Abstract
Background:
Currently, the treatment of large hepatocellular carcinoma (HCC) is still a challenging problem. Transcatheter arterial chemoembolization (TACE) is the main treatment for intermediate end-stage HCC, while it is only a palliative and not a curative treatment due to the existence of residual tumors, and radiofrequency ablation (RFA) has limitations in complete ablation of large HCC. We hypothesized that TACE combined with simultaneous RFA (herein referred to as TACE + RFA) could improve the efficacy and survival of large HCC. This study aimed to investigate the feasibility, efficacy, and safety of TACE + RFA on single large HCC.
Methods:
A total of 66 patients with single large HCC (≥5 cm in diameter) were recruited between February 2010 and June 2016. TACE was first performed and computed tomography was performed immediately after TACE, and the lesions with poor lipiodol deposition were subjected to simultaneous RFA. The success rate, technique-related complications, liver and kidney functions, serum alpha-fetoprotein (AFP) levels, progression-free survival (PFS), median survival time (MST), focal control rate, and long-term survival rate were evaluated.
Results:
TACE + RFA were performed smoothly in all the patients with the success rate of 100%. Intra- and post-operative severe complications were not observed. There were no marked differences in mean alanine transaminase or aspartate transaminase before TACE + RFA compared with 7 days after TACE + RFA (all P > 0.05). In 57 AFP-positive patients, the levels of serum AFP were reduced by 100.0%, 100.0%, and 94.7% at 1, 3, and 6 months after TACE + RFA, respectively; the tumor control rates (complete remission + partial remission) were 100.0% (66/66), 92.4% (61/66), 87.9% (58/66), and 70.1% (39/55) at 1, 3, 6, and 12 months after TACE + RFA, respectively. Patients were followed up for 7–82 months after TACE + RFA. The MST was 18.3 months, PFS was 14.2 ± 6.2 months, and the 1-, 3-, and 5-year survival rates were 93.2% (55/59), 42.5% (17/40), and 27.2% (9/33), respectively.
Conclusion:
TACE + RFA is safe, feasible, and effective in enhancing the focal control rate and survival rate of patients with large HCC.
Keywords: Computed Tomography, Large Hepatocellular Carcinoma, Radiofrequency Ablation, Simultaneous, Transcatheter Arterial Chemoembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies and has a high morbidity and mortality, it is difficult to detect at an early stage. There are more than 1,000,000 new cases of HCC annually worldwide, and more than 55% of new cases are found in mainland China. Large HCC is defined as a tumor >5 cm in diameter. Currently, surgical resection is still the first choice for the treatment of HCC, but most patients are diagnosed with advanced HCC at initial diagnosis and, thus, are not surgical candidates. Resectable HCC is found in only about 20% of HCC patients.[1]
Transcatheter arterial chemoembolization (TACE) is an important technique for the treatment of unresectable HCC. However, TACE alone is usually unable to produce complete necrosis of large HCC due to the presence of collateral circulation, the opening of communicating branch vessels, a blood supply that extends to the edge of a large HCC from the portal vein, and vascular recanalization, which increases the difficulty of a second TACE.[2,3] Radiofrequency ablation (RFA) is a minimally invasive technique for the treatment of liver tumor and has the advantages of minimal invasiveness, rapid postoperative recovery, and proven efficacy. Thus, RFA has been an important nonsurgical alternative for the treatment of liver cancer. Liver cancers <3 cm in diameter can be radically cured by RFA, but radical treatment is not easy for large HCC.[4,5,6] Thus, for the treatment of large HCC, single nonsurgical interventional method usually has limited efficacy, and increasing attention has been paid to combination therapy of large HCC.
Studies have revealed that TACE combined with RFA may achieve better efficacy as compared with TACE alone or RFA alone,[7,8,9] but the timing and methodology of combined therapy are still controversial. Some clinicians proposed sequential treatment with TACE and RFA at an interval of 1–3 weeks with TACE being performed first, while others suggested RFA to be given first. In the recent years, some clinicians have attempted to employ cone-beam computed tomography (CBCT)-guided TACE in combination with immediate RFA for the treatment of large HCC.[10,11,12]
This study retrospectively reviewed 66 patients with large HCC who received CT-angiography-guided TACE combining with simultaneously RFA (TACE + RFA) to investigate the feasibility, efficacy, and safety of this combined treatment. The hybrid imaging system combines angiography and CT Sliding Gantry with both systems sliding over the same table to quickly switch between modalities. Using the system, TACE was performed first, and plain CT was performed immediately thereafter, and then precise RFA targeted the lesions with poor lipiodol deposition.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Chinese People's Liberation Army General Hospital. Informed written consent was obtained from all patients prior to their enrollment in this study.
Clinical characteristics
HCC treatment was based on the guidelines of the American Association for the Study of Liver Diseases (AASLD) for the clinical treatment of HCC. HCC was confirmed by ultrasonography, CT, magnetic resonance imaging (MRI), serum alpha-fetoprotein (AFP) levels, and pathological examination. A total of 66 patients with large HCC, who received TACE + RFA between February 2010 and June 2016 in Chinese People's Liberation Army General Hospital, were included in this study.
The inclusion and exclusion criteria were based on National Comprehensive Cancer Network Guidelines for the treatment of primary liver cancer (2015).[13] The inclusion criteria were as follows: (1) imaging examinations that showed a single lesion in the liver, the maximal diameter was ≥5 cm, and there were no indications for surgical resection or the patient refused to receive surgery; (2) Child-Pugh Grade was A or B; and (3) the expected survival time was longer than 3 months, and the Karnofsky score was ≥70. The exclusion criteria were as follows: (1) there was cancer embolus within the main portal vein or its left/right major branch, bile duct, inferior vena cava, or hepatic vein; (2) there was a hepatic arterial-portal venous fistula or hepatic arteriovenous fistula; (3) there was extrahepatic metastasis; (4) there was severe coagulation dysfunction; (5) acute infection or chronic infection at an acute phase was present; (6) patients who had implantation of cardiac pacemaker; (7) patients who had mental disorder, a history of mental disorder, or a history of epilepsy; and (8) patients who were unable to receive TACE or RFA.
Each patient underwent a full physical examination, review of medical history, routine laboratory examinations (including routine blood tests, routine urine tests, routine stool tests, blood biochemistries, coagulation tests, D-dimer detection, serum evaluation, detection of tumor markers, serum cholinesterase detection, and quantification of HBV load), head CT, chest CT, plain and enhanced MRI of the liver, gallbladder and spleen, or plain and enhanced abdominal CT, and bone scan or positron emission tomography CT. The maximal diameter was determined by MRI or CT.
All patients received TACE guided by Miyabi DSA-CT (Artis Zee DSA and Emotion 16-slice CT, SIEMENS MIYABI, Munich, Germany) in the Department of Interventional Radiology, Chinese People's Liberation Army General Hospital, and received plain CT immediately after TACE without being transferred, and lipiodol deposition was observed. The imaging findings were compared with those taken before TACE, and the residual lesions were determined. If necessary, enhanced CT was performed by injecting contrast agent via the cannulated hepatic artery to confirm the residual lesions, based on the region designated for simultaneous RFA.
Interventional procedures
Transcatheter arterial chemoembolization
TACE was performed using the DSA of Miyabi DSA-CT by specialists with at least 5 years of experience in interventional treatment. After routine sterilization, focal anesthesia with 1% lidocaine (10 ml) was given, and femoral arterial puncture was performed using the modified Seldinger technique. A 4F arterial sheath was inserted, followed by insertion of 4F arterial catheter (RH, Terumo, Japan) for selective celiac artery-hepatic arteriography and superior mesenteric artery angiography. A 3F microcatheter (RH, Terumo, Japan) was inserted for superselective chemoembolization. Three to five drugs were administered for chemoembolization including fluorouracil (500–1000 mg), calcium folinate (200–300 mg), pirarubicin hydrochloride (30–50 mg), oxaliplatin (100–150 mg), mitomycin (8–14 mg), and hydroxycamptothecin (10–14 mg). Liquid drugs were administered directly, and powdered drugs were mixed with lipiodol for injection. If necessary, gelatin sponge and polyvinyl alcohol (PVA) granules (300–500 μm) were used for embolization. If collateral circulation was observed (from the thoracic internal artery, subphrenic artery, adrenal artery, or intercostal artery), collateral arterial embolization was performed simultaneously.[14,15]
Radiofrequency ablation
After TACE, patients were asked to lie in the supine position, followed by plain CT examination. The CT images were carefully reviewed and the regions with poor lipiodol deposition were identified by comparing the images obtained before TACE + RFA, and their relationship with the surrounding blood vessels, tissue structure, and major organs was analyzed. Then, the puncture site and approach were determined. After routine sterilization, focal anesthesia with or without basic anesthesia was performed. Under CT of Miyabi DSA-CT guidance, the RFA needle (Model 1500, RITA Medical System, Mountain View, CA, USA) was used to puncture the tumor with a radiofrequency of 460 kHz. The electrode needles were classified as either multipolar or unipolar. A multipolar electrode needle had a 14G external catheter and an internal 9 umbrella-like hook-shaped cluster electrode with a maximal diameter of 5 cm at its opening. The distance between needle tip and distal cancer was 3 cm. The RFA needle was localized in real time under CT guidance, and then the electrode needle was inserted into cancer and push out internal umbrella-like hook-shaped cluster electrode 3–5 cm slowly according to ablation area and the adjacent structure. Make sure that there is a safe distance from cluster electrode tip to other important organs and tissues (i.e., the diaphragm, gastrointestinal tract, gallbladder, hilar area containing large blood vessels, and bile ducts). When the lesion was close to an important structure or organ, lidocaine in saline was injected to separate the lesion from the organ or important structure. The RFA power used was 150–200 W, and RFA was performed for 15–20 min at a temperature of 105°C. The RFA coverage extended to the edge of each lesion, and then the needle was withdrawn and its direction was adjusted, followed by further RFA. When the lesion was at the edge of the liver or close to the important organ, ablation could be performed with the unipolar electrode. The UniBlate™ unipolar electrode with a 17G external catheter (10–25 cm in length) had an area of ablation of 3 cm × 3 cm. After ablation, the needle was withdrawn, and the needle tunnel was ablated at 70°C–90°C to reduce the risk of hemorrhage, biliary fistula, or implantation metastasis via the needle tunnel. After RFA, the sheath in the femoral artery was removed and pressure was applied to the puncture for 10–15 min for hemostasis. Sterilized gauze was used as a pressure dressing at abdominal and groin puncture sites.
Postoperative management and follow-up
After surgery, routine electrocardiogram monitoring was continued for 24 h, and oxygen was administered at a low flow rate. Hydration, anti-infection precautions, liver protection, jaundice reduction, gastrointestinal motility promotion, laxatives, urine alkalization, and nutritional support were also administered after treatment. In the present study, antiviral therapy was administered to all patients who were positive for hepatitis B surface antigen. Analgesic drugs were prescribed if necessary. Detection of kidney and liver function, blood electrolytes, and routine blood tests were performed at 3–7 days after treatment. If fever accompanied by shaking chills was observed, blood was collected for culture, and antibiotics were adjusted according to the results of the bacterial culture. Liver and kidney function tests, blood electrolytes, routine blood tests, and cancer markers including AFP, carcino-embryonic antigen (CEA), and carbohydrate antigen 199 levels were measured 1 month after RFA. Either enhanced MRI or CT was performed to evaluate for residual cancer. If residual cancer was present, a second TACE + RFA, TACE alone, or RFA alone was performed, depending on the condition of the residual cancer. If there was no residual cancer, reexamination was performed once every 2–3 months (pre-, intra-, and post-operative images of a 69-year-old female receiving TACE + RFA twice [Figure 1]; pre-, intra-, and post-operative images of a 54-year-old male receiving TACE + RFA twice [Figure 2]). The progression-free survival (PFS) and overall survival were determined, and cancer markers were detected.
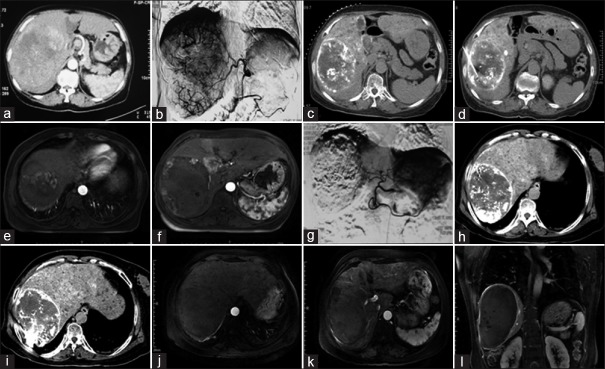
Figure 1.
Pre-, intra-, and post-operative images of a 69-year-old female receiving TACE + RFA twice. (a) A large HCC was observed by CT before the first treatment. (b) The blood supply to the cancer was observed during the first TACE. (c,d) Plain CT immediately after TACE, regions with poor lipiodol deposition were treated by simultaneous RFA. (e,f) MRI 1 month after treatment showed that residual lesions were observed at the top of the diaphragm and the edge of the tumor. (g) DSA during the second TACE. (h,i) RFA immediately after the second TACE. (j-l) MRI 3 months after the second treatment, the cancer was completely necrotic, and no cancer enhancement was present during arterial-phase imaging. TACE: Transcatheter arterial chemoembolization; RFA: Radiofrequency ablation; HCC: Hepatocellular carcinoma; DSA: Digital subtraction angiography; CT: Computed tomography; MRI: Magnetic resonance imaging.
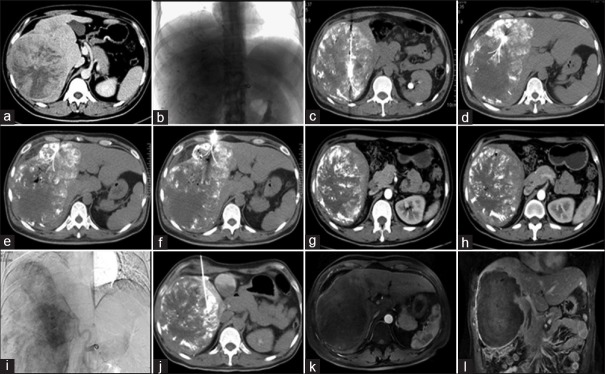
Figure 2.
Pre-, intra-, and post-operative images of a 54-year-old male receiving TACE + RFA twice. (a) A large HCC was observed by CT before the first treatment. (b) The blood supply to the cancer is observed during the first TACE. (c-f) Plain CT immediately after TACE, the cancer lesions were treated by simultaneous RFA at multiple sites. (g,h) CT 1 month after treatment; residual lesions were observed at the edge of cancer. (i) DSA during the second TACE. (j) Unipolar RFA immediately after the second TACE was performed in the residual lesion at the edge of cancer. (k,l) MRI 3 months after the second treatment; the cancer was completely necrotic and no arterial-phase enhancement was present. TACE: Transcatheter arterial chemoembolization; RFA: Radiofrequency ablation; HCC: Hepatocellular carcinoma; DSA: Digital subtraction angiography; CT: Computed tomography; MRI: Magnetic resonance imaging.
Evaluation of therapeutic efficacy
The modified response evaluation criteria in solid tumor of AASLD were employed for the evaluation of therapeutic efficacy. On the basis of findings from enhanced MRI or CT, the therapeutic efficacy was evaluated and focal control rate was analyzed. Evaluation was performed as follows: (1) complete remission (CR): all the target lesions disappeared; (2) partial remission (PR): the maximal diameter was reduced by at least 30% as compared to that at baseline; (3) disease progression (PD): the maximal diameter increased by 20% as compared to the maximal diameter of the smallest lesion, or one or more lesions were observed; (4) stable disease: the condition between PR and PD.[16] The efficacy (response rate) was calculated as CR + PR.[17]
Statistical analysis
SPSS version 19.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Quantitative data were expressed as mean ± standard deviation and compared using Student's t-test. A P < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
A total of 66 patients (39 males and 27 females) were included in this study. The mean age was 54.0 ± 11.3 years (range: 28–78 years). In addition, 57 patients were positive for AFP (>20 ng/ml) before TACE + RFA, and the remaining nine patients were negative for AFP. Forty-seven patients were positive for hepatitis B surface antigen, 10 patients were positive for hepatitis C antigen, and nine patients were negative for both. The maximal tumor diameter ranged from 5.2 cm to 19.7 cm (mean: 10.0 ± 3.3 cm). HCC measuring 5–10 cm in diameter was found in 38 patients and HCC >10 cm in diameter in 28 patients. Child-Pugh Grade A was found in 21 patients and Grade B in 45 patients.
Follow-up time
All the patients were followed up until they died or the cutoff date (January 2017). The shortest follow-up time was 6 months and the longest was 82 months. A total of 59 cases achieved 1-year follow-up after their first operations, 40 cases achieved 5-year follow-up after their first operations, and 33 cases achieved 5-year follow-up after their first operations. None was lost to follow-up.
Technique assessment
All the procedures were done smoothly in all the patients with a success rate of 100%, and severe complications were not observed during surgery.
Alpha-fetoprotein level
The change in postoperative AFP levels in 57 AFP positive patients at 1, 3, and 6 months after the first treatment is shown in Table 1. Nine AFP-negative patients were also negative for AFP at 1, 3, and 6 months after TACE + RFA.
Table 1.
Serum alpha-fetoprotein levels at 1, 3, and 6 months after the first treatment in 57 alpha-fetoproteinpositive patients, n (%)
| Time point | Returned to normal | Reduction of ≥50% | Reduction of <50% | Increase |
|---|---|---|---|---|
| 1 month | 19 (33.3) | 38 (66.7) | 0 (0.0) | 0 (0.0) |
| 3 months | 28 (49.1) | 27 (47.4) | 2 (3.5) | 0 (0.0) |
| 6 months | 25 (43.9) | 26 (45.6) | 3 (5.3) | 3 (5.3) |
Short-term focal cancer control rate
Focal cancer control rates (equivalent to efficiency) were the sum of CR and PR rates. At 12 months, 7 patients had been followed up for a period of <12 months and four patients had died within 12 months and were excluded from the analysis. Short-term (within 12 months postoperation), focal cancer control rates were shown in Table 2.
Table 2.
Focal cancer control at 1, 3, 6, and 12 months after TACE + RFA in 66 patients, n/N (%)
| Time point | Efficacy | SD | PD | |
|---|---|---|---|---|
| CR | PR | |||
| 1 month | 30/66 (45.5) | 36/66 (54.5) | 0/66 (0.0) | 0/66 (0.0) |
| 3 months | 29/66 (43.9) | 32/66 (48.5) | 3/66 (4.5) | 2/66 (3.0) |
| 6 months | 27/66 (40.9) | 31/66 (47.0) | 5/66 (7.6) | 7/66 (4.5) |
| 12 months | 18/55 (32.7) | 21/55 (38.2) | 10/55 (18.2) | 6/55 (10.9) |
CR: Compete remission; PR: Partial remission; SD: Stable disease; PD: Disease progression; TACE: Transcatheter arterial chemoembolization; RFA: Radiofrequency ablation.
Liver function change
Transient increases in both alanine transaminase (ALT) and aspartate transaminase (AST) were observed in 66 patients at approximately 3 days after TACE + RFA, but they returned to normal levels at 5–7 days after TACE + RFA. The mean ALTs were 62.3 ± 17.1 U/L before TACE + RFA, and 156.9 ± 122.5 U/L and 63.4 ± 15.6 U/L at 3 and 7 days after TACE + RFA, respectively; and the mean ASTs were 81.3 ± 16.3 U/L before TACE + RFA, and 345.4 ± 178.2 U/L and 92.1 ± 21.3 U/L at 3 and 7 days after TACE + RFA, respectively. ALTs before TACE + RFA were significantly different from those at 3 days after TACE + RFA (t = −6.449, P < 0.001), but similar to those at 7 days after TACE + RFA (t = 0.126, P = 0.755). ASTs before TACE + RFA were significantly different from those at 3 days after TACE + RFA (t = −17.776, P < 0.001), but similar to those at 7 days after TACE + RFA (t = 0.177, P = 0.860).
Median survival time and progression-free survival
All patients subsequently did not receive other antitumor treatments thereafter such as chemotherapy, immunotherapy, radiotherapy, and targeted therapies during their follow-up period. Patients were followed up for 7–82 months and the survival time of all the patients was longer than 6 months. The shortest survival time was 7 months and the longest survival time was 82 months; the median survival time (MST) was 18.3 months; the mean PFS was 14.2 ± 6.2 months.
Survival rate
The 1-, 3-, and 5-year survival rates were 93.2% (55/59), 42.5% (17/40), and 27.2% (9/33), respectively. There were 30 dead cases, among which six cases died of acute esophageal variceal bleeding, five cases died of hepatic encephalopathy, three cases died of multiple metastases of pulmonary combined with infection and respiratory failure, three cases died of obstructive jaundice, two cases died of hepatopulmonary syndrome, and the other 11 cases died of other complications of liver cancer, such as cachexia, rupture, and hemorrhage.
Postoperative complications
Severe complications related to TACE and RFA (such as severe infection, skin burn, heavy bleeding, liver abscess, ectopic embolization, diaphragmatic fistula, gallbladder and intestinal necrosis perforation, liver failure, or renal failure) were not observed in any of the 66 patients. The major postoperative complication was embolization syndrome. Varying severities of fever appeared in all the 66 patients. Moreover, the body temperature fluctuated between 38.1°C and 39.5°C. On the basis of numerical rating scale (NRS) criteria of pain, mild pain was diagnosed in 36 patients, moderate pain in 25, and pain radiating to the right shoulder was noted in five patients, but pain was significantly relieved after 2–5 days of analgesic treatment. In 5 patients, intraoperative hemoglobinuria was noted which abated 2–3 days after hydration and urine alkalization. Creatinine and urea nitrogen rose in 6 cases but all returned to normal after 5 days. Intra- and post-operative increases in blood pressure were found in seven patients which were normalized 6–12 h after symptomatic treatment. In 19 patients, moderate nausea and vomiting were observed after TACE + RFA which were relieved after symptomatic treatment, and no evident of nausea or vomiting was noted in the majority of patients. Six patients had hiccups which relieved 2–3 days after symptomatic treatment. Grades 3 and 4 side effects (CTCEA) were not observed after TACE + RFA.
DISCUSSION
Surgical resection is the first choice for treatment of large HCC, but the cancer is usually unresectable or the patients often refuse surgery due to the large size of the tumor, improper localization, poor liver function, postoperative complications, and high recurrence rate. TACE is an alternative treatment for unresectable large liver cancer. It can reduce the blood supply to the tumor, and induce tumor necrosis, but TACE may not achieve complete pathological necrosis and the cancer will develop collateral circulation over time. In addition, the edge of a large liver cancer is often supplied by the portal vein, which also affects the efficacy of TACE. Thus, TACE is usually performed repeatedly, with a subsequent increase in the dose of drugs used for embolization and chemotherapy which causes damage to the hepatic blood vessels and liver function. Moreover, repeated TACE may cause stenosis and discontinuation of large blood vessels within the cancer, which may make delivery of the drugs difficult. TACE may cause ischemia and hypoxia in the cancer, leading to the elevation of hypoxia-inducible factor (HIF), which then upregulates vascular endothelial growth factor (VEGF), resulting in intrahepatic recurrence and distal metastasis. Thus, disease control is difficult to achieve, leading to the presence of postoperative residual cancer and high incidence of recurrence,[18,19,20] resulting in a poor prognosis.
RFA may cause coagulative necrosis of the cancer by inducing ion oscillation and frictional heat while killing cancer cells. As a minimally invasive treatment for liver cancer, RFA has minimal invasiveness, rapid recovery, and documented efficacy, and has become one of the common treatments for liver cancer. Studies have shown that the 5-year survival of patients with small liver carcinomas after RFA was comparable to that after surgical resection.[4,5,6] However, for liver cancers >5 cm in diameter (especially >10 cm), RFA alone usually fails to radically cure the cancer. For patients with large liver cancer, any single interventional treatment is often unable to radically cure the cancer. Evidences showed that TACE combined with simultaneous RFA was superior to TACE alone or RFA alone in the treatment of large HCC,[21,22,23] but the timing of combined therapy is still controversial. Some clinicians recommended that RFA should be performed first and subsequent TACE may target the residual cancer to kill cancer cells,[24] while the majority of clinicians recommended that RFA should be performed 1 week to 1 month after TACE because they speculated that RFA would cause damage to the arteries supplying the cancer, which was detrimental to subsequent TACE, and the interval between TACE and RFA might allow recovery of liver function.[25] TACE may cause ischemia and hypoxia in the cancer, leading to the elevation of HIF, which then upregulates VEGF, resulting in intrahepatic recurrence and distal metastasis. Thus, simultaneous RFA following TACE is necessary for radical cure of residual cancer after TACE.[26] In addition, the blood vessels are completely occluded immediately after TACE and the reduction in blood supply is the most obvious within the cancer while heat loss via the circulation is the lowest. Thus, simultaneous RFA immediately after TACE will achieve the largest extent of ablation, which increases the complete ablation rate and reduces the recurrence rate. In addition, simultaneous RFA following TACE may minimize the clearance of lipiodol and angiogenesis within the cancer, which maximizes ablation efficacy. Our previous studies showed that TACE combined with simultaneous CBCT-guided RFA achieved better clinical efficacy in patients with large liver cancer.[10,27]
TACE with RFA may exert synergistic effects on large liver cancer as follows: (1) TACE not only blocks the blood supply to the cancer, but reduces the influence of blood circulation on heat ablation. Lipiodol has a heat conduction effect, which is helpful for the conduction of heat. Thus, the focal temperature may increase rapidly and an even temperature within the cancer may induce complete coagulation necrosis and effectively kill cancer cells. In addition, TACE can be employed to treat lesions that are not identified on the imaging examinations and subclinical lesions; (2) CT with lipiodol shows a high contrast. Comparison of images after TACE with those before TACE allows for precise determination of the regions with poor lipiodol deposition, which improves the safety of puncture. The radiofrequency temperature control system may detect the extent of ablation and the temperature of surrounding organs in a real-time manner, which is helpful for the protection of organs and tissues at risk and avoids the damage to these organs or tissues. Thus, the liver tissues not invaded by the cancer are preserved, which also improves liver reserve capacity; (3) the lipiodol deposition region forms a circle. The heat conduction effect of lipiodol may be employed to concentrate the heat in the lipiodol region and surrounding tissues,[28,29] forming an “oven phenomenon.” This expands the extent of ablation and inactivates the edge of the cancer, which reduces recurrence; (4) the necrotic tissues after RFA may induce immune response to cancer cells;[30,31] and (5) intensive chemotherapy may exert synergistic effects with heat ablation. Chemotherapeutics may inhibit the tolerance of cancer cells to heat, and RFA will increase the sensitivity of cancer cells to chemotherapeutics;[32] and (6) normal hepatocytes will proliferate after treatment, leading to compensatory enlargement of the liver, which improves liver function and reduces cancer stage.
Precise TACE is important. In this study, TACE + RFA were performed by specialists with at least 5 years of experience in the field. They were skilled at maximizing the identification of collateral circulation. Collateral angiography can be performed at the diaphragmatic artery, intercostal artery, adrenal artery, or the thoracic artery, besides the routine celiac artery and superior mesenteric arteries, if necessary, and super-selective embolization can be achieved.[14,15] Chemotherapeutics targeting different phases of the cell cycle are recommended, and combined use of chemotherapeutics is preferred. Granule embolization (such as PVA 300–500 μm for permanent embolization) may improve efficacy. The blood vessels should be carefully checked during angiography before embolization, and lipiodol and embolic agents should be slowly and evenly injected to prevent reflux of lipiodol, leading to ectopic embolization. CT is performed immediately after TACE to observe lipiodol deposition. The images are compared with those before TACE + RFA to observe lipiodol deposition, aiming to identify residual lesions. If necessary, enhanced CT may be performed after injection of contrast via the catheterized hepatic artery. Residual lesions are identified and the extent of RFA is determined.
During TACE + RFA, focal anesthesia and intravenous anesthesia are often employed. In routine ablation, pethidine (50 mg) and phenergan (25 mg) may be administered to alleviate pain when ablation is performed close to the diaphragm or the edge of the liver. Focal injection of 1% lidocaine in saline (10–30 ml) will not only attenuate pain, but also reduce impedance, leading to an increase in ablation efficiency. In addition, injection of normal saline (100–500 ml) into the regions surrounding the liver, close to the top of the diaphragm, gastrointestinal tract, and gallbladder will not only attenuate pain, but also achieve isolative effect, which may assure tolerance to long ablation, avoid damage to the surrounding abdominal wall, diaphragm, gastrointestinal tract, gallbladder, and right kidney, and reduce postoperative complications.[33] If fluid fails to be retained in effective spaces in the body, it flows into the abdominal cavity and therefore cannot play a role in isolation. Then, puncture is done with a cone needle to the area beside the stomach, gallbladder, and gastrointestinal tract, and the blunt head of the needle is used to push the cavity organs dorsally, which may protect these organs and also assure sufficient ablation of intrahepatic lesions. In addition, for lesions close to the diaphragm, puncture is performed from caudal to cranial direction to avoid damage to the lung and diaphragm; for lesions close to the gallbladder and pericardium, puncture is done parallel to the gallbladder and pericardium, and vertical puncture should be avoided or it will cause damage to these organs. The distance to the edge of the lesion should be precisely calculated before the use of ablation needle, which may avoid damage to at-risk organs. Patients are asked to hold their breath to avoid damage to the liver capsule. When the needle is withdrawn, hemostasis of needle tunnel should be performed to avoid implantation metastasis via the needle tunnel.[34] Clinicians should also communicate with their patients during the ablation and closely monitor their vital signs.
The major side effect of TACE + RFA is the postembolization syndrome, including postoperative fever, pain, nausea and vomiting, transient liver dysfunction, and hiccups. These side effects will resolve within 2–7 days after symptomatic treatment. In the present study, simultaneous RFA following TACE did not increase the incidence of postembolization syndrome, but attenuated this response. This might be explained by the fact that, as liquefaction necrosis is induced by TACE, simultaneous RFA might induce coagulation necrosis of cancer tissues, which is effective in reducing the absorption of necrotic tissues. Thus, TACE combined with simultaneous RFA would attenuate postembolization syndrome as compared with TACE alone. After TACE, the blood vessels are occluded within the cancerous tissues, which reduce the risk of hemorrhage. Nevertheless, if damage is caused to the intercostal vessels, blood vessels in the liver capsule, and phrenic vessels during RFA, it is not necessary to move the patient, and clinicians may perform hemostasis by embolization under the guidance of DSA of SIEMENS Miyabi DSA-CT. Under this condition, ablation may stimulate the diaphragm and pleura to cause a responsive effusion. Intraoperative hemoglobinuria was noted in five patients, which might be related to the use of contrast, extensive cancer necrosis after ablation, and subsequent occlusion of renal tubules by macromolecules. The hemoglobinuria resolved within 2–3 days after hydration and urine alkalization treatments, and severe complications such as renal failure were not observed. In the present study, skin burn was not observed. The electrode should be attached to the base of the leg where the soft tissues are the thickest. The site where the electrode is placed should be monitored during TACE + RFA, and ice should be applied to lower the focal temperature, if necessary.
This was a retrospective, single-arm study without a tight comparative parallel-controlled group, including TACE alone group or RFA alone group, which should be improved in the future research. The sample size in the present study was small, and we need to carry out further multicenter studies with a large simple size and longer follow-up. However, this study had an advantage in the results of MST, PFS, and survival rates over the findings of other research, whose treatment methods included TACE alone or RFA alone. The novelties of the present study were as follows:first, this study focused on large (>5 cm in diameter) and huge (>10 cm in diameter) HCC, of which the treatment was even more complicated. Second, previous studies mainly investigated sequential treatment with TACE and RFA at an interval of 1–3 weeks, with TACE being performed first, and others arranged RFA first, while this study was TACE combined with simultaneous RFA.
In conclusion, TACE + RFA is an effective, safe, and precise technique for the treatment of large HCC and could improve the focal control rate and survival rate. Thus, this technique is worthy of being promoted in clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753–70. doi: 10.1016/j.bpg.2014.08.007. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.de Baere T, Arai Y, Lencioni R, Geschwind JF, Rilling W, Salem R, et al. Treatment of liver tumors with lipiodol TACE: Technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39:334–43. doi: 10.1007/s00270-015-1208-y. doi: 10.1007/s00270-015-1208-y. [DOI] [PubMed] [Google Scholar]
- 3.Massmann A, Rodt T, Marquardt S, Seidel R, Thomas K, Wacker F, et al. Transarterial chemoembolization (TACE) for colorectal liver metastases – Current status and critical review. Langenbecks Arch Surg. 2015;400:641–59. doi: 10.1007/s00423-015-1308-9. doi: 10.1007/s00423-015-1308-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhu ZX, Huang JW, Liao MH, Zeng Y. Treatment strategy for hepatocellular carcinoma in China: Radiofrequency ablation versus liver resection. Jpn J Clin Oncol. 2016;46:1075–80. doi: 10.1093/jjco/hyw134. doi: 10.1093/jjco/hyw134. [DOI] [PubMed] [Google Scholar]
- 5.Hocquelet A, Balageas P, Laurent C, Blanc JF, Frulio N, Salut C, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: A study of 281 Western patients. Int J Hyperthermia. 2015;31:749–57. doi: 10.3109/02656736.2015.1068382. doi: 10.3109/02656736. [DOI] [PubMed] [Google Scholar]
- 6.Francica G, Saviano A, De Sio I, De Matthaeis N, Brunello F, Cantamessa A, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: A retrospective analysis of 363 patients. Dig Liver Dis. 2013;45:336–41. doi: 10.1016/j.dld.2012.10.022. doi: 10.1016/j.dld.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: A prospective randomized trial. Radiology. 2012;262:689–700. doi: 10.1148/radiol.11110637. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 8.Hou YF, Wei YG, Yang JY, Wen TF, Xu MQ, Yan LN, et al. Combined hepatectomy and radiofrequency ablation versus TACE in improving survival of patients with unresectable BCLC stage B HCC. Hepatobiliary Pancreat Dis Int. 2016;15:378–85. doi: 10.1016/s1499-3872(16)60089-9. doi: 10.1016/S1499-3872(16)60089-9. [DOI] [PubMed] [Google Scholar]
- 9.Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC) Eur Radiol. 2006;16:661–9. doi: 10.1007/s00330-005-0029-9. doi: 10.1007/s00330-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Chang ZF, et al. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. World J Gastroenterol. 2013;19:4192–9. doi: 10.3748/wjg.v19.i26.4192. doi: 10.3748/wjg.v19.i26.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. The tumor marker score is an independent predictor of survival in patients with recurrent hepatocellular carcinoma. Surg Today. 2015;45:1513–20. doi: 10.1007/s00595-014-1102-2. doi: 10.1007/s00595-014-1102-2. [DOI] [PubMed] [Google Scholar]
- 12.Arai T, Kobayashi A, Ohya A, Takahashi M, Yokoyama T, Shimizu A, et al. Assessment of treatment outcomes based on tumor marker trends in patients with recurrent hepatocellular carcinoma undergoing trans-catheter arterial chemo-embolization. Int J Clin Oncol. 2014;19:871–9. doi: 10.1007/s10147-013-0634-6. doi: 10.1007/s10147-013-0634-6. [DOI] [PubMed] [Google Scholar]
- 13.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: Hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–91. doi: 10.6004/jnccn.2009.0027. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Wang M, Duan F, Song P, Liu F. Bile duct injury after transcatheter arterial chemoembolization: Risk factors and clinical implications. Hepatogastroenterology. 2014;61:947–53. doi: 10.5754/hge121342. [PubMed] [Google Scholar]
- 15.Zhao Y, Fang Z, Luo J, Liu Q, Xu G, Pan H, et al. Evaluation of extrahepatic collateral arteries in hepatocellular carcinoma in three independent groups in a single center. Exp Ther Med. 2015;10:2366–74. doi: 10.3892/etm.2015.2822. doi: 10.3892/etm.2015.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Ng SH, Lin CY, Chan SC, Yen TC, Liao CT, Chang JT, et al. Dynamic contrast-enhanced MR imaging predicts local control in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiotherapy. PLoS One. 2013;8:e72230. doi: 10.1371/journal.pone.0072230. doi: 10.1371/journal.pone.0072230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho Y, Sinn DH, Yu SJ, Gwak GY, Kim JH, Yoo YJ, et al. Survival analysis of single large (>5 cm) hepatocellular carcinoma patients: BCLC A versus B. PLoS One. 2016;11:e0165722. doi: 10.1371/journal.pone.0165722. doi: 10.1371/journal.pone.0165722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, Li RS, Wang J, Huang YQ, Li PY, Wang J, et al. The therapeutic efficacy and safety of compound Kushen injection combined with transarterial chemoembolization in unresectable hepatocellular carcinoma: An update systematic review and meta-analysis. Front Pharmacol. 2016;7:70. doi: 10.3389/fphar.2016.00070. doi: 10.3389/fphar.2016.000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins MC, Soulen MC. Combining locoregional therapies in the treatment of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30:74–81. doi: 10.1055/s-0033-1333656. doi: 10.1055/s-0033-1333656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhanasekaran R, Khanna V, Kooby DA, Kauh JS, Carew JD, Kim HS. Chemoembolization Combined with RFA for HCC:Survival Benefits and Tumor Treatment Response. JCT. 2013;04:493–9. doi:10.4236/jct.2013.42060. [Google Scholar]
- 22.Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: A time-to-event meta-analysis. Korean J Radiol. 2016;17:93–102. doi: 10.3348/kjr.2016.17.1.93. doi: 10.3348/kjr.2016.17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: A meta-analysis. Dig Dis Sci. 2013;57:3026–31. doi: 10.1007/s10620-012-2212-6. doi: 10.1007/s10620-013-2570-8. [DOI] [PubMed] [Google Scholar]
- 24.Xu RC, Liu HC, Li JL, Li K, Ou SY, Yu ZY, et al. Long-term outcome of transcatheter arterial chemoembolization after radiofrequency ablation as a combined therapy for Chinese patients with hepatocellular carcinoma. Curr Med Res Opin. 2015;31:1553–60. doi: 10.1185/03007995.2015.1058249. doi: 10.1185/03007995. [DOI] [PubMed] [Google Scholar]
- 25.Azuma S, Asahina Y, Nishimura-Sakurai Y, Kakinuma S, Kaneko S, Nagata H, et al. Efficacy of additional radiofrequency ablation after transcatheter arterial chemoembolization for intermediate hepatocellular carcinoma. Hepatol Res. 2016;46:312–9. doi: 10.1111/hepr.12566. doi: 10.1111/hepr.12566. [DOI] [PubMed] [Google Scholar]
- 26.Slomiany BL, Slomiany A. Helicobacter pylori-induced gastric mucosal TGF-α ectodomain shedding and EGFR transactivation involves Rac1/p38 MAPK-dependent TACE activation. Inflammopharmacology. 2016;24:23–31. doi: 10.1007/s10787-015-0254-z. doi: 10.1007/s10787-015-0254-z. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Wang Y, et al. Clinical application of transcatheter arterial chemoembolization combined with synchronous C-arm cone-beam CT guided radiofrequency ablation in treatment of large hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:1649–54. doi: 10.7314/apjcp.2013.14.3.1649. doi: 10.7314/APJCP.2013.14.3.1649. [DOI] [PubMed] [Google Scholar]
- 28.Akhundi A, Habibi-Yangjeh A. Novel g-C3N4/Ag2SO4 nanocomposites: Fast microwave-assisted preparation and enhanced photocatalytic performance towards degradation of organic pollutants under visible light. J Colloid Interface Sci. 2016;482:165–74. doi: 10.1016/j.jcis.2016.08.002. doi: 10.1016/j.jcis.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 29.DeSantis M, Bowne W, Ferretti JA, Dalessandro T, Kumar A, Sclafani SJ, et al. In situ nanocarbon assisted microwave therapy (NAMT) for application of targeting human prostate tumor cells in nude mice causing cytotoxic thermal ablation using extremely short cycle microwave energy as primary therapy. J Vasc Interv Radiol. 2013;24:4. doi: 10.1016/j.jvir.2013.01.287. [Google Scholar]
- 30.McDonnell AM, Nowak AK, Lake RA. Contribution of the immune system to the chemotherapeutic response. Semin Immunopathol. 2011;33:353–67. doi: 10.1007/s00281-011-0246-z. doi: 10.1007/s00281-011-0246-z. [DOI] [PubMed] [Google Scholar]
- 31.Liu SR, Xiao YY, Le Pivert PJ, Wu B, Zhang X, Ma XY, et al. CT-guided percutaneous chemoablation using an ethanol-ethiodol-doxorubicin emulsion for the treatment of metastatic lymph node carcinoma: A comparative study. Technol Cancer Res Treat. 2013;12:165–72. doi: 10.7785/tcrt.2012.500254. doi: 10.7785/tcrt.2012.500254. [DOI] [PubMed] [Google Scholar]
- 32.Vignati G. Pediatric arrhythmias: Which are the news? J Cardiovasc Med (Hagerstown) 2007;8:62–6. doi: 10.2459/01.JCM.0000247438.12817.9e. doi: 10.2459/01.JCM.0000247438.12817.9e. [DOI] [PubMed] [Google Scholar]
- 33.Deng Y, Naeini PS, Razavi M, Collard CD, Tolpin DA, Anton JM. Anesthetic management in radiofrequency catheter ablation of ventricular tachycardia. Tex Heart Inst J. 2016;43:496–502. doi: 10.14503/THIJ-15-5688. doi: 10.14503/THIJ-15-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbold T, Wahba R, Bangard C, Demir M, Drebber U, Stippel DL. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma – Indication, technique and results. Langenbecks Arch Surg. 2013;398:47–53. doi: 10.1007/s00423-012-1018-5. doi: 10.1007/s00423-012-1018-5. [DOI] [PubMed] [Google Scholar]