Abstract
Background:
Estimating the grades of liver inflammation is critical in the determination of antiviral therapy in patients chronically infected with hepatitis B virus (HBV). The aim of this study was to investigate the correlation of serum levels of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) with the liver inflammation grades in treatment-naïve patients with chronic HBV infection.
Methods:
We retrospectively enrolled 584 treatment-naïve HBeAg-positive patients who underwent liver biopsy in Ditan Hospital from January 2008 to January 2016. Based on the severity of liver inflammation, the patients were divided into minimal, mild, and moderate groups. SPSS software was used for statistical analysis of all relevant data.
Results:
The liver histological examinations showed that 324, 194, and 66 patients had minimal, mild, and moderate liver inflammation, respectively. The median age of the three groups was 30, 33, and 38 years, respectively (χ2 = 26.00, P < 0.001). The median HBsAg levels in minimal, mild, and moderate inflammation groups were 4.40, 4.16, and 3.67 log U/ml, respectively, and the median HBeAg levels in the three groups were 3.12, 2.99, and 1.86 log sample/cutoff, respectively; both antigens tended to decrease as the grade of inflammation increased (χ2 = 99.68 and χ2 = 99.23, respectively; both P < 0.001). The cutoff values of receiver operating characteristic curve in the age, HBsAg and HBeAg levels were 36 years, 4.31 log U/ml, and 2.86 log S/CO, respectively, l to distinguish minimal grade and other grades of treatment-naïve HBeAg-positive patients with chronic HBV infection.
Conclusions:
Serum HBsAg and HBeAg quantitation might gradually decrease with aggravated liver inflammation and the corresponding cutoff values might help us to distinguish minimal grades and other grades and detect those who do not need antiviral therapy in treatment-naïve HBeAg-positive patients with chronic HBV infection.
Keywords: Chronic Hepatitis B Virus Infection, Hepatitis B e Antigen, Hepatitis B Surface Antigen, Liver Inflammation
INTRODUCTION
The natural history of chronic hepatitis B virus (HBV) infection is usually divided into four states: immune tolerance, immune clearance or hepatitis B e antigen (HBeAg) positive immune activation, inactive hepatitis B surface antigen (HBsAg) carrier, and HBeAg negative chronic hepatitis B (CHB), according to the American Association for the Study of Liver Diseases (AASLD) and Asian Pacific Association for the Study of the Liver (APASL).[1,2] The new guidelines of European Association for the Study of the Liver (EASL) of 2017 emphasize the liver inflammation, and accordingly the chronic HBV-infected patients could be divided into infection or hepatitis stages according to the presence or absence of liver inflammation and its severity.[3] In the natural history of chronic HBV-infected patients, the evolution of liver inflammation was accompanied by changes in age, serum HBsAg, and HBeAg quantitation under a regular pattern in the absence of antiviral treatment intervention.[4] We attempted to study the correlation of serum HBsAg and HBeAg levels with liver inflammation grades and find out whether some of the treatment-naïve HBeAg-positive patients with chronic HBV-infection in China do not need the antiviral therapy.
METHODS
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institute (No. 201703601). Written informed consent was obtained from all patients before their enrollment in this study.
Patients and study design
We retrospectively enrolled 584 treatment-naïve HBeAg-positive patients who underwent liver biopsy in Ditan Hospital from January 2008 to January 2016. Entry standards: patients who were infected with CHB for more than 6 months, e antigen positive without antiviral treatment and experienced liver biopsy were recruited. The enrolled patients also had alanine aminotransferase (ALT) levels ≤2 folds of upper limit normal (ULN, 80 U/L) for half a year and HBV DNA ≥103 U/ml. Exclusion criteria: patients with liver cirrhosis, with other combined viral hepatitis, i.e., hepatitis A, C, D, E, or HIV infection, alcoholic liver diseases, drug-related liver injury, autoimmune hepatitis, metabolic liver disease, or liver cancer were excluded. Pregnant female patients were also excluded. All patients had data of following parameters: the quantitative HBsAg/HBeAg, blood routine tests, liver function tests, HBV DNA quantitation and blood coagulation determined using blood samples. All stained specimens of liver biopsy were scored using Knodell scoring system (0–18) by three pathologists. These patients were grouped according to the Knodell scores (0–18) of pathology: minimal group (0–3); mild group (4–8); moderate group (9–12); and severe group (13–18).[5]
Serological assays
Serum HBsAg and HBeAg levels were measured by an Abbott Architect i2000 detection reagent (Abbott Diagnostics, Abbott Park, IL, USA); the HBsAg dynamic range was 0.05–250 U/ml. Samples with HBsAg levels >250 U/ml were diluted to 1:500–1:1000. The positive HBeAg levels were defined as >1 sample/cutoff (S/CO).
Liver biopsy
Liver biopsy was performed with the guidance of color Doppler ultrasound (Siemens Company, NJ, USA) using a puncture needle of 16G named Max-Core (BARD, Peripheral Vascular, Inc., USA). Nikon Eclipse 80i Microscope (Nikon, Inc., Japan) was used for examination of the stained slices of liver biopsy specimens. The specimens of liver biopsy were independently read by three pathologists, and the average scores were taken as the final Knodell scores.
Statistical analysis
Serum HBsAg and HBeAg concentrations were logarithmically transformed for analysis. Continuous variables were expressed as median (interquartile). Comparisons were performed with Kruskal-Wallis and Mann-Whitney U-tests for nonparametric data. Fisher's exact or Chi-square test was applied for comparison of categorical data. Two-tailed tests were used, P < 0.05 was considered statistically significant and P < 0.01 was considered highly statistically significant. The correlation between liver inflammation and age, serum HBsAg, and HBeAg were analyzed by ordinal logistic regression analysis. Statistical analysis of receiver operating characteristic curve (ROC) and cutoff values was carried out by SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics
Totally, 584 patients were included in this study. The liver histopathological examinations showed that 324, 194, and 66 patients had minimal, mild, and moderate liver inflammation, respectively. The median age of the patients in the three groups was 30, 33, and 38 years, respectively (χ2 = 26.00, P < 0.001). The median HBsAg levels in minimal, mild, and moderate inflammation groups were 4.40, 4.16, and 3.67 log U/ml respectively, and the median HBeAg levels in the three groups were 3.12, 2.99, and 1.86 log S/CO, respectively; both antigens tended to decrease as the inflammation increased [all P < 0.001, Table 1].
Table 1.
Baseline characteristics of age, serum HBsAg, HBeAg quantitation in the minimal, mild, moderate grades in treatment-naïve HBeAg-positive chronic HBV-infected patients
| Parameters | Minimal group (n = 324) | Mild group (n = 194) | Moderate group (n = 66) | χ2 | P |
|---|---|---|---|---|---|
| Gender (female:male) | 97:227 | 71:123 | 15:51 | 5.23 | 0.073 |
| Age (years) | 30.00 (25.00, 38.00) | 33.00 (27.00, 41.00) | 38.00 (31.00, 43.00) | 26.00 | <0.001 |
| HBsAg (log U/ml) | 4.40 (4.21, 4.63) | 4.16 (3.57, 4.40) | 3.67 (3.37, 3.96) | 99.68 | <0.001 |
| HBeAg (log S/CO) | 3.12 (2.99, 3.18) | 2.99 (1.55, 3.13) | 1.86 (0.87, 2.61) | 99.23 | <0.001 |
Data are shown as n or median (interquartile). HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; S/CO: Sample/cutoff; HBV: Hepatitis B virus.
The results showed that 55.48% (324/584) of patients with HBeAg-positive chronic HBV-infection were in minimal liver lesion stage and 44.52% (260/584) of patients were in other stages (mild and moderate).
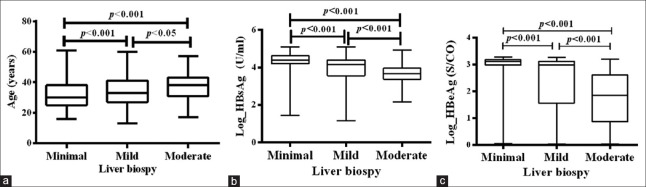
As age increased, liver inflammation grades increased. As the quantitation of serum HBsAg and HBeAg decreased, inflammation grades increased [Figure 1].
Figure 1.
Comparison of the baseline characteristics of age, serum HBsAg, HBeAg quantitation in the minimal, mild, moderate grades of liver inflammation in treatment-naïve HBeAg-positive chronic HBV-infected patients. (a) The comparison results of the baseline age of the minimal, mild, moderate grade in treatment-naïve HBeAg-positive chronic HBV-infected patients. As age increases, liver inflammation grades increase. Minimal versus mild (z = −51.870, P < 0.001); minimal versus moderate (z = −103.178, P < 0.001); mild versus moderate (z = −51.308, P = 0.033). (b) The comparison results of the baseline HBsAg of the minimal, mild, moderate grade in treatment-naïve HBeAg-positive chronic HBV-infected patients. As the quantitation of HBsAg decrease, inflammation grades increase. Minimal versus mild (z = 100.788, P < 0.001); minimal versus moderate (z = 199.578, P < 0.001); mild versus moderate (z = 98.790, P < 0.001). (c) The comparison results of the baseline HBeAg the minimal, mild, moderate grade in HBeAg-positive CHB. As the quantitation of HBeAg decrease, inflammation grades increase. Minimal versus mild (z = 94.092, P < 0.001); minimal versus moderate (z = 207.829, P < 0.001); mild versus moderate (z = 113.804, P < 0.001). HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; S/CO: Sample/cutoff; CHB: Chronic hepatitis B.
Correlation between liver inflammation grades and age, serum hepatitis B surface antigen, and hepatitis B e antigen quantitation
Data of correlation between liver inflammation grades and age, serum HBsAg, and HBeAg quantitation by ordinal logistic regression analysis are shown in Table 2.
Table 2.
Correlation between different liver inflammation grades and age, serum HBsAg and HBeAg quantitation in treatment-naïve HBeAg-positive chronic HBV-infected patients
| Parameters | B | SE | Wald | P | 95% CI |
|---|---|---|---|---|---|
| Age (years) | 0.018 | 0.007 | 6.981 | 0.008 | 0.005–0.031 |
| HBsAg (log U/ml) | −0.267 | 0.107 | 6.184 | 0.013 | −0.478–−0.057 |
| HBeAg (log S/CO) | −0.362 | 0.074 | 24.067 | <0.001 | −0.506–−0.217 |
| Sex (female/male) | −0.114 | 0.135 | 0.711 | 0.399 | −0.380–0.151 |
HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; S/CO: Sample/cutoff; CI: Confidence interval; HBV: Hepatitis B virus; SE: Standard error.
A positive correlation was found between age and liver inflammation grades, P = 0.008 (B = 0.018, 95% confidence interval [CI], 0.005, 0.031). A negative correlation was found between serum HBsAg or HBeAg quantitation and liver inflammation grades, P = 0.013 (B = −0.267, 95% CI [−0.478, −0.057]) and P < 0.001 (B = −0.362, 95% CI [−0.506, −0.217]). No correlation was found between sex and the liver inflammation.
ROC and cutoff value of differentiating minimal grade and other (mild and moderate) grades in treatment-naïve HBeAg-positive chronic HBV-infected patients [Table 3 and Figure 2].
Table 3.
The AUC and CO values of age, serum HBsAg, HBeAg quantitation for minimal grade and other grades (mild and moderate) in HBeAg-positive chronic HBV-infected patients
| Parameters | CO value for discriminating minimal grade | AUC (95% CI) | SEN (%) | SPE (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Age (years) | 36.00 | 0.613 (0.567–0.658) | 68.94 | 50.31 | 55 | 62 |
| HBsAg (log U/ml) | 4.31 | 0.710 (0.667–0.753) | 67.42 | 72.22 | 72 | 69 |
| HBeAg (log S/CO) | 2.86 | 0.711 (0.668–0.755) | 55.68 | 83.64 | 65 | 73 |
AUC: Area under receiver operating characteristic curve; SEN: Sensitivity; SPE: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; CI: Confidence interval; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; S/CO: Sample/cutoff; HBV: Hepatitis B virus.
Figure 2.
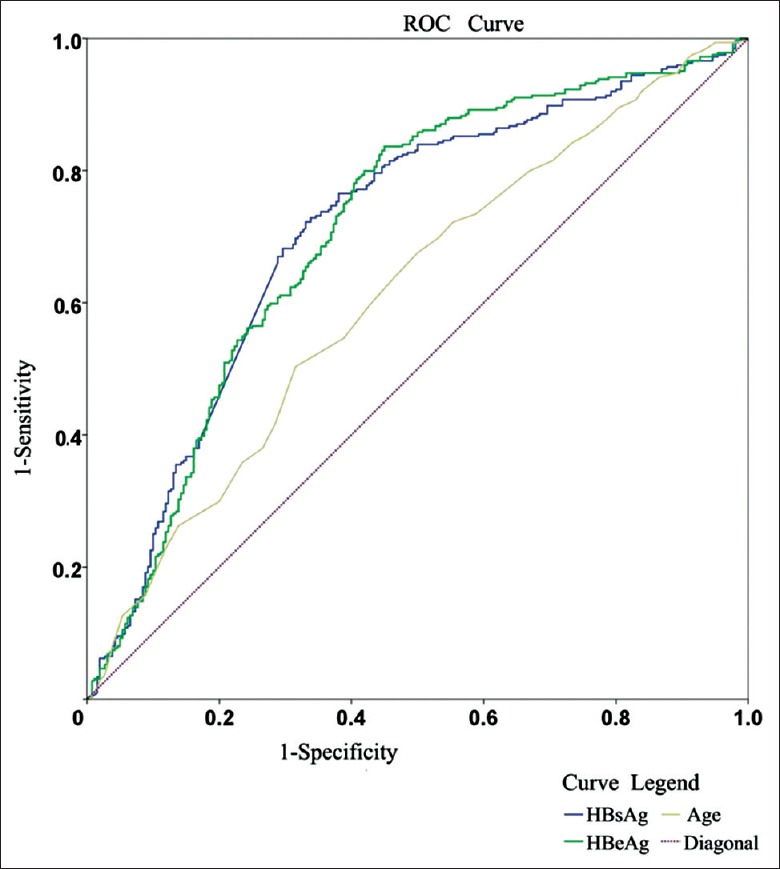
AUC of age, serum HBsAg (log U/ml), HBeAg (log S/CO) for minimal grade and other (mild and moderate) grades in treatment-naïve HBeAg-positive chronic HBV-infected patients. AUCs of HBsAg and HBeAg at cutoff values of 4.31 log U/ml (P<0.001) and 2.86 log S/CO (P<0.001) for differentiation of minimal grade and other (mild and moderate) grades in HBeAg-positive chronic HBV-infected patients are 0.710 and 0.711, with sensitivity 67.42 and 55.58%, and specificity 72.22 and 83.64%, respectively. AUC: Area under ROC; ROC: Receiver operating characteristic curve; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; S/CO: Sample/cutoff.
As shown in Table 3, the cutoff for differentiating in minimal grade were the age of 36 years (the positive predictive value [PPV] value 55% and the negative predictive value [NPV] value 62%), HBeAg 2.86 log S/CO (the PPV value 65% and the NPV value 73%), HBsAg 4.31 log U/ml (the PPV value 72% and the NPV value 69%) respectively in treatment-naïve HBeAg-positive chronic HBV-infected patients.
DISCUSSION
After a person was infected with HBV from maternal-neonatal transmission, the compromise and struggle between human immune system and HBV caused the evolution stages of immune tolerance, HBeAg-positive immune activation, inactive HBsAg carrier state, HBeAg-negative CHB in several years or even decades. At the initial stage liver pathology showed minimal inflammation and the disease gradually progresses to mild, moderate, and severe inflammation and even liver fibrosis, and serum HBV infection markers (HBsAg, HBeAg, and HBV DNA) also evolve at the same time.[1,2,3,6,7] The only effective way to prevent CHB patients from cirrhosis is antiviral treatment. Effective antiviral therapy could even result in the control of liver inflammation and even HBsAg loss in a proportion of patients with lower baseline HBsAg level.[8] The clinical guidelines of 2015 AASLD, APASL, 2017 EASL all agreed that active liver inflammation of pathologic features was one of the main symbols of the beginning of antiviral treatment.[1,2,3] Which parameter could represent the degree of liver inflammation? The most direct method was liver biopsy. In treatment-naïve chronic HBV-infected patients, liver biopsy was the golden standard to evaluate the degree of activity of liver inflammation in chronic HBV-infected patients. In the previous antiviral evaluation, we concluded that the immune tolerance phase roughly corresponds to the minimal phase of liver pathology.[1,2,3,4,5] The severe phase was not involved in this study because other serum indicators such as more than 2 folds of ULN ALT in this grade maybe indicate the active liver inflammation. A variety of guidelines agreed that antiviral therapy was not necessary in minimal liver inflammation in CHB patients, and the mark of beginning antiviral therapies was obvious liver inflammation such as mild or moderate conditions.[1,2,3]
We acknowledged that liver biopsy was helpful in judging the liver inflammation, and liver biopsy might not be performed in every CHB patient with its shortcoming of invasiveness and lack of reproducibility. In the clinical guideline of 2015 AASLD, APASL, 2015 guidelines for the prevention and treatment of CHB in China, 2017 EASL, the adopted direct parameters for active liver inflammation were ALT and HBV DNA. ALT (>2 ULN) had been accepted as a milestone of active liver inflammation grades and starting point for antiviral therapy in HBeAg-positive chronic HBV-infected patients in all the above-mentioned guidelines. The normal ULNS of ALT was 40U/L in Asia and Europe, which is different from 30 U/L for male and 19 U/L for female in the USA.[1,2,3] A person with ALT ≤2 ULN (80 U/L) need to be treated according to all guideline? The answer was no. All the guidelines[1,2,3] pointed out that liver biopsy might be applied for detecting active liver inflammation when chronic HBV-infected patients with 1 ULN ≤ ALT ≤2 ULN (40≤ ALT ≤80 U/L in Asia and Europe; 30 U/L ≤ ALT ≤60 U/L for male and 19≤ ALT ≤38 U/L for female in the USA), especially when age was over 40 years. There were data suggesting that people with 0.5 ULN ≤ ALT <1 ULN still had liver inflammation other than the minimal stage.
Therefore, ALT ≤80 U/L might be seen in many chronic HBV-infected patients with active liver inflammation, and we should have other parameters to judge who would need antiviral therapy. The liver biopsy should be suggested in people with ALT ≤80 U/L which conform to the interests of chronic HBV-infected patients without a doubt in most cases. In this study, we enrolled chronic HBV-infected patients with ALT ≤80 U/L. Quantitative data of serum HBV DNA (roughly >1 million U/ml in 2015 AASLD; >107 U/ml in 2017 EASL; >200,000 U/ml in guidelines for the prevention and treatment of CHB in China, 2015 Edition)[9] might be considered a marker of immune tolerance. The use of specific HBV DNA cutoff value to determine breaking immune tolerance had rarely been reported in the literature. All guidelines pointed out that the research on new markers was necessary in the future. Why we had studied the influence of age on liver inflammation? There were age-dependent changes in immunity of chronic HBV-infected patients.[6,10] After reviewing the literature, we found that serum HBsAg and HBeAg were associated with HBV replication capability, the former was widely studied. In previous reports, serum HBsAg was more representative of the active level of covalently closed circular DNA which was the primary replication template of HBV. The evolution of HBsAg and HBeAg quantitation in chronic HBV-infected patients maybe potential parameter to reflect the liver inflammation, but specific and accurate data from larger sample size are still needed.[11,12,13,14,15] Eventually, age, the quantitation of HBsAg and HBeAg, were included in this study for the exact judgment of liver inflammation grades in chronic HBV-infected patients in China.
The cutoff value of 36 years might be mainly related to mother-to-child transmission which resulted in relatively an immune tolerance period of up to 30 years in treatment-naïve HBeAg-positive chronic HBV-infected patients in China. The exact mechanism might be related to the decline of the immune tolerance factor of e antigen and the activation of active and passive immune response with the age increases.
In this study, we also found that serum HBsAg and HBeAg were negatively correlated with inflammation in treatment-naïve HBeAg-positive chronic HBV-infected patients. For nonparametric data, Jaroszewicz et al.,[16] summarized that the median HBsAg in immune tolerance are 4.96 log U/ml in European in genotype D. In immune tolerance, the mean HBsAg was 4.7 log U/ml in the North of China reported by Wang et al.,[17] the median HBsAg was 4.53 log U/ml on Asia by Tin Nguyen et al.,[15] and the median HBsAg of the study was 4.40 log U/ml. Why the mean or median HBsAg in immune tolerance of Asia were all lower than Jaroszewicz et al.'s results in Europeans?[16] The reason of different median of HBsAg might mainly be related to the major genotype of B and C in Asia. It needs to be reminded that the proportion of young people might also affect the median of HBsAg. There was little information about the median HBeAg in immune tolerance and the research found the median HBeAg was 3.12 log S/CO. The significance of the median was far less than the ROC and cutoff values in clinical analysis. The cutoff values of serum HBsAg and HBeAg might be rarely influenced by proportion of young people, but might be influenced by various races, regions, different HBV subtypes. The key finding of this study was that the ROC for predicting the minimal grade was 0.710 (HBsAg 4.31 log U/ml, sensitivity 67.42%, specificity 72.22%, respectively) and 0.711 (HBeAg 2.86 log S/CO, sensitivity 55.68%, specificity 83.64%, respectively) in the sample population. Wang et al.,[17] also concluded that the cutoff values for differentiating immune tolerance from immune active phase of HBsAg and HBeAg were 4.41 log U/ml and 1118.96 S/CO (3.05 log U/ml) slightly higher than our results. Zeng et al.[18] mentioned that the AUC of ROC for predicting the IT phase was 0.831 (HBsAg 4.398 log U/ml, sensitivity 87.5%, specificity 73.2%) in chronic HBV-infected patients of Southern China by liver biopsy in 2016. As a study of ROC for predicting the minimal liver inflammation of chronic HBV-infected patients in Asia, the results are strikingly similar to those of Wang et al.[17] and Zeng et al.[18] The number of chronic HBV-infected patients enrolled in Wang et al.[17] and Zeng et al.'s[18] research was less than those in this research for immune tolerance and immune active grade.
The clinical significance of this study is its practical value. The infection rate of chronic HBV infection in China was about 7.18% in 2016. The effective antiviral therapy for chronic HBV-infected patients were interferon and nucleoside analogs, the cost of the latter was <50–100 dollars per month in China. All the relevant guidelines[1,2,3] suggested that patients were included in the observational cohort unless there were clear evidences of immune tolerance or minimal liver inflammation for chronic HBV-infected patients. Clinicians should make more positive judgments whether antiviral treatment was necessary or not when we found that the possible age of breaking the immune tolerance for the overall population was advanced to 36 years and in most cases, ALT <0.5 ULN might be real minimal liver inflammation in chronic HBV-infected patients. Because of various ethnicity, and HBV genotype, we needed to be more cautious about chronic HBV-infected patients with ALT ≤80 U/L. After 44.52% chronic HBV-infected patients with ALT ≤80 U/L were in stages other than minimal inflammation and should begin antivirus therapy in China. The cutoff values of 4.31 log U/ml for HBsAg, 2.86 log S/CO for HBeAg might help us to make decision for antiviral therapy without histological data.
The advantages of this study were mainly four aspects. First, there were 584 liver biopsy specimens which were enough for data analysis. Second, three cutoff values of age, HBsAg, HBeAg were determined for minimal grade and other (mild and moderate) grades. The median index was more affected by the proportion of young patients than cutoff value in HBeAg-positive chronic HBV-infected patients. Third, Knodell scoring system was adopted in this present study was easily to compare in similar research. Last, in a similar study[15] of serum HBsAg and HBeAg, the standards by which groups were divided into immune tolerance and HBeAg positive immune activation stages were complicated, and indirectly, but division by pathology of liver inflammation would be more accurate and intuitive.
The shortcomings of the study are as following: First, it was a retrospective study. Second, the severe phase was not involved in the research, because ALT ≥80 U/L could predict antiviral therapy. Third, no genotype detection for HBV was conducted for enrolled patients. Nguyen et al.[15] have reported that the median of HBsAg quantitation has resemblance between genotype B and C which were mainly the genotypes seen in Northern China. Forth, AUC of ROCs in age, serum HBsAg, and HBeAg quantitation for determining minimal liver inflammation were 0.613, 0.710, and 0.711, respectively. It is a difficult task to judge liver inflammation only using a single parameter of ALT, or HBV DNA, or age, or serum HBsAg or HBeAg in chronic HBV-infected patients with ALT ≤80 U/L. We should make efforts to make comprehensive judgment based on ALT, HBV DNA, age, serum HBsAg, or HBeAg. We should create scoring systems by a combination of age, HBV DNA, serum HBsAg, and HBeAg quantitation together in clinical diagnosis and treatment in the next studies. Last, the clinical significance of HBeAg is slightly less than HBsAg because of the possible variations in the precore G1896A mutation and basal core promoter A1762T/G1764A mutation in chronic HBV-infected patients.
In summary, serum HBsAg and HBeAg quantitation gradually decreased accompanied by liver inflammation grades increase in treatment-naïve HBeAg-positive chronic HBV-infected patients. There was a positive correlation between age and liver inflammation grades and a negative correlation between quantitation and liver inflammation grades. The HBsAg or HBeAg cutoff values for distinguishing minimal grade and the other grades were 36 years, 4.31 log U/ml, 2.86 log S/CO, respectively, in treatment-naïve HBeAg-positive chronic HBV-infected patients, which would help us to detect those who do not need antiviral therapy in China; 44.52% chronic HBV-infected patients with ALT ≤80 U/L might need antiviral therapy and 55.48% should be in regular clinical follow-up.
Financial support and sponsorship
This work was supported by grants from the fund of Beijing Ditan Hospital of Capital Medical University (No. DTQH201610), and Beijing Municipal Administration of Hospital Clinical Medicine Development of Special Funding Support (No. ZY201402).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. doi: 10.1016/j.jhep.2017.03.021. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: What we knew in 1981 and what we know in 2005. Hepatology. 2006;43(2 Suppl 1):S173–81. doi: 10.1002/hep.20956. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 5.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 6.Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept. Cell Mol Immunol. 2015;12:258–63. doi: 10.1038/cmi.2014.79. doi: 10.1038/cmi.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng LY, Lian JS, Chen JY, Jia HY, Zhang YM, Xiang DR, et al. Hepatitis B surface antigen levels during natural history of chronic hepatitis B: A Chinese perspective study. World J Gastroenterol. 2014;20:9178–84. doi: 10.3748/wjg.v20.i27.9178. doi: 10.3748/wjg.v20.i27.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MH, Zhang L, Qu XJ, Lu Y, Shen G, Wu SL, et al. Kinetics of hepatitis B surface antigen level in chronic hepatitis B patients who achieved hepatitis B surface antigen loss during pegylated interferon alpha-2a treatment. Chin Med J. 2017;130:559–65. doi: 10.4103/0366-6999.200554. doi: 10.4103/0366-6999.200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update (In Chinese) Chi J Hepatology. 2015;23:888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Andreani T, Serfaty L, Mohand D, Dernaika S, Wendum D, Chazouillères O, et al. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: Histologic findings and outcome. Clin Gastroenterol Hepatol. 2007;5:636–41. doi: 10.1016/j.cgh.2007.01.005. doi: 10.1016/j.cgh.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HL, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398–411. doi: 10.1016/j.jhep.2016.08.009. doi: 10.1016/j.jhep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, et al. Early serum HBsAg drop: A strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–7. doi: 10.1002/hep.22744. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Lee JM, Seo JH, Kim HS, Ahn SH, Kim DY, et al. Predictive value of HBsAg quantification for determining the clinical course of genotype C HBeAg-negative carriers. Liver Int. 2012;32:796–802. doi: 10.1111/j.1478-3231.2011.02693.x. doi: 10.1111/j.1478-3231.2011.02693.x. [DOI] [PubMed] [Google Scholar]
- 14.Jang JW, Yoo SH, Kwon JH, You CR, Lee S, Lee JH, et al. Serum hepatitis B surface antigen levels in the natural history of chronic hepatitis B infection. Aliment Pharmacol Ther. 2011;34:1337–46. doi: 10.1111/j.1365-2036.2011.04888.x. doi: 10.1111/j.1365-2036.2011.04888.x. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: A perspective on Asia. J Hepatol. 2010;52:508–13. doi: 10.1016/j.jhep.2010.01.007. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: A European perspective. J Hepatol. 2010;52:514–22. doi: 10.1016/j.jhep.2010.01.014. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zou ZQ, Wang K, Yu JG, Liu XZ. Role of serum hepatitis B virus marker quantitation to differentiate natural history phases of HBV infection. Hepatol Int. 2016;10:133–8. doi: 10.1007/s12072-015-9657-6. doi: 10.1007/s12072-015-9657-6. [DOI] [PubMed] [Google Scholar]
- 18.Zeng DW, Zhang JM, Liu YR, Dong J, Jiang JJ, Zhu YY. A retrospective study on the significance of liver biopsy and hepatitis B surface antigen in chronic hepatitis B infection. Medicine (Baltimore) 2016;95:e2503. doi: 10.1097/MD.0000000000002503. doi: 10.1097/MD.0000000000002503. [DOI] [PMC free article] [PubMed] [Google Scholar]