Abstract
Background:
Luminescent rare-earth-based nanoparticles have been increasingly used in nanomedicine due to their excellent physicochemical properties, such as biomedical imaging agents, drug carriers, and biomarkers. However, biological safety of the rare-earth-based nanomedicine is of great significance for future development in practical applications. In particular, biological effects of rare-earth nanoparticles on human's central nervous system are still unclear. This study aimed to investigate the potential toxicity of rare-earth nanoparticles in nervous system function in the case of continuous exposure.
Methods:
Adult ICR mice were randomly divided into seven groups, including control group (receiving 0.9% normal saline) and six experimental groups (10 mice in each group). Luminescent rare-earth-based nanoparticles were synthesized by a reported co-precipitation method. Two different sizes of the nanoparticles were obtained, and then exposed to ICR mice through caudal vein injection at 0.5, 1.0, and 1.5 mg/kg body weight in each day for 7 days. Next, a Morris water maze test was employed to evaluate impaired behaviors of their spatial recognition memory. Finally, histopathological examination was implemented to study how the nanoparticles can affect the brain tissue of the ICR mice.
Results:
Two different sizes of rare-earth nanoparticles have been successfully obtained, and their physical properties including luminescence spectra and nanoparticle sizes have been characterized. In these experiments, the rare-earth nanoparticles were taken up in the mouse liver using the magnetic resonance imaging characterization. Most importantly, the experimental results of the Morris water maze tests and histopathological analysis clearly showed that rare-earth nanoparticles could induce toxicity on mouse brain and impair the behaviors of spatial recognition memory. Finally, the mechanism of adenosine triphosphate quenching by the rare-earth nanoparticles was provided to illustrate the toxicity on the mouse brain.
Conclusions:
This study suggested that long-term exposure of high-dose bare rare-earth nanoparticles caused an obvious damage on the spatial recognition memory in the mice.
Keywords: Mouse Brain, Rare-earth Nanoparticles, Spatial Recognition Memory
INTRODUCTION
The development of nanotechnology in medicine has increasingly created many opportunities for effective treatments in clinical diagnosis and therapy.[1,2] In the past 10 years, many nanoparticles have been used in nanomedicines as diagnostic and therapeutic agents. In particular, luminescent rare-earth-based nanoparticles are showing great promises for practical biomedical applications due to their unique advanced properties.[3] For example, Yb/Er-codoped upconversion luminescence nanoparticles have been used to carry out optical imaging in vivo and controllable drug release under a near-infrared illumination.[4] NaGdF4-based nanoparticles can be used as an excellent agent for magnetic resonance imaging (MRI) or computed tomography.[5] When loading with anticancer drugs, they could provide a therapy platform in a multi-model diagnostic therapy fashion. However, the biological safety and risk of such rare-earth nanoparticle-based nanomedicine become a major concern.
Many previous studies have reported that rare-earth nanoparticles have relatively low toxicity both in cells and in animal models in a short term[6] These methods for cytotoxicity assessment were typically conducted by incubating cells with nanoparticles about a time frame of 48 h. The toxicity assays were usually conducted on nanoparticles coated with a thin layer of silica.[7] The surface coating can change the behavior of the rare-earth nanoparticles. Toxicity evaluation on the basis of such short-term assays is generally not well effective in the practical usage. Recent studies have indicated that lanthanide-doped nanoparticles without a surface protection do exhibit potential cytotoxicity, because it was found that adenosine triphosphate (ATP) quenching can be induced by strong binding between the lanthanide ions and the phosphate group of intracellular ATP.[8] Furthermore, Vedunova et al.[9] found that bare rare-earth nanoparticles are toxic to hippocampal cells in vitro. It is well known that ATP is the most important neurotransmitter in the mouse brain. The hippocampal cells are the substantial reconfigurations in stages characterized by morphological and functional changes. Thus, we recognized that it has become increasingly important to obtain more information on the biological effects of these rare-earth nanomaterials to forestall any possible deleterious effect caused by use.
In this study, we reported an experimental investigation on rare-earth nanoparticle-induced cytotoxicity in mouse brain through the tests of spatial cognition. The rare-earth-induced neurotoxicity in mice was evaluated by two different sizes of rare-earth nanoparticles. The rare-earth nanoparticles-induced toxicity and damages on the mouse brain were accessed by evaluating the functionality of neural network activity through the tests of spatial recognition memory and histopathological analysis. Consequently, the study of intrinsic toxicity of these rare-earth nanoparticles may offer an important theoretical basis for evaluating the potential toxicity of nanomaterials on animals or humans.
METHODS
Ethical approval
The use of animals in this study was approved by the Institutional Animal Care and Use Committee of Fujian Medical University. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Chemicals and reagents
Oleic acid (90%), 1-octadecene (90%), NH4F (99%), and NaOH (98%) were purchased from Sigma-Aldrich, USA. All acetic lanthanide nanoparticles, including Y(CH3CO2)3·xH2O (99.9%), Gd(CH3CO2)3·xH2O (99.9%), Er(CH3CO2)3·xH2O (99.9%), and Yb(CH3CO2)3·4H2O (99.9%), were also obtained from Sigma-Aldrich.
Synthesis of rare-earth-based NaY (Gd) F4 nanoparticles
The synthesis of luminescent rare-earth-based nanoparticles was achieved by a conventional co-precipitation method.[8] Two different sizes of rare-earth nanoparticles were synthesized by doping the Gd3+ and Yb3+ ions. In a typical experiment, NaLnF4 (Ln = Yb, Gd, Y, Er) nanoparticles were synthesized according to the previous reports.[8] An aqueous solution (2 ml) of 0.2 mol/L Gd (CH3CO2)3, 0.2 mol/L Y (CH3CO2)3, 0.2 mol/L Er (CH3CO2)3, and 0.2 mol/L Yb (CH3CO2)3 was prepared in a 50-ml two-neck flask. Then, 3 ml oleic acid and 7 ml 1-octadecene were added to mix with them. The resulting mixture was heated at 150°C for 2 h under an oil bath condition. The precursor solution was cooled and moved to the heating mantle with a temperature controller. The precursor was kept at 50°C, and a fresh mixture of 1 mmol NaOH and 1.6 mmol NH4F was added and stirred for 30 min. After that, the reaction was heated to 110°C and degassed for 10 min under the argon condition to remove the methanol and oxygen. The resulting solution was raised to 280°C for 3 h reaction. The products were precipitated out with ethanol, collected by 2800 × g centrifugation for 10 min, and rewashed with ethanol, and finally dispersed in 4 ml cyclohexane. Our experiments prepared two different upconversion nanoparticles, NaGdF4: Yb/Er (18/2 mol%) and NaYbF4:Er (2 mol%).
Ligand free of rare-earth nanoparticles
To make the nanoparticles soluble for cell and animal experiments, we used a ligand-free protocol to transfer hydrophobic rare-earth nanoparticles into hydrophilic nanoparticles. The as-prepared nanoparticles were precipitated out with ethanol by centrifugation and transferred into a 2-ml tube. Then, 1 ml HCl (2 mol/L) was added, and the mixture was treated with ultrasonic for 5 min. The nanoparticles were precipitated out with 1 ml ethanol by 30,492 × g centrifugation for 30 min. The resulting ligand-free nanoparticles were washed with ethanol for twice, and finally dried at 80°C.
Characterization
Fluorescence spectrum was obtained under an excitation of a 980 nm-diode laser. Both the fluorescence spectrum and luminescence lifetime were measured by a phosphorescence lifetime spectrometer (FSP920, Edinburgh Instruments, UK). Transmission electron microscopy (TEM) measurements were carried out on a 100 kV JEM-2100F TEM (JEOL, SHIMADZU, Japan). Powder X-ray diffraction was measured by an X-ray powder diffractometer (Techcomp [China] Ltd., Shanghai, China) with Cu Kα radiation. The MRI characterization (7 T, 20-cm bore magnet, 70/20 USR Bruker, Germany) was carried out for recording nanoparticle taken up in the mouse liver.
Animals and treatment
Adult male ICR mice, weighing 20–25 g at the beginning of the experiments, were purchased from the laboratory animal center of Fujian Medical University. All animals were housed in separate cages, with access to standard laboratory food and water, and kept in a regulated environment (22°C) under a 12 h:12 h light/dark cycle starting at 7:00 a.m.
Mice were randomly divided into seven groups, including control group (receiving 0.9% normal saline) and six experimental groups (10 mice in each group). In a typical experiment, NaGdF4:Yb/Er and NaYbF4:Er-based nanoparticles were injected with a dosage of 0.5, 1.0, and 1.5 mg/kg body weight. Nanoparticle solutions or the same volume normal saline was administered through caudal vein injection once daily in the morning for consecutive 7 days. Animals were weighed before intravenous injection every day. The symptoms were observed and recorded carefully every day for 28 days. The animals were regularly handled and weighed before behavioral experiments.
Behavioral examination
After 28 days, the effects on spatial recognition memory in all animals were evaluated by a Morris water maze.[10] The Morris water maze is a circular pool (1220-cm diameter and 30-cm height) filled with water (20.0 ± 1.0°C). The pool was divided into four quadrants. A circular escape platform (7-cm diameter) was located in one quadrant with 1.5 cm below the surface of the water. Each mouse was subjected to a series of 5 trials per day. For each trial, mice were randomized to 1 of the 4 directional starting locations (north, south, east, and west) and placed in the pool facing the wall. If the mouse was found to climb onto the platform in 60 s, the trial will be stopped with a recorded escape latency by real time. However, if the mouse did not climb onto the platform within 60 s, the trial will be stopped and the mouse will be guided to the platform by us. An escape latency of 60 s was only recorded. Swimming was videotaped, and the navigation parameters were analyzed using the Smart software (Panlab, Barcelona, Spain).
At the end of day 5, the platform was removed, and a 60-s probe trial was carried out. The probe trial was used to assess the spatial memory retention of the location of the hidden platform 24 h after the last acquisition session. The mouse was allowed to search the pool for 60 s. The following parameters were evaluated during the probe trial: the traversing times in the target quadrant and the distance to the platform. If the traversing times were more, it means that the mice swam closer to the zone platform during the probe test. The distance to the platform was used to evaluate motor functions.
Histopathological examination of brain
For pathological studies, all histopathological tests were performed using standard laboratory procedures. The brain tissues were fixed in situ through saline perfusion for 3 min, followed by 4% formaldehyde for 10 min, immersion in 4% formaldehyde for 24 h, then removed from the skulls by a nontraumatic technique, and finally immersion fixed in 4% formaldehyde for more than 24 h. The brains were embedded in paraffin blocks, then sliced into 5 μm in thickness, and placed onto glass slides. After hematoxylin and eosin staining, the slides were observed and the photographs were taken using optical microscope (Nikon U-III Multi-point Sensor System, USA). The identity and analysis of the pathology slides were blind to the pathologist.
Statistical analysis
Experiments were repeated at least three times (n ≥ 3), and each in the behavior studies had at least ten mice. Data are expressed as mean ± standard deviation (SD), and processed with one-way analysis of variance (ANOVA) followed by a two-way ANOVA test. Statistical analysis was performed using SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA). Significance was set at P < 0.05.
RESULTS
Characterization of rare-earth nanoparticles
In this study, we synthesized two types of rare-earth nanoparticles with different sizes to study the nanoparticle toxicity, which would be used to examine the effects of particle sizes on the toxicity. As shown in Figure 1, the as-prepared rare-earth nanoparticles were characterized by the TEM. The results indicated that the sizes of the nanoparticles were 10 nm for NaGdF4:Yb/Er and 100 nm for NaYbF4:Er. The as-prepared rare-earth nanoparticles were treated into hydrophilic properties by a ligand-free method. Further, fluorescence spectra were measured under an excitation of a 980-nm diode laser [Figure 2]. The results demonstrated the successful preparation of previously desired components. Thus, the rare-earth nanoparticles could be further used for the next experiments.
Figure 1.
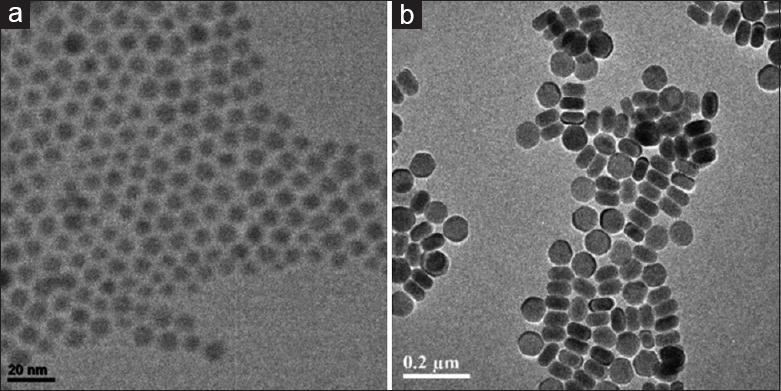
Transmission electron microscopy image of the rare-earth nanoparticles: NaGdF4:Yb/Er (a) and NaYbF4:Er (b). The rare-earth nanoparticles were uniform and well dispersed in H2O.
Figure 2.
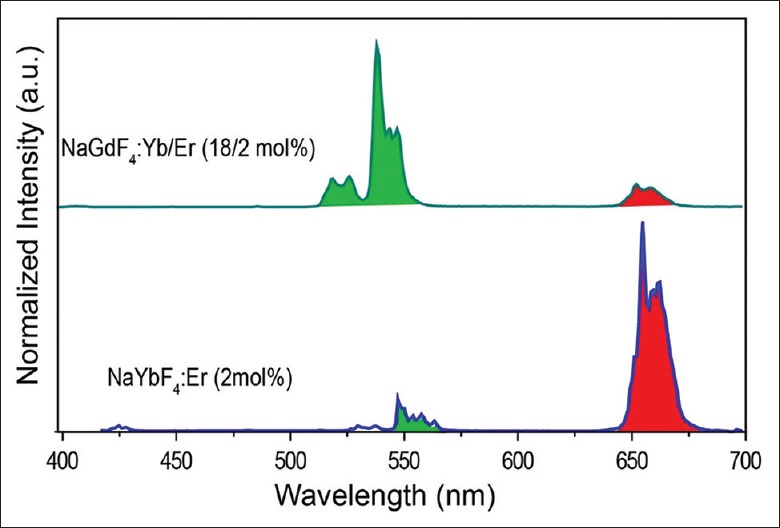
Fluorescence spectra of two different rare-earth nanoparticles: NaGdF4:Yb/Er (18/2 mol%) and NaYbF4:Er (2 mol%). The spectra were measured under the excitation by a 980-nm diode laser.
Evaluation of the effects of nanoparticle toxicity on spatial recognition memory
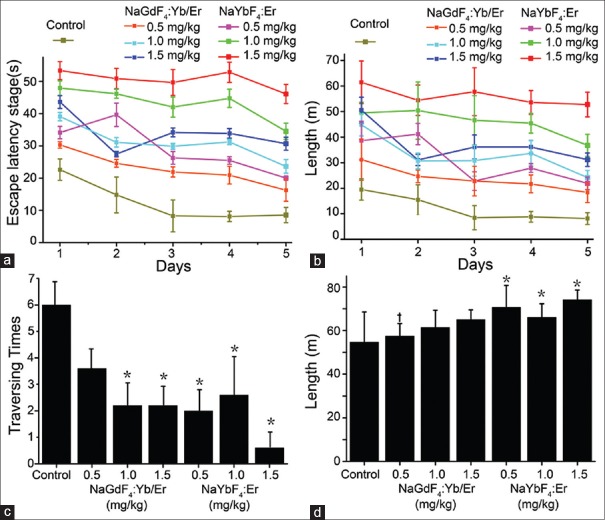
To verify whether rare-earth nanoparticles have influence on cognitive deficits in mice, the mice were evaluated by a Morris water maze test. As shown in Figure 3a and 3b, the mice receiving the injection of the NaYbF4:Er and NaGdF4:Yb/Er nanoparticles resulted in an increased escape latency times in the target quadrant when compared to control group, which also referred to the swimming length. A high dose of 1.5 mg/kg NaGdF4:Yb/Er nanoparticles (or NaYbF4:Er nanoparticles) obviously showed an abnormal change in escape latency times compared to the experiment groups with 0.5 and 1.0 mg/kg NaGdF4:Yb/Er nanoparticles (or NaYbF4:Er nanoparticles). Note that the escape latency time and swimming length in the control group were shorter than those in the other six experimental groups (all P < 0.05). In addition, we observed that the increased concentrations of nanoparticle exposure indeed increased in the escape latency time and swimming length [Figure 3a and 3b]. In the 6th day of the Morris water maze test, we found that, both in the NaGdF4:Yb/Er and NaYbF4:Er treatment groups (except the 0.5 mg/kg NaGdF4:Yb/Er nanoparticles group), the times of traversing from the target quadrant were decreased compared to the control group during the probe trial [all P < 0.05; Figure 3c]. Furthermore, under the exposure of NaYbF4:Er nanoparticles in the mice, the control group without exposure of any nanoparticles indicated a shorter distance of the surrounding swimming length than the experimental groups [Figure 3d; all P < 0.05]. The results also suggested that there were no significant differences between the NaYbF4:Er nanoparticle groups and NaGdF4:Yb/Er nanoparticle groups at the same dose (except 0.5 mg/kg) in the surrounding swimming length [all P > 0.05; Figure 3d].
Figure 3.
Evaluation of spatial recognition memory in Morris water maze test. Escape latency time in the target quadrant (a) and the swimming length (b) of the mice in different groups during the consecutive 5 days. The traversing times of the target quadrant (c) and the surrounding swimming length (d) of mice in the different groups. *P < 0.05, compared with the control group. †P < 0.05, compared with the same concentrations in different nanoparticle groups.
Brain histopathological observation
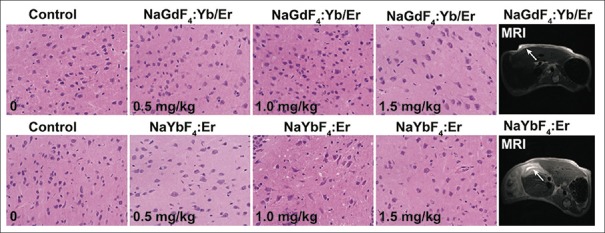
The results of brain histopathological experiments are shown in Figure 4. In the experiments of using the NaGdF4:Yb/Er nanoparticles, the tests of low-dose nanoparticles at 0.5 mg/kg and 1.0 mg/kg had a similar pathology with the control group. However, at a higher dose of 1.5 mg/kg, NaGdF4:Yb/Er nanoparticles obviously showed an abnormal pathology change compared to the control group. We further found that the experimental group using 0.5, 1.0, and 1.5 mg/kg NaYbF4:Er nanoparticles showed an abnormal pathology change compared to the control group. This also resulted in more intercellular space among the neurocytes. Moreover, the neurocyte cells under the experiments of high-dose rare-earth nanoparticles were decreased compared to control group, as shown in Figure 4. Meanwhile, the MRI results indeed demonstrated that the rare-earth nanoparticles were concentrated and remained in the mouse liver, which caused the toxicity in the mice. This might induce deficits of the spatial recognition memory in the mice.
Figure 4.
Histopathology results of the brain tissue in ICR mice caused by nanoparticle injection (original magnification, ×400). Magnetic resonance images for identification of the rare-earth nanoparticles in the mice.
DISCUSSION
In this study, the neurotoxicity study was carried out in mice models using two different rare-earth nanoparticles. In many previous studies, rare-earth nanoparticles were surface coated with polymers or silica layers that exhibited low cytotoxicity in brain cells. Considering that nanoparticles might undergo degradation within lysosomes and the coating layers may be released in complex cellular environments, the intrinsic cytotoxicity of the bare nanoparticles should be used regardless.[11] In this study, to reveal the intrinsic toxicity of rare-earth nanoparticles, surface-coated ligands were removed by acid treatment to achieve the bare nanoparticles. Through TEM characterization and fluorescence spectrum measurement, the rare-earth nanoparticles were successfully synthesized. Although ligand-free lanthanide-doped nanoparticles are known to induce a decreased cell viability due to intracellular ATP deprivation in the live cells, little study is reported to investigate the neurotoxicity effects of the bare nanoparticles in vitro.[8,9] However, almost these studies used silica-coated rare-earth nanoparticles to study their biological toxicity in a short-term period. This could not reveal their toxicity in the practical applications.
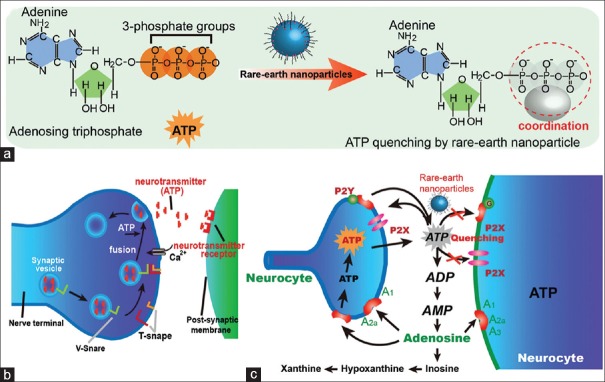
It is well known that the Morris water maze test is widely used in behavioral neuroscience to study the neural mechanisms of spatial learning and memory. To evaluate the neurotoxicity caused by the treatment with bare rare-earth nanoparticles, the Morris water maze test was used to evaluate ICR mice exposed to the nanoparticles in this study. Meanwhile, the escape latency time in the target quadrant and the swimming length of ICR mice exposed to the nanoparticles were statistically significant different from those of the control mice. The results indicated that rare-earth nanoparticles impaired the spatial recognition memory in the mice.[2,12,13] We thus have completed the first assessment of the effects of rare nanoparticles on animal spatial recognition memory. To illustrate the mechanism of rare-earth nanoparticle-induced toxicity in mouse brain, we have provided a proposed mechanism for the pathway of ATP quenched by the rare-earth nanoparticles [Figure 5]. In particular, the rare-earth nanoparticles could strongly coordinate with the ATP molecules, thus causing an ATP quenching in cells. As a result, the strong quenching of ATP by rare-earth nanoparticles led to the neuron toxicity.
Figure 5.
Schematic illustration of rare-earth nanoparticle-induced ATP quenching (a). ATP molecules acting as a neurotransmitter between post-synaptic membrane and nerve terminal (b), and proposed mechanism of neurotoxicity induced by the rare-earth nanoparticles through ATP quenching (c). ATP: Adenosine triphosphate.
The damage of the spatial recognition memory in the mice also suggested that rare-earth nanoparticles can cause brain injury and change the contents of electrolyte in the mouse brain after nanoparticle injection, which have been also demonstrated by the morphological examination of the brain tissue.[12] After the mice were exposed to the rare-earth nanoparticles, the high-dose nanoparticles mice exhibited abnormal pathology changes, which was consistent with the results of the Morris water maze test. Moreover, our experimental investigation found that most of the rare-earth nanoparticles remained in the liver through body metabolism in the mouse. This leads to a fact that the rare-earth nanoparticles were concentrated in the liver, while the concentration of the rare-earth nanoparticles was very low in the brain. It should be noted that this case has been also demonstrated using other nanoparticles in many previous studies.[14,15] However, the size-dependent cell uptakes of rare-earth nanoparticles at the cell level are still not clear. The interactions between the rare-earth nanoparticles and cells cannot be directly studied by the animal test.
In conclusion, this study has observed an obvious decline of neurobehavioral performance and morphological signs of brain damage in the mice caused by rare-earth nanoparticle exposure. The negative effects on spatial recognition memory behavior under the exposure of rare-earth nanoparticles have been demonstrated by our studies. These findings could provide a fundamental understanding of the intrinsic toxicity associated with bare rare-earth nanoparticles in animals. Moreover, this study would arose typical attentions on the future applications of rare-earth nanoparticle on human, which is valuable for long-term and high-dose treatment in nanomedicines.
Financial support and sponsorship
This work was supported by grants from the Provincial Health and Family Planning Research of Fujian (No. 2016-1-43 and No. 2017-ZQN-32).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Xu J, Kuang Y, Lv R, Yang P, Li C, Bi H, et al. Charge convertibility and near infrared photon co-enhanced cisplatin chemotherapy based on upconversion nanoplatform. Biomaterials. 2017;130:42–55. doi: 10.1016/j.biomaterials.2017.03.041. doi: 10.1016/j.biomaterials.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, Xie X, Huang B, Liang L, Han S, Yi Z, et al. Confining excitation energy in Er3+ -sensitized upconversion nanocrystals through Tm3+ -mediated transient energy trapping. Angew Chem Int Ed Engl. 2017;56:7605–9. doi: 10.1002/anie.201703012. doi: 10.1002/anie.201703012. [DOI] [PubMed] [Google Scholar]
- 3.Gorris HH, Wolfbeis OS. Photon-upconverting nanoparticles for optical encoding and multiplexing of cells, Biomolecules, and Microspheres. Angew Chem Int Ed Engl. 2013;52:3584–600. doi: 10.1002/anie.201208196. doi: 10.1002/anie.201208196. [DOI] [PubMed] [Google Scholar]
- 4.Lei PP, Zhang P, Yuan QH, Wang Z, Dong LL, Song SY, et al. Yb3+/Er3+-codoped Bi2O3 nanospheres: Probe for upconversion luminescence imaging and binary contrast agent for computed tomography imaging. Acs Appl Mater Interfaces. 2015;7:26346–54. doi: 10.1021/acsami.5b09990. doi: 10.1021/acsami.5b09990. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Liu Z, Li F. Upconversion nanophosphors for small-animal imaging. Chem Soc Rev. 2012;41:1323–49. doi: 10.1039/c1cs15187h. doi: 10.1039/c1cs15187h. [DOI] [PubMed] [Google Scholar]
- 6.Teng X, Zhu Y, Wei W, Wang S, Huang J, Naccache R, et al. Lanthanide-doped Na(x)ScF(3+x) nanocrystals: Crystal structure evolution and multicolor tuning. J Am Chem Soc. 2012;134:8340–3. doi: 10.1021/ja3016236. doi: 10.1021/ja3016236. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Lee A, Ji Z, Huang C, Chang CH, Wang X, et al. Reduction of pulmonary toxicity of metal oxide nanoparticles by phosphonate-based surface passivation. Part Fibre Toxicol. 2017;14:13. doi: 10.1186/s12989-017-0193-5. doi: 10.1186/s12989-017-0193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian J, Zeng X, Xie X, Han S, Liew OW, Chen YT, et al. Intracellular adenosine triphosphate deprivation through lanthanide-doped nanoparticles. J Am Chem Soc. 2015;137:6550–8. doi: 10.1021/jacs.5b00981. doi: 10.1021/jacs.5b00981. [DOI] [PubMed] [Google Scholar]
- 9.Vedunova MV, Mishchenko TA, Mitroshina EV, Ponomareva NV, Yudintsev AV, Generalova AN, et al. Cytotoxic effects of upconversion nanoparticles in primary hippocampal cultures. RSC Adv. 2016;6:33656–65. doi: 10.1039/c6ra01272h. [Google Scholar]
- 10.Tanyeri P, Buyukokuroglu ME, Mutlu O, Ulak G, Akar FY, Celikyurt IK, et al. Effects of ziprasidone, SCH23390 and SB277011 on spatial memory in the Morris water maze test in naive and MK-801 treated mice. Pharmacol Biochem Behav. 2015;138:142–7. doi: 10.1016/j.pbb.2015.09.014. doi: 10.1016/j.pbb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Naccache R, Vetrone F, Mahalingam V, Cuccia LA, Capobianco JA. Controlled synthesis and water dispersibility of hexagonal phase NaGdF4:Ho3+/Yb3+ nanoparticles. Chem Mater. 2009;21:717–23. doi: 10.1021/cm803151y. [Google Scholar]
- 12.Hu R, Gong X, Duan Y, Li N, Che Y, Cui Y, et al. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31:8043–50. doi: 10.1016/j.biomaterials.2010.07.011. doi: 10.1016/j.biomaterials.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Liu L, Jiang P, Chen C, Zhang T. Levodopa improves learning and memory ability on global cerebral ischemia-reperfusion injured rats in the Morris water maze test. Neurosci Lett. 2017;636:233–40. doi: 10.1016/j.neulet.2016.11.026. doi: 10.1016/j.neulet.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Maldiney T, Bessière A, Seguin J, Teston E, Sharma SK, Viana B, et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat Mater. 2014;13:418–26. doi: 10.1038/nmat3908. doi: 10.1038/nmat3908. [DOI] [PubMed] [Google Scholar]
- 15.Getts DR, Shea LD, Miller SD, King NJ. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36:419–27. doi: 10.1016/j.it.2015.05.007. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]