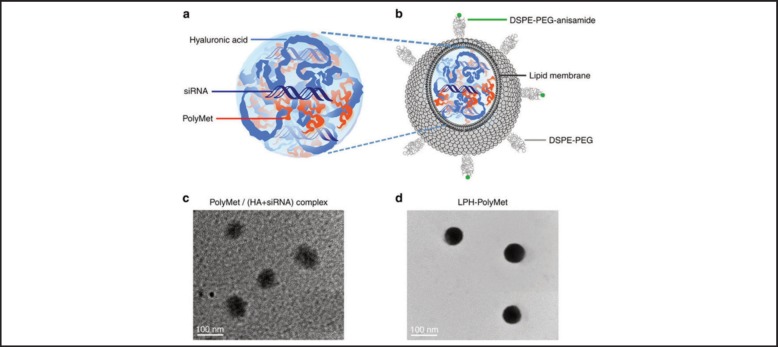
Metformin is a well-known diabetic medication that has been studied as an anti-cancer drug. Its mechanism is believed to rely on the ability to reduce insulin resistance and indirectly lower levels of insulin, a known cancer cell growth promoter, as well as through direct inhibition of cancer cell growth.71 Zhao, et al, prepared a polymer conjugate of Metformin, coined PolyMetformin through dicyandiamide conjugation to linear PEI.72 The positive charge of PolyMetformin facilitated the entrapment of the negatively charged siRNA into a core-membrane structured lipid-polycation-hyaluronic acid nanoparticle as shown in Figure 2. Vascular endothelial growth factor (VEGF) is a signaling protein that stimulates blood vessel formation, and if expressed by cancer cells can cause tumor growth and metastasis.73 LPH-PolyMetformin nanoparticles facilitated VEGF siRNA delivery in a human lung cancer xenograft, leading to hindrance of tumor tissue growth. Without RNAi, the lipid-polycation-hyaluronic acid-PolyMetformin nanoparticles were able to induce antitumor efficacy similarly to Metformin. PolyMetformin was combined with siRNA to further improve the therapeutic activity of an anti-oncogene and oncoprotein therapy.
Figure 2.
Schematic illustration and representative TEM images of PolyMet nanoparticles. Anionic HA + siRNA mixture was condensed by cationic PolyMet into negatively charged PolyMet (HA+ siRNA) complex (a, c). DOTAP/cholesterol cationic liposomes were added to the complex to form lipid coating, then DSPE-PEG and DSPE-PEG-anisamide were used to prepare a liposome using the post-insertion method to form LPH-PolyMet nanoparticles (b,d). By Zhao, et al, 2016. Used under Creative Commons Attribution 4.0.72
Examples of Lipid-based siRNA Nanocarriers
Several siRNA loaded lipid nanoparticle delivery systems have undergone evaluation as cancer therapies within clinical trials. However none have specifically targeted lung cancer. Lipid-based siRNA nanocarriers include liposomes, lipid complexes, and solid lipid nanoparticles.74 Phase I clinical trials of stable nucleic acid lipid particles (SNALP) encompassing siRNA's directed towards serine/threonine-protein kinase (PLK1), a mitosis regulating gene, for cancer therapy was initiated by Tekmira Pharmaceuticals Corporation (NCT01262235, BC, Canada).75,76 The Anderson Cancer Center (Texas, USA) initiated a Phase I clinical trial where siRNAs encapsulated within a neutral liposome composed of 1,2-dioleoyl-sn-glycero-3-phosphatydylcholine for oncoprotein Eph2 suppression (NCT01591356) for delivery for the treatment of advanced cancers.
Lung cancer and mesothelioma tumorigenesis, or the onset of tumor growth, has been associated with the receptor EphA2 overexpression. A liposomal cisplatin formulation, known as Lipoplatin™, has been administered against cisplatin resistant cancers. To further enhance the sensitivity of lung cancer cells to Lipoplatin, Lee, et al, combined receptor EphA2 siRNA with Lipoplatin and targeted them to tumor cells.77 This group demonstrated that silencing EphA2 significantly enhanced the cellular sensitivity of lung tumor and malignant pleural mesothelioma cells to Lipoplatin.77
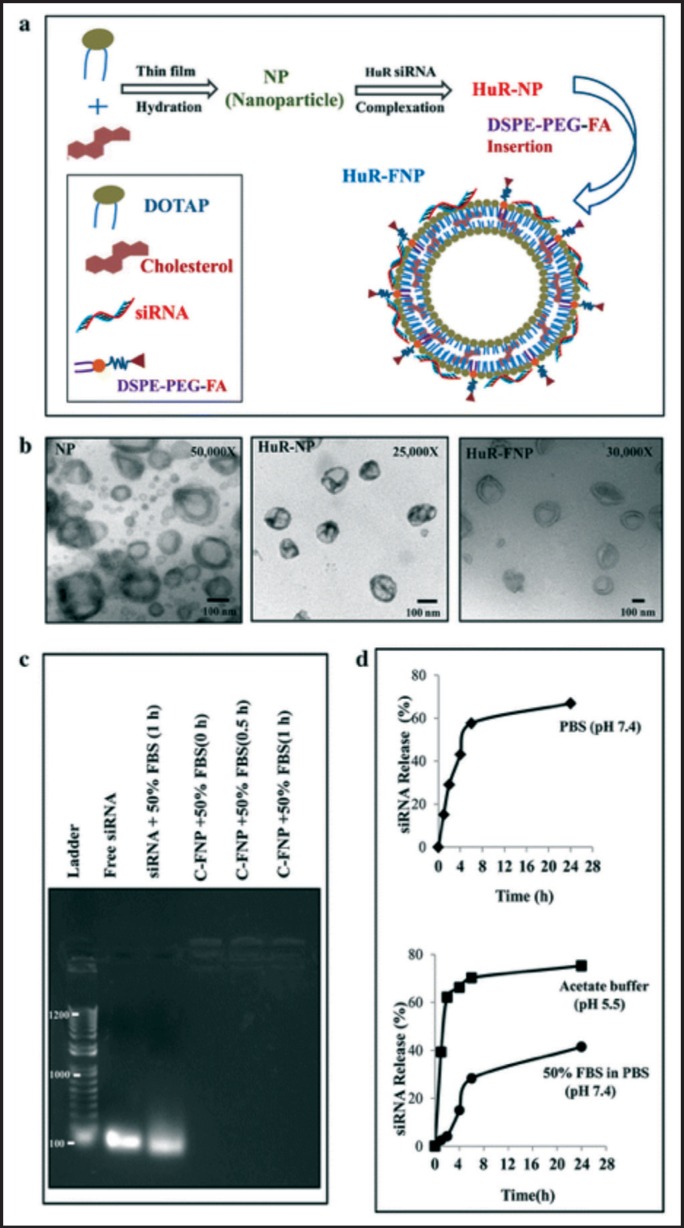
Human antigen R (HuR), a RNA binding protein, has been shown to be overexpressed in various cancers, has demonstrated its role in several oncoprotein expression regulations, and has been linked to overall resistance and poor-prognosis. Muralidharan, et al, hypothesized that cancer cell-targeted inhibition of HuR would suppress oncoproteins that would thus result in effective lung cancer therapy.78 To examine this proposition, folate receptor-α (FRA)-targeted DOTAP:Cholesterol lipid nanoparticles carrying HuR siRNA (HuR-FNP) against human lung cancer cells were prepared and tested for stability release as shown in Figure 3. The prepared particles had a particle size of approximately 100 nm, adequately protected the siRNA from degradation, and displayed good release profiles (Figure 3b–d). HuR-FNP was shown to induce apoptotic cell death in H1299 cells that resulted in noteworthy growth inhibition and higher cell cytotoxicity.78
Figure 3.
Synthesis and physicochemical characterization of siRNA-FNP. a Scheme showing HuR-FNP preparation. b TEM image of NP, HuR-NP, and HuR-FNP. Scale bar denotes 100 nm. c Agarose gel electrophoretogram showing siRNA protection by FNP at different time (0, 0.5, and 1 hr) points of incubation compared to naked siRNA exposed to serum for 1 hr. Free siRNA not exposed to serum was used as an internal marker. d siRNA release profile over time from siRNA-FNP in PBS (pH 7.4) measured by Quanti-iT Picogreen Assay (top figure); and from fluorescently labeled siRNA (siGLO)-FNP in acetate buffer (pH 5.5) and in 50% FBS containing PBS (pH 7.4) (bottom figure). By Muralidharanet, al, 2016. Used under Creative Commons Attribution 4.0.78
Examples of Metallic- and Carbon-based siRNA Nanocarriers
Iron oxide nanoparticles, carbon nanotubes, gold nanoparticles, and quantum dots have been developed for siRNA delivery as lung cancer therapies and diagnostic agents. Iron oxide nanoparticles theranostics have the ability to be used as a drug delivery system and a contrast agent for magnetic resonance imaging (commonly known as MRI) through the selective delivery of therapeutics agents to target sites. Carbon nanotubes have been used as drug delivery carriers because they can enter cells. Gold nanoparticles are interesting drug or siRNA delivery systems because they can be easily formed into a desired shape or size, they have exclusive surface plasmon resonance, and their surfaces may be modified through conjugation of thiolated targeting molecules.58 Quantum dots are nanoparticulate materials having semiconductive nature which may be incorporated into living cells or tissue for experimental purposes but have been proposed for therapeutic applications as well.79
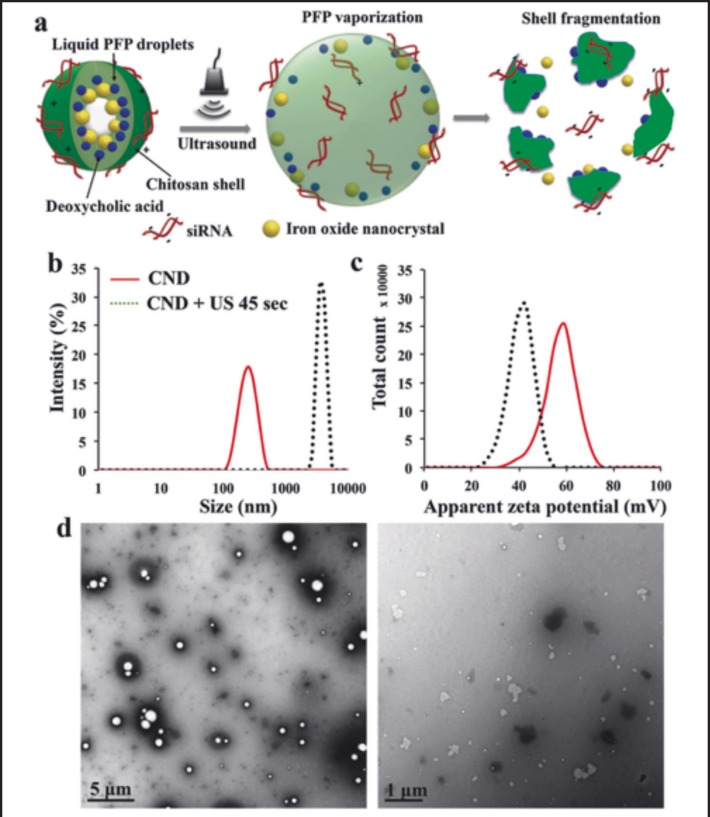
Lee, et al, determined whether chitosan-deoxycholic acid nanoparticles containing perfluoropentane and iron oxide can be used as an siRNA delivery system with the use of ultrasound exposure as shown in Figure 4.80 The results show that the polymer coated iron oxide nanoparticles were able to successfully promote siRNA uptake, leading to significant apoptosis 3 days following ultrasound treatment.
Figure 4.
a Schematic representation of chitosan-deoxycholic acid coated perfluoropentane nanodroplets (CNDs). b Hydrodynamic diameters of CNDs before (solid line) and after (dotted line) exposure to ultrasound for 45 s in 10% glycerol in water. c Zeta potential of CNDs before and after ultrasound exposure. d TEM images of CND before (left) and after (right) ultrasound exposure for 45 s. By Lee, et al, 2017. Used under Creative Commons Attribution 4.0.80
Li, et al, developed a single vehicle l-arginine and hydroxypropyl-cyclodextrin quantum dot nanoparticulate combination drug delivery system containing siRNA directed towards B-cell lmphoma 2 (Bcl-2), which is a regulator of apoptosis, combined with carboplatin, doxorubicin, and paclitaxel for treatment in an A549 lung cancer cell line.81 When compared to treatments consisting of only the free chemotherapeutics, the use of siRNA and chemotherapeutic combination loaded quantum dot nanocarriers exerted a 3 to 4 times increase in A549 cell cytotoxicity, implying improved treatment efficacy for the combination of siRNA and chemotherapeutic. These multifunctional quantum dot nanocarriers may potentially be a worthwhile method for delivering siRNA and chemotherapeutics for the lung cancer combination therapies and due to their fluorescent properties, may also serve as a diagnostic agent.
Kamrani Moghaddam, et al, studied the ability of siRNA loaded hexagonal selenium nanoparticles (HSNM-siRNA) to prevent EGFR signaling in human NSCLC.82 Plain hexagonal selenium nanoparticles and HSNM-siRNA were each separately used to treat NSCLC cell lines, and then onco-gene and -protein expression levels were evaluated. HSNM-siRNA was shown to downregulate the EGFR signaling gene expression and increase in a number of apoptotic cells.82
Mi, et al, developed a porous silicon-based nanocomposite material that simultaneously delivered chemotherapeutic agents and siRNA to the lungs after i.v. injection.83 The silicon microparticles entrapped B-Raf proto-oncogene serine/threonine kinase siRNA-loaded liposomes and contained docetaxel entrapped polymeric nanoparticles conjugated to their surface. A synergistic antitumor effect was demonstrated when siRNA/docetaxel nanocarriers were used to treat melanoma cell cultures and also showed synergistic efficacy in vivo using a melanoma lung metastasis mouse model. The siRNA/docetaxel nanocarriers displayed higher accumulation in the lungs of the mouse model that exhibited metastatic melanoma lesions.
Wu, et al, designed multi-functionalized, integrated theranostic folate-conjugated reducible polyethylenimine passivated carbon dots (fc-rPEI-Cdots) which were used to encapsulate EGFR and cyclin B1 siRNA.84 These particles were capable of emitting visible blue photoluminescence and siRNA intracellular delivery. In vitro cell culture studies suggested that the developed fc-rPEI-Cdots were capable of targeted siRNA delivery and were biocompatible.
Iron-oxide nanoparticles modified with biodegradable polyester nanoparticles composed of the polymers poly(lactic-co-glycolic acid) (PLGA) and PEG were loaded with telomerase siRNA. Telomerase expression is responsible for inhibition of apoptosis and cancer mutations associated with lung cancer malignant cells. This study demonstrated that the self-assembly of magnetic diblock copolymers encapsulated telomerase siRNA and resulted in reduced telomerase expression when compared to that of naked siRNA. The reduction of telomerase gene expression leads to increased tumor cell apoptotic death in lung cancer cells treated with siRNA magnetic copolymers than compared to naked siRNA treated cells.85
Summary and Future Directions
Nanoparticles carrying siRNA molecules have revealed high transfection rates and targeting ability for lung carcinoma tumors through systemic intravenous or localized inhaled administration. Therapeutic efficiency of gene therapy can be improved by active targeting on specific lung cancer tumors or metastases through modification or conjugation of targeting agents on the surface of the nanoparticles. The use of polymer, lipid, metals, and carbon-based nanoparticle systems in the field of targeted siRNA delivery has grown tremendously and has demonstrated promising in vitro and in vivo therapeutic efficacy results. The challenges of delivering nanoparticle mediated siRNA therapy within the body, such as maintaining the nanoparticle stability and siRNA stability, controlling the biodistribution and pharmacokinetics, penetrating biological barriers and minimizing the potential toxicity of the nanoparticles needs to be considered and overcome before entering clinical trials. The field of nanomedicine will continue to expand in the areas of cell or tissue targeting ability, circulation longevity, improved aerosol pulmonary delivery, enhanced intracellular penetration, stimuli sensitivity, and carrier-mediated visualization through using different nanocarrier properties or surface functional moieties. To increase the application of nanoparticle systems in siRNA therapy to the clinic, standards in the examination of nanoparticle safety and evaluation of therapeutic efficacy should be established to guide the direction of research and development of siRNA loaded nanoparticle therapeutic interventions.
Acknowledgements
The authors acknowledge the support of the National Institute of General Medical Science of the National Institutes of Health under award number SC3GM109873; the Hawai‘i Community Foundation, Honolulu, HI 96813, USA, for research support on lung cancer (LEAHI FUND for Pulmonary Research Award; ID# 15ADVC-74296); the 2013 George F. Straub Trust and Robert C. Perry Fund of the Hawai‘i Community Foundation, Honolulu, HI, for research support on lung cancer; a seed grant from the Research Corporation of the University of Hawai‘i at Hilo, Hilo, HI; The Daniel K. Inouye College of Pharmacy, the University of Hawai‘i at Hilo, Hilo, HI, for providing start-up financial support to their research group; and the Department of Pharmaceutics and Drug Delivery, School of Pharmacy, University of Mississippi, University, MS, for providing start-up support to Dr. Chougule's lab.
Conflict of Interest
None of the authors identify any conflict of interest.
References
- 1.HCCC, author; Coalition HCC, editor. Hawai'i State Cancer Plan 2016–2020. 2016. [Google Scholar]
- 2.Detailed Guide to Lung Cancer (Non-Small Cell), author American Cancer Society, 2014. 2014. [10/22/14]. http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics.
- 3.Torre LA, Sauer AMG, Chen MS, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA: a cancer journal for clinicians. 2016 doi: 10.3322/caac.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawaii at a Glance. Cancer Statistics Center 2017. 2017. [09/19/2017]. https://cancerstatisticscenter.cancer.org/#/state/Hawaii.
- 5.American Cancer Society, author. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 6.Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2011. 2014. [March 19, 2015]. http://seer.cancer.gov/csr/1975_2011/
- 7.Reade CA, Ganti AK. EGFR targeted therapy in non-small cell lung cancer: potential role of cetuximab. Biologics: targets & therapy. 2009;3:215. [PMC free article] [PubMed] [Google Scholar]
- 8.Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. American Society of Clinical Oncology. 2006 doi: 10.1200/JCO.2006.07.3841. [DOI] [PubMed] [Google Scholar]
- 9.Muscat JE, Wynder EL. Lung cancer pathology in smokers, ex-smokers and never smokers. Cancer letters. 1995;88(1):1–5. doi: 10.1016/0304-3835(94)03608-l. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS. Lung carcinogenesis by tobacco smoke. International Journal of Cancer. 2012 Dec 15;131(12):2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieber J, Deeg A, Ullrich E, et al. Outcome and prognostic factors of postoperative radiation therapy (PORT) after incomplete resection of non-small cell lung cancer (NSCLC) Lung Cancer (Amsterdam, Netherlands) 2016 Jan;91:41–47. doi: 10.1016/j.lungcan.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: Results of a phase I/II trial. Journal of Clinical Oncology. 2012;30(32):3953–3959. doi: 10.1200/JCO.2012.41.9820. [DOI] [PubMed] [Google Scholar]
- 13.Farhat FS, Houhou W. Targeted therapies in non-small cell lung carcinoma: what have we achieved so far? Therapeutic Advances in Medical Oncology. 2013;5(4):249–270. doi: 10.1177/1758834013492001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. Journal of Clinical Oncology. 2010;28(25):3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 15.Metro G, Crinò L. The LUX-Lung clinical trial program of afatinib for non-small-cell lung cancer. Expert Review of Anticancer Therapy. 2011;11(5):673–682. doi: 10.1586/era.11.34. [DOI] [PubMed] [Google Scholar]
- 16.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. The Lancet Oncology. 2012;13(5):528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 17.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cellular and MolecularLife Sciences : CMLS. 2008;65(10):1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke JM, Hurwitz HI. Targeted inhibition of VEGF Receptor-2: An update on Ramucirumab. Expert Opinion on Biological Therapy. 2013 Jun 26;13(8):1187–1196. doi: 10.1517/14712598.2013.810717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanash S. Disease proteomics. Nature. 2003;422(6928):226–232. doi: 10.1038/nature01514. [DOI] [PubMed] [Google Scholar]
- 20.Bagasra O, Prilliman KR. RNA interference: The molecular immune system. Journal of Molecular Histology. 2004 Aug;35(6):545–553. doi: 10.1007/s10735-004-2192-8. [DOI] [PubMed] [Google Scholar]
- 21.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nature Reviews Molecular Cell Biology. 2007 Jan;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 22.DeVincenzo JP. Harnessing RNA interference to develop neonatal therapies: from Nobel Prize winning discovery to proof of concept clinical trials. Early Hum Dev. 2009 Oct;85(10 Suppl):S31–S35. doi: 10.1016/j.earlhumdev.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Ali HM, Urbinati G, Raouane M, Massaad-Massade L. Significance and applications of nanoparticles in siRNA delivery for cancer therapy. Expert Review of Clinical Pharmacology. 2012-Jul. 2012;5(4):403–412. doi: 10.1586/ecp.12.33. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Design, Development and Therapy. 2015 Jul 24;9:3767–3777. doi: 10.2147/DDDT.S88023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houghton PJ, Kurmasheva RT, Kolb EA, et al. Initial Testing (Stage 1) of the Tubulin Binding Agent Nanoparticle Albumin-Bound (nab) Paclitaxel (Abraxane(®)) by the Pediatric Preclinical Testing Program (PPTP) Pediatric Blood & Cancer. 2015 Mar 23;62(7):1214–1221. doi: 10.1002/pbc.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao M, Lei C, Yang Y, et al. Abraxane, the Nanoparticle Formulation of Paclitaxel Can Induce Drug Resistance by Up-Regulation of P-gp. PloS One. 2015 Jul 16;10(7):e0131429. doi: 10.1371/journal.pone.0131429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A. Big Moment for Nanotech: Oncology Therapeutics Poised for a Leap, 2013. 2017. http://www.onclive.com/publications/oncology-live/2013/june-2013/big-moment-for-nanotech-oncology-therapeutics-poised-for-a-leap.
- 29.Magforce, author. Press releases: MagForce Nanotechnologies AG receives European regulatory approval for its Nano Cancer® therapy. 2010. http://www.etp-nanomedicine.eu/public/news-events/news-archive-1/press-releases-magforce-nanotechnologies-ag-receives-european-regulatory-approval-for-its-nano-cancerae-therapy.
- 30.Hrkach J, Von Hoff D, Ali MM, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Science Translational Medicine. 2012;4(128):128ra139–128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 31.A Study of BIND-014 Given to Patients with Advanced or Metastatic Cancer, 2016. 2017. https://clinicaltrials.gov/ct2/show/NCT01300533?term.
- 32.Davis ME, Zuckerman JE, Choi CHJ, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010 Apr 15;464(7291):1067–U1140. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US National Library of Medicine, author. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00689065?term.
- 34.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. 1976 doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 35.Leserman LD, Barbet J, Kourilsky F, Weinstein JN. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature. 1980;288(5791):602–604. doi: 10.1038/288602a0. [DOI] [PubMed] [Google Scholar]
- 36.Heath TD, Fraley RT, Papahdjopoulos D. Antibody targeting of liposomes: cell specificity obtained by conjugation of F (ab') 2 to vesicle surface. Science. 1980;210(4469):539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- 37.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 38.Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics. Clinical Pharmacokinetics. 2003;42(13):1089–1105. doi: 10.2165/00003088-200342130-00002. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvascular Research. 1986;31(3):288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 41.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008 Jul-Aug;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folkman J, Long DM. The use of silicone rubber as a carrier for prolonged drug therapy. Journal of Surgical Research. 1964;4(3):139–142. doi: 10.1016/s0022-4804(64)80040-8. [DOI] [PubMed] [Google Scholar]
- 43.Yokoi K, Tanei T, Godin B, et al. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Letters. 2014;345(1):48–55. doi: 10.1016/j.canlet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Research. 2014;74(16):4239–4246. doi: 10.1158/0008-5472.CAN-13-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 46.Miller MA, Gadde S, Pfirschke C, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Science Translational Medicine. 2015;7(314):314ra183–314ra183. doi: 10.1126/scitranslmed.aac6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? Journal of Controlled Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. Journal of controlled release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 50.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Advanced Drug Delivery Reviews. 2013;65(1):121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. Journal of Controlled Release. 2014;190:485–499. doi: 10.1016/j.jconrel.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glebova KV, Marakhonov AV, Baranova AV, Skoblov MI. Therapeutic siRNAs and non-viral systems for their delivery. Molekuliarnaia Biologiia. 2012;46(3):371–386. 2012. [PubMed] [Google Scholar]
- 53.Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010 Jan;267(1):44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nature Reviews Immunology. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SY, Zheng Y, Chen JY, et al. Comprehensive study of cationic liposomes composed of DC-Chol and cholesterol with different mole ratios for gene transfection. Colloids Surf B Biointerfaces. Vol 101C: 2012 Elsevier B.V. 2012:6–13. doi: 10.1016/j.colsurfb.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 56.Kim NH, Batton D, Thakur A, Lum L, Bassett DP, Merkel OM. Targeted SiRNA Delivery to Activated T Cells for Anti-Inflammatory Therapy of Asthma. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2013 Apr;26(2):A46–A47. [Google Scholar]
- 57.Sundar S, Kundu J, Kundu SC. Biopolymeric nanoparticles. Science and Technology of Advanced Materials. 2016 doi: 10.1088/1468-6996/11/1/014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Liu G, Zheng H, Chen X. Rigid nanoparticle-based delivery of anti-cancer siRNA: challenges and opportunities. Biotechnology Advances. 2014;32(4):831–843. doi: 10.1016/j.biotechadv.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youngren-Ortiz SR. Development and Evaluation of siRNA Loaded Gelatin Nanocarriers for the Treatment of Asthma. 2016 [Google Scholar]
- 60.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. Journal of the American Chemical Society. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 61.Werner ME, Cummings ND, Sethi M, et al. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. International Journal of Radiation Oncology, Biology, Physics. 2013;86(3):463–468. doi: 10.1016/j.ijrobp.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan Y, Zhou K, Xiong H, et al. Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors. Biomaterials. 2017 Feb;118:84–93. doi: 10.1016/j.biomaterials.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seifi-Najmi M, Hajivalili M, Safaralizadeh R, et al. SiRNA/DOX lodeded chitosan based nanoparticles: Development, Characterization and in vitro evaluation on A549 lung cancer cell line. Cellular and Molecular Biology (Noisy-le-Grand, France) 2016 Sep 30;62(11):87–94. [PubMed] [Google Scholar]
- 64.Fung M, Cheung HW, Ling M, Cheung A, Wong YC, Wang X. Role of MEK/ERK pathway in the MAD2-mediated cisplatin sensitivity in testicular germ cell tumour cells. British Journal of Cancer. 2006;95(4):475. doi: 10.1038/sj.bjc.6603284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B, Amiji MM. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol Pharm. 2014 Oct 6;11(10):3515–3527. doi: 10.1021/mp5002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B, Amiji MM. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomaterialia. 2017 Jan 1;47:71–80. doi: 10.1016/j.actbio.2016.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nascimento AV, Gattacceca F, Singh A, et al. Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models. Nanomedicine (Lond) 2016 Apr;11(7):767–781. doi: 10.2217/nnm.16.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu C, Tian H, Wang P, Wang Y, Chen X. The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and Survivin siRNA. Biomaterials Science. 2016 Oct 18;4(11):1646–1654. doi: 10.1039/c6bm00601a. [DOI] [PubMed] [Google Scholar]
- 69.Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998 Dec 1;58(23):5315–5320. [PubMed] [Google Scholar]
- 70.Srikar R, Suresh D, Zambre A, et al. Targeted nanoconjugate co-delivering siRNA and tyrosine kinase inhibitor to KRAS mutant NSCLC dissociates GAB1-SHP2 post oncogene knockdown. Sci Rep. 2016 Aug 17;6:30245. doi: 10.1038/srep30245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahra IB, Le Marchand-Brustel Y, Tanti J-F, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Molecular Cancer Therapeutics. 2010;9(5):1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y, Wang W, Guo S, et al. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nature Communications. 2016 Jun 6;7:11822. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends in Pharmacological Sciences. 2001;22(4):201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 74.Bangham AD, Horne R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. Journal of Molecular Biology. 1964;8(5):660IN662–668IN610. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 75.McCarroll JA, Dwarte T, Baigude H, et al. Therapeutic targeting of polo-like kinase 1 using RNA-interfering nanoparticles (iNOPs) for the treatment of non-small cell lung cancer. Oncotarget. 2014 Dec 20; doi: 10.18632/oncotarget.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Z, Sun Q, Wang X. PLK1, A Potential Target for Cancer Therapy. Translational Oncology. 2017;10(1):22–32. doi: 10.1016/j.tranon.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee HY, Mohammed KA, Goldberg EP, Kaye F, Najmunnisa N. Silencing Receptor EphA2 Enhanced Sensitivity to Lipoplatin in Lung Tumor and MPM Cells. Cancer Investigation. 2016 Aug 8;34(7):293–304. doi: 10.1080/07357907.2016.1201678. [DOI] [PubMed] [Google Scholar]
- 78.Muralidharan R, Babu A, Amreddy N, et al. Folate receptor-targeted nanoparticle delivery of HuR-RNAi suppresses lung cancer cell proliferation and migration. Journal of Nanobiotechnology. 2016 Jun 21;14(1):47. doi: 10.1186/s12951-016-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zrazhevskiy P, Sena M, Gao X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chemical Society Reviews. 2010;39(11):4326–4354. doi: 10.1039/b915139g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JY, Crake C, Teo B, et al. Ultrasound-Enhanced siRNA Delivery Using Magnetic Nanoparticle-Loaded Chitosan-Deoxycholic Acid Nanodroplets. Advanced Healthcare Materials. 2017 Feb 13; doi: 10.1002/adhm.201601246. [DOI] [PubMed] [Google Scholar]
- 81.Li J, Wang Y, Xue S, et al. Effective combination treatment of lung cancer cells by single vehicular delivery of siRNA and different anticancer drugs. Int J Nanomedicine. 2016;11:4609–4624. doi: 10.2147/IJN.S107345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamrani Moghaddam L, Ramezani Paschepari S, Zaimy MA, Abdalaian A, Jebali A. The inhibition of epidermal growth factor receptor signaling by hexagonal selenium nanoparticles modified by SiRNA. Cancer Gene Therapy. 2016 Sep;23(9):321–325. doi: 10.1038/cgt.2016.38. [DOI] [PubMed] [Google Scholar]
- 83.Mi Y, Mu C, Wolfram J, et al. A Micro/Nano Composite for Combination Treatment of Melanoma Lung Metastasis. Advanced Healthcare Materials. 2016 Apr 20;5(8):936–946. doi: 10.1002/adhm.201500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu YF, Wu HC, Kuan CH, et al. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci Rep. 2016 Feb 16;6:21170. doi: 10.1038/srep21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fekri Aval S, Akbarzadeh A, Yamchi MR, Zarghami F, Nejati-Koshki K, Zarghami N. Gene silencing effect of SiRNA-magnetic modified with biodegradable copolymer nanoparticles on hTERT gene expression in lung cancer cell line. Artificial Cells, Nanomedicine, and Biotechnology. 2016;44(1):188–193. doi: 10.3109/21691401.2014.934456. [DOI] [PubMed] [Google Scholar]