Abstract
Background:
Damage of the medial prefrontal cortex (mPFC) results in similar characteristics to the cognitive deficiency seen with the progress of Parkinson's disease (PD). Since the course of mPFC damage is still unclear, our study aimed to investigate the effects of melatonin (MT) on neurotoxicity in the mPFC of a rat model of PD.
Methods:
One hundred and fifty-four normal, male Wistar rats were randomly divided into the following five groups: normal + normal saline (NS), normal + 6-hydroxydopamine (6-OHDA), sham pinealectomy (PX) + 6-OHDA, PX + 6-OHDA, and MT + 6-OHDA. 6-OHDA was injected into the right substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) of each group, except normal + NS, 60 days after the PX. In the MT treatment group, MT was administered immediately after the intraperitoneal injection at 4 p.m. every day, for 14 days. Neuronal apoptosis in the mPFC was examined using the TUNEL method, while the expression of tyrosine hydroxylase (TH), Bax, and Bcl-2 in this region was measured using immunohistochemistry. The concentration of malondialdehyde (MDA) in the mPFC was examined using the thiobarbituric acid method.
Results:
Rats in the normal + 6-OHDA and sham PX + 6-OHDA groups were combined into one group (Group N + 6-OHDA) since there was no significant discrepancy between the groups for all the detected parameters. Apoptosis of cells in the NS, MT + 6-OHDA, N + 6-OHDA, and PX + 6-OHDA groups was successively significantly increased (Hc = 256.25, P < 0.001). The gray value of TH (+) fibers in the NS, MT + 6-OHDA, N + 6-OHDA, and PX + 6-OHDA groups was also successively significantly increased (F = 99.33, P < 0.001). The staining intensities of Bax and Bcl-2 were as follows: Group NS +/+, Group MT + 6-OHDA ++/+, Group N + 6-OHDA ++/+, and PX + 6-OHDA +++/+. The concentrations of MDA in the NS, MT + 6-OHDA, N + 6-OHDA, and PX + 6-OHDA groups were significantly increased in sequence (Hc = 296.309, P < 0.001).
Conclusions:
Neuronal damage of the VTA by 6-OHDA might induce VTA-mPFC nerve fibers to undergo anterograde nerve damage, in turn inducing transneuronal damage of the mPFC. PX significantly exacerbated the neurotoxicity in the mPFC, which was induced by the neuronal injury of the VTA. However, MT replacement therapy significantly alleviated the neurotoxicity in the mPFC.
Keywords: Medial Prefrontal Cortex, Melatonin, Pinealectomy, Tyrosine Hydroxylase, Ventral Tegmental Area
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disease, where the reduction of dopamine (DA) in the substantia nigra pars compacta (SNc)-striatal, ventral tegmental area (VTA)-cortex, and VTA-limbic pathways leads to the motor and nonmotor symptoms (NMS) of the disease.[1] Being a crucial part of the cortex-limbic system-striatal pathway, the medial prefrontal cortex (mPFC) connects with many nuclear groups in the brain. It was mainly innervated by dopaminergic nerve fiber projections from the midbrain VTA.[2] DA is an important neurotransmitter for mPFC function. Damages in the dopaminergic nerve system of the mPFC can result in persistent cognitive and behavioral disorder. Further, cognitive disorder is one of the major manifestations of PD NMS.[3,4] A recent meta-analysis demonstrated that the emergence of NMS (such as memory loss, depression, apathy, and anxiety) often occurs earlier than motor symptoms. The NMS of approximately 50% of patients with PD has not been given enough attention.[5]
Although the mechanisms underlying this disease are still unclear, PD is widely believed to be caused by multiple factors. Melatonin (MT) is an indole neural endocrine hormone that is mainly secreted by the pineal gland. It regulates the circadian rhythm. Studies have shown that the incidence of PD increases with ages, while the level of MT in vivo reduces with ages.[6] Further, the level of MT is lower in patients with PD than their normal, aged counterparts.[6] Thus, it has been speculated that the level of MT is potentially related to the occurrence of PD. Currently, only a few studies have focused on the relationship between MT levels in vivo and PD. Further, the results of basic studies led to different conclusions.[7] Previous studies have also emphasized movement symptoms of PD, with little focus on the NMS of PD. Up to now, the relationship between MT levels in vivo and mPFC pathological variation in rat model of the disease or patients with PD has not been reported.
6-hydroxydopamine (6-OHDA) can selectively damage catecholamine neurons and their fiber tips, resulting in neurotoxicity via multiple routes that lead to symptoms similar to PD.[8] 6-OHDA can damage hemi-midbrain SNc and VTA DA neurons, which allows the rat model of PD to better simulate disease pathogenesis. Tyrosine hydroxylase (TH) is the rate-limiting enzyme of DA biosynthesis, while Bax promotes cell apoptosis and Bcl-2 inhibits it. Malondialdehyde (MDA) is a degradation product of lipid peroxidation, which indirectly reflects the degree of cell damage. It is an important indicator of oxidative stress in the human body. In the current study, we aimed to further understand the effect of MT levels in vivo on the neurotoxicity of DA neurons in the mPFC of the 6-OHDA hemi-PD rat model. To do this, cell apoptosis, the expression of TH(+) fibers, Bax, and Bcl-2, and the MDA content were analyzed, following a pinealectomy (PX) and MT replacement therapy.
METHODS
Materials
A total of 154 normal male Wistar rats, weighing 301–320 g each and aged 12 months, were provided by the animal experiment center of Shandong University. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Shandong University. The rats were placed in a quiet environment and could freely drink water and eat food. The rats were housed under a light/dark environment, with 12 h of light (8:00–20:00) and 12 h of darkness (20:00–8:00).
Animal model
The animals were randomly divided into the following five groups: normal + normal saline (NS; n = 30), normal + 6-OHDA (n = 30), sham PX + 6-OHDA (n = 30), PX + 6-OHDA (n = 32), and MT + 6-OHDA (n = 32). The same experimental protocol was performed on control rats, except that the pineal gland was not removed. The rats were bred for 60 days after the operation, and then anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.5 mg/100 g). The head of each rat was horizontally fixed onto the mouse stereotaxic instrument and the three-dimensional coordinates of the right SNc and VTA were determined in reference to the atlas by Ming and Yun.[9] A total of 10 μg of 6-OHDA was injected at each point, with the equivalent dose of NS injected into rats in the normal + NS group. Rats in the MT treatment group were immediately administered MT (dissolved into 10% ethanol NS, dosage of 0.5 mg/100 g), via an intraperitoneal injection, and then continued to receive an MT injection at 16:00 every afternoon, for 14 days. Behavioral observation was performed at 14 days after the 6-OHDA injection. Apomorphine (0.5 mg/kg) was administered with an intraperitoneal injection, and the number of total rotating circles within 30 min was recorded by taking notes oneself for each rat. An average value >7 r/min indicated that a successful PD model had been established. Rats that failed to meet these standards were excluded from the analysis.
Main reagents and equipment
The main reagents used in the current study included the following: in situ cell apoptosis kits (Roche, USA), MT (Sigma, USA), TH primary antibody (Santa Cruz Biotechnology, USA), and Bax, Bcl-2, SP, DAB kits, and primary antibody (Beijing Zhongshan Reagents, China). We also used MDA kits that were provided by Boster Biological Technology (Wuhan, China). The following equipment were also used: Synergy-HT multi-function microplate reader (Bio-Tek, USA), ultrasonic homogenizer (Omni, USA), and rat brain stereotactic instrument (NeuroStar, Germany).
Sample selection and pathological observation
A total of 16 PD rats in each group were anesthetized and perfused with 4% paraformaldehyde for 2 h, 14 days after 6-OHDA was injected. Thereafter, they were decapitated and the cerebra were removed. Samples from Bregma of 4.6–1.6 mm were selected, fixed for 6 h, dehydrated till transparent, and embedded in wax. The sample was then sliced into 4-mm sections. A total of 20 sections, within 4.6–2.6 mm Bregma, were selected and five sections were then dyed. Cell apoptosis was detected by TUNEL staining. The expressions of TH, Bax, and Bcl-2 were analyzed using immunohistochemistry (IHC). A total of ten residual rats in each group were decapitated. Their brains were removed, and the residual blood was washed by saline and blotted with filter paper. The right forebrain was preserved at −20°C. The brains were then added to NS in an ice bath, with a brain tissue weight: saline volume of 1:9. The tissue was then homogenized with an ultrasonic homogenizer, and the homogenate, which consisted of 10% of tissue, was centrifuged for 10 min at 1369.55 ×g, at 4°C. The concentration of MDA in some of the supernatants was measured using thiobarbituric acid within 10 h. It must be noted that the pineal gland was not incompletely removed when each brain was picked up. During feeding, nine rats in Group N + 6-OHDA, six rats in Group PX + 6-OHDA, and six rats in Group MT + 6-OHDA were excluded due to postoperative acroparalysis, difficulty in feeding and death, or failure to effectively model PD.
Statistical analysis
Since there were no significant differences between groups such as normal + 6-OHDA and sham PX + 6-OHDA, these two groups were combined into one group named N + 6-OHDA. The mPFC in the hemisphere that was administered with 6-OHDA injection was analyzed. The number of apoptotic cells in 5 mPFC slices was counted, using 10 × 20-fold light microscope. Three fields were selected for each slice, and the numbers were averaged. The Tangier digital medical figure analyzer was used to measure the TH (+) fiber gray value in the mPFC. Note that, according to the law of Lambert–Beer, the actual concentration of substances is directly proportional to the optical density of the material, but inversely proportional to fiber gray value of the material. Thus, larger gray values represented a smaller actual concentration of the measured substance, and vice versa. Semi-quantitative standards were adopted for Bax and Bcl-2 IHC as follows: no negative (−) expression; weak positive (+) expression in several cells, where positive cells accounted for 0–25% of the whole field of view, staining was light; positive (++), where positive cells accounted for 25–50% of the whole field of view, staining was medium; and strong positive (+++), where positive cells accounted for more than 50% of the whole field of view, staining was deep. Data are expressed as mean ± standard deviation (SD). Comparisons among multiple sets were based on a completely random variance analysis, which was performed using SPSS 18.0 statistical software package (SPSS Inc., USA). The comparison between the two groups was based on the least significant difference method. If the variance analysis was inconclusive, the Kruskal–Wallis test was adopted and a comparison between the two groups was performed based on the Nemenyi method. The level of statistical significance was set at P < 0.05.
RESULTS
TUNEL staining
The characteristics of cell apoptosis under light microscopy were as follows: nuclear condensation, dyed brown, uneven coloring, and typically ring form. A few apoptotic cells in the NS group were not in a constant position. Compared with the NS group, the number of apoptotic cells was apparently increased in all the PD groups and scattered among the cortex layers, most in layers III–VI. The number of apoptotic cells in the NS, MT + 6-OHDA, N + 6-OHDA, and PX + 6-OHDA groups was significantly increased successively (Hc = 256.25, P < 0.001) [Table 1 and Figure 1a–1d].
Table 1.
Comparison of the number of apoptosis and TH(+) fiber gray values in mPFC in each group (mean ± SD)
| Group | Number of animals | TUNEL | Hc | P | TH(+) fibers | F | P | |
|---|---|---|---|---|---|---|---|---|
| Count | Mean rank | |||||||
| NS | 16 | 1.33 ± 0.11* | 40.50 | 256.25 | <0.001 | 141.23 ± 15.69† | 99.330 | <0.001 |
| MT + 6-OHDA | 16 | 11.21 ± 2.98* | 134.23 | 157.56 ± 14.33† | ||||
| N + 6-OHDA | 16 | 15.88 ± 3.02* | 205.09 | 169.41 ± 16.76† | ||||
| PX + 6-OHDA | 16 | 20.36 ± 3.97* | 262.18 | 181.96 ± 15.42† | ||||
*Comparison between two groups and †comparison between two groups, all P<0.05. TH: Tyrosine hydroxylase; 6-OHDA: 6-hydroxydopamine; N + 6-OHDA: Normal + 6-OHDA and sham PX + 6-OHDA; PX: Pinealectomy; MT: Melatonin; mPFC: Medial prefrontal cortex; SD: Standard deviation; NS: Normal saline.
Figure 1.
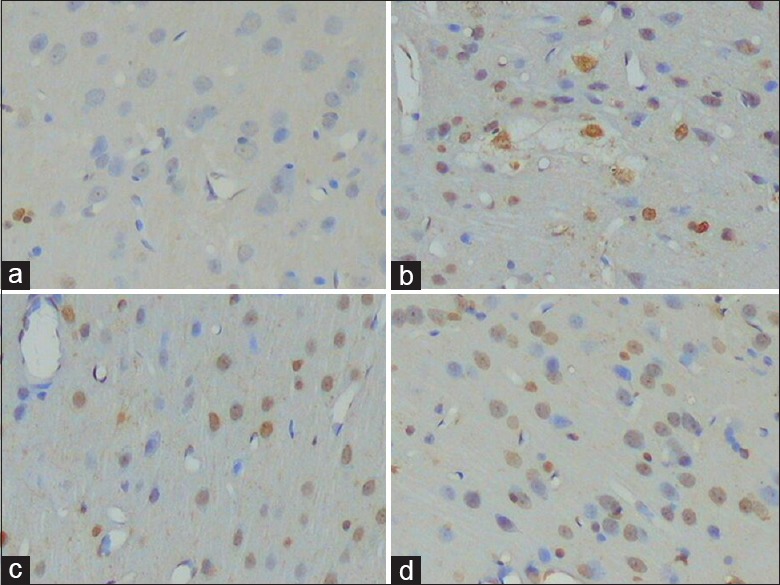
TUNEL staining for neuronic apoptosis in mPFC (original magnification ×200). (a) Group NS. (b) Group MT + 6-OHDA. (c) Group N + 6-OHDA. (d) Group PX + 6-OHDA. mPFC: Medial prefrontal cortex; NS: Normal saline; MT: Melatonin; 6-OHDA: 6-hydroxydopamine; N: Normal + Sham pinealectomy; PX: Pinealectomy.
Tyrosine hydroxylase immunohistochemistry staining
Sparse and fine TH(+) nerve fibers in the NS group were scattered in the cortical layers, mainly in layer IV. In the PD model groups, TH(+) nerve fibers were more sparse and fine, with only a few TH(+) dots. The TH(+) gray values in the nerve fibers in the NS, MT + 6-OHDA, N + 6-OHDA, and PX + 6-OHDA groups were significantly increased successively (F = 99.33, P < 0.001) [Table 1 and Figure 2a–2d].
Figure 2.
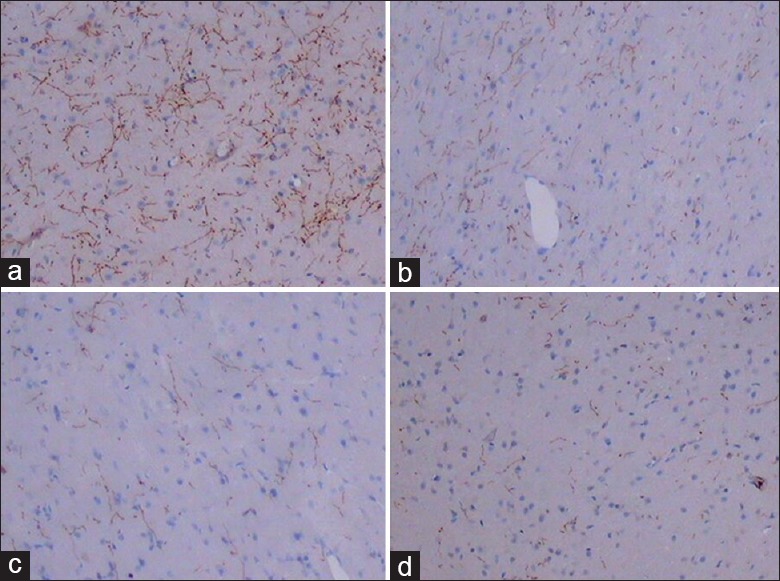
Immunohistochemistry for expression of TH(+) fibers in mPFC (SP, original magnification ×100). (a) Group NS. (b) Group MT + 6-OHDA. (c) Group N + 6-OHDA. (d) Group PX + 6-OHDA. mPFC: Medial prefrontal cortex; NS: Normal saline; MT: Melatonin; 6-OHDA: 6-hydroxydopamine; N: Normal + Sham pinealectomy; PX: Pinealectomy; TH: Tyrosine hydroxylase.
Bax and Bcl-2 immunohistochemistry staining
Bax and Bcl-2 were scattered in the cortical layers, Bax was most in layers III–VI. The results were as follows: Bax: Group NS+, Group MT + 6-OHDA ++, Group N + 6-OHDA ++, Group PX + 6-OHDA +++; Bcl-2: all groups +.
Determination of malondialdehyde concentration
Compared with the NS group, the concentration of MDA in the mPFC of the PD group was significantly increased. The concentrations of MDA in the NS, MT + 6-OHDA, N + 6-OHDA, and PX + 6-OHDA groups were significantly increased in sequence (Hc = 296.309, P < 0.001) [Table 2].
Table 2.
Comparison of the concentrations of MDA in mPFC in each group, mean ± SD (nmol/mg protein)
| Group | Number of animals | The concentrations of MDA | Mean rank | Hc | P |
|---|---|---|---|---|---|
| NS | 10 | 110.74 ± 7.45* | 40.50 | 296.309 | <0.001 |
| MT + 6-OHDA | 10 | 154.12 ± 5.05* | 122.28 | ||
| N + 6-OHDA | 10 | 169.22 ± 5.24* | 198.82 | ||
| PX + 6-OHDA | 10 | 198.58 ± 8.11* | 280.40 |
*Comparison between two groups, all P<0.05; MDA: Malondialdehyde; 6-OHDA: 6-hydroxydopamine; N + 6-OHDA: Normal + 6-OHDA and sham PX + 6-OHDA; PX: Pinealectomy; MT: Melatonin; SD: Standard deviation; mPFC: Medial prefrontal cortex; NS: Normal saline.
DISCUSSION
The PFC is a part of the cerebral cortex, which refers to the full frontal cortex, with the exception of the primary motor cortex and secondary motor cortex. The PFC contains areas that differ in structure and function, and is mainly divided into the mPFC and orbitofrontal cortex. The mPFC is additionally divided into the infralimbic cortex, prelimbic cortex, and dorsal anterior cingulate.[10] The mPFC is mainly innervated by dopaminergic nerve fiber projections from the VTA and forms the midbrain cortex dopaminergic system, which controls recognition and thinking ability.[10] Cognitive deficiency can occur at different stages during the course of PD, and its characteristics are similar to mPFC damage.[11] Previous imaging studies demonstrated a significant reduction in the volume of the frontal lobe of patients with PD.[12] Pathological changes in the mPFC in PD could be attributed to either the reduction of mPFC DA fiber projections, due to VTA DA damage, or to other factors leading to neurotoxicity in these brain area, which has not yet been reported.
Anatomical studies have demonstrated that mPFC cells are divided into six layers. The VTA-mPFC dopaminergic nerve fibers are mainly projected into layer IV, namely in the granule cell layer. The results of this experiment demonstrated the following: TH(+) nerve fibers in Group NS were scattered throughout the cortical layers, most in layer IV, which corroborate with a previous anatomical study. Compared with the NS group, there was an increase in gray values of TH(+) nerve fibers in Group N + 6-OHDA, which indicated a decrease in TH(+) nerve fibers. This result indicated that VTA damage resulting from the injection of 6-OHDA into the midbrain could cause anterograde nerve damage to the projected nerve fibers. Tompkins et al.[13] adopted the TUNEL approach to determine apoptosis-like changes in mesencephalic dopaminergic neurons in patients with PD. In the current study, compared with the NS group, the number of apoptotic cells in the mPFC of the PD group was increased and scattered throughout the cortical layers, mainly in layers III–IV. This indicated that damage to the VTA could cause postsynaptic neurons with synaptic contact to elicit neurotoxic effects, i.e., orthograde transneuronal damage. This can be defined as the phenomenon by which neurons completely lose the innervation of afferent neuron, consequently leading to nerve cell atrophy and degeneration. Although it is known that cell apoptosis participates in this process of neurotoxicity, the mechanisms that underlie this are still not clear. Further, non-VTA-mPFC dopaminergic postsynaptic neurons in other layers of the mPFC cortex also caused cells to be apoptotic. Therefore, we postulated that post- and pre-synaptic transneuronal damage between each cortical layer could be one of the reasons. This finding corroborated with previous studies by Sweet et al.[14,15] who demonstrated that damage of the SNc and VTA was associated with a decrease in nerve fibers in a wide range of brain regions. Further, its projection fiber terminal neurons were also significantly reduced.
The Bax/Bcl-2 is the main pathway that modulates cell apoptosis. Its expression in the brains of patients with PD, however, was reported to be different.[16,17] In the current experiment, the expression of these proteins in the cortical layers of the mPFC was as follows: Bax/Bcl-2 in Group NS was +/+, indicating that the apoptosis of cells in normal brain tissues is regulated and modulated by the balance between Bax and Bcl-2; Bax/Bcl-2 in Group N + 6-OHDA was ++/+, which verified that VTA damage caused by 6-OHDA could promote apoptosis in the mPFC, by enhancing Bax expression. The latter finding corroborated with the apparent increase of apoptotic cells in the mPFC of Group N + 6-OHDA, compared with that in the NS group, which verified that the imbalance of Bax/Bcl-2 is a possible reason for the apparent increase in cell apoptosis induced by VTA damage in mPFC. MDA is a degradation product of lipid peroxidation. The level of MDA indirectly reflects the degree of cell damage, with a high concentration of MDA leading to apoptosis. In the current experiment, the content of MDA in the mPFC of the N + 6-OHDA group was significantly higher than that in the NS group, indicating that 6-OHDA-induced VTA damage caused an oxidative stress reaction in the mPFC. The reason for this is not clear. We postulated that the marked increase in MDA is one of the ways in which VTA damage induces increased cell apoptosis in the mPFC.
Previous studies[18,19] demonstrated that MT shortage in vivo was related to PD, with MT replacement therapy partially improving PD symptoms. Other studies,[7] however, have yielded contrary results. Some therapeutic trials demonstrated that, although MT was effective in impeding the progression of Alzheimer's disease, it was not an effect for PD.[20] In the current study, compared with Group N + 6-OHDA, the number of apoptotic cells in the mPFC in Group MT + 6-OHDA was significantly reduced, while the number of TH(+) nerve fibers was significantly increased (i.e., since the TH(+) nerve fiber gray values were significantly reduced). The results demonstrated that MT apparently alleviated the effect, i.e., the apparent increase of apoptosis of mPFC cells and reduction of TH(+) nerve fibers in mPFC, which was induced by VTA damage. This suggested that MT replacement therapy may have a protective effect on PD. The putative mechanisms by which MT reduces neurotoxicity have been outlined below. First, the antioxidant stress response, i.e., the ability of MT to scavenge free radicals, is 2, 4, and 14 times that of Vitamin E, glutathione, and mannitol, respectively.[21,22] MT is selectively occupied by the respiratory chain and not shared by other antioxidants. In the antioxidant system, MT is the only hormone that matches age.[7] Second, MT inhibits the inflammatory response.[23] Third, MT inhibits aggregation of α-Synuclein and other abnormal proteins, consequently reducing their neurotoxicity.[24] Further, MT can increase the mRNA and protein expression of neurotrophic factors (i.e., GDNF), whereas 6-OHDA inhibits the expression of GDNF.[25] Finally, MT can inhibit apoptosis by regulating Bax/Bcl-2 gene expression.[26] In the current study, compared to the N + 6-OHDA group, the apoptosis of cells in the mPFC of the PX + 6-OHDA group was significantly higher. Further, the number of TH(+) fibers was significantly reduced (i.e., since the gray value of TH[+] fibers was significantly increased). This finding indicated that MT lacks an apparently aggravated effect on the increase of apoptosis of mPFC cells and reduction of TH(+) nerve fibers in the mPFC, which were induced by VTA damage, supporting the theory that there is an association between MT deficiency and PD onset. MT is mainly produced in the pineal gland. After PX, the level of MT decreased significantly in rats, which reduced its neuroprotective effect. Compared to other tissues, brain tissue contains a large amount of polyunsaturated fatty acids and has more iron ions that have a catalytic activity. Further, the brain contains very low level of antioxidant enzymes, making it susceptible to neuronal toxic factors.[27] Thus, mPFC neurotoxicity that was induced by a 6-OHDA injection to the VTA was aggravated after PX.
In the current study, the expression of Bax/Bcl-2 in mPFC was as follows: Group N + 6-OHDA was ++/+ and Group PX + 6-OHDA was +++/+, indicating that PX aggravated the upregulation of Bax/Bcl-2, which was induced by the 6-OHDA injection to the SNc and VTA. This validated the theory that PX worsened the cell apoptosis induced by VTA damage by modulating the expression of Bax/Bcl-2. This experiment indicated that MT reduced the mPFC cell apoptosis induced by VTA damage. However, since the expression of Bax/Bcl-2 was ++/+ in the MT + 6-OHDA group and ++/+ in the N + 6-OHDA, this suggested that MT reduced apoptotic cells in mPFC by a mechanism that was not dependent on Bax or Bcl-2. This finding did not corroborate with previous findings. In the current study, the content of mPFC MDA in the MT + 6-OHDA group was significantly lower than that in the N + 6-OHDA group. Further, the content of MDA, in the PX + 6-OHDA group, was significantly increased, and thus, for the significant increase in the level of lipid peroxides of mPFC by the 6-OHDA injection to the VTA, MT alleviated this effect, while a deficiency in MT had an aggravated effect. The results proved that MT had an anti-lipid peroxidation effect, which is consistent with previous findings.
Although we studied the relationship between MT level and mPFC nerve damage in a 6-OHDA rat model of PD, we only focused on the lipid peroxidation and Bax/Bcl-2 expression in relation to the mechanism of MT action. Thus, we did not analyze other mechanism in depth, which remains to be elucidated in future experimental studies.
We confirmed that a deficiency in MT could aggravate mPFC nerve damage in a 6-OHDA rat model of PD, and that MT replacement therapy could alleviate this damage, providing a certain reference for the early diagnosis and treatment of PD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Sgambato-Faure V, Buggia V, Gilbert F, Lévesque D, Benabid AL, Berger F, et al. Coordinated and spatial upregulation of arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64:936–47. doi: 10.1097/01.jnen.0000186922.42592.b7. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- 2.Hajós M, Hajós-Korcsok E, Sharp T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br J Pharmacol. 1999;126:1741–50. doi: 10.1038/sj.bjp.0702510. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraus MF, Maki PM. Effect of amantadine hydrochloride on symptoms of frontal lobe dysfunction in brain injury: Case studies and review. J Neuropsychiatry Clin Neurosci. 1997;9:222–30. doi: 10.1176/jnp.9.2.222. doi: 10.1176/jnp.9.2.222. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology. 2010;75:1062–9. doi: 10.1212/WNL.0b013e3181f39d0e. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson's disease to health care professionals: An international study using the nonmotor symptoms questionnaire. Mov Disord. 2010;25:704–9. doi: 10.1002/mds.22868. doi: 10.1002/mds.22868. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan V, Spence DW, Pandi-Perumal SR, Brown GM, Cardinali DP. Melatonin in mitochondrial dysfunction and related disorders. Int J Alzheimers Dis 2011. 2011:326320. doi: 10.4061/2011/326320. doi: 10.4061/2011/326320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis GL, Turner EJ. Primary and secondary features of Parkinson's disease improve with strategic exposure to bright light: A case series study. Chronobiol Int. 2007;24:521–37. doi: 10.1080/07420520701420717. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- 8.Hwang CK, Chun HS. Isoliquiritigenin isolated from licorice Glycyrrhiza uralensis prevents 6-hydroxydopamine-induced apoptosis in dopaminergic neurons. Biosci Biotechnol Biochem. 2012;76:536–43. doi: 10.1271/bbb.110842. doi: 10.1271/bbb.110842. [DOI] [PubMed] [Google Scholar]
- 9.Ming BX, Yun SS. The Stereotaxic Atlas of the Rat Brain. 1st ed. Beijing: People's Medical Publishing House; 1991. [Google Scholar]
- 10.Spencer RC, Devilbiss DM, Berridge CW. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol Psychiatry. 2015;77:940–50. doi: 10.1016/j.biopsych.2014.09.013. doi: 10.1016/j.biopsych.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 12.Nishio Y, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Suzuki K, et al. Corticolimbic gray matter loss in Parkinson's disease without dementia. Eur J Neurol. 2010;17:1090–7. doi: 10.1111/j.1468-1331.2010.02980.x. doi: 10.1111/j.1468-1331.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 13.Tompkins MM, Basgall EJ, Zamrini E, Hill WD. Apoptotic-like changes in Lewy-body-associated disorders and normal aging in substantia nigral neurons. Am J Pathol. 1997;150:119–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Sweet JA, Walter BL, Gunalan K, Chaturvedi A, McIntyre CC, Miller JP, et al. Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: Implications for targeting in deep brain stimulation. J Neurosurg. 2014;120:988–96. doi: 10.3171/2013.12.JNS131537. doi: 10.3171/2013.12.JNS131537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anaya-Martinez V, Martinez-Marcos A, Martinez-Fong D, Aceves J, Erlij D. Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: Implications for abnormal motor behavior. Neuroscience. 2006;143:477–86. doi: 10.1016/j.neuroscience.2006.08.033. doi: 10.1016/j.neuroscience.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann A, Michel PP, Troadec JD, Mouatt-Prigent A, Faucheux BA, Ruberg M, et al. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson's disease? J Neurochem. 2001;76:1785–93. doi: 10.1046/j.1471-4159.2001.00160.x. doi: 10.1046/j.1471-4159.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 18.Litvinenko IV, Krasakov IV, Tikhomirova OV. Sleep disorders in Parkinson's disease without dementia: A comparative randomized controlled study of melatonin and clonazepam. Zh Nevrol Psikhiatr Im S S Korsakova. 2012;112:26–30. [PubMed] [Google Scholar]
- 19.Gutierrez-Valdez AL, Anaya-Martínez V, Ordoñez-Librado JL, García-Ruiz R, Torres-Esquivel C, Moreno-Rivera M, et al. Effect of chronic L-dopa or melatonin treatments after dopamine deafferentation in rats: Dyskinesia, motor performance, and cytological analysis. ISRN Neurol. 2012;2012:360379. doi: 10.5402/2012/360379. doi: 10.5402/2012/360379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olakowska E, Marcol W, Kotulska K, Lewin-Kowalik J. Role of melatonin in neurodegenerative diseases. Neurotox Res. 2005;7:293–318. doi: 10.1007/BF03033887. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- 21.Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: A peroxyl radical scavenger more effective than Vitamin E. Life Sci. 1994;55:PL271–6. doi: 10.1016/0024-3205(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 22.Ozsoy O, Yildirim FB, Ogut E, Kaya Y, Tanriover G, Parlak H, et al. Melatonin is protective against 6-hydroxydopamine-induced oxidative stress in a hemiparkinsonian rat model. Free Radic Res. 2015;49:1004–14. doi: 10.3109/10715762.2015.1027198. doi: 10.3109/10715762.2015.1027198. [DOI] [PubMed] [Google Scholar]
- 23.Pinato L, da Silveira Cruz-Machado S, Franco DG, Campos LM, Cecon E, Fernandes PA, et al. Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct Funct. 2015;220:827–40. doi: 10.1007/s00429-013-0686-4. doi: 10.1007/s00429-013-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sae-Ung K, Uéda K, Govitrapong P, Phansuwan-Pujito P. Melatonin reduces the expression of alpha-synuclein in the dopamine containing neuronal regions of amphetamine-treated postnatal rats. J Pineal Res. 2012;52:128–37. doi: 10.1111/j.1600-079X.2011.00927.x. doi: 10.1111/j.1600-079X.2011.00927. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Zhang HQ, Liang XY, Zhang HF, Zhang T, Liu FE, et al. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: Role of oxidative stress, BDNF and caMKII. Behav Brain Res. 2013;256:72–81. doi: 10.1016/j.bbr.2013.07.051. doi: 10.1016/j.bbr.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Yildirim FB, Ozsoy O, Tanriover G, Kaya Y, Ogut E, Gemici B, et al. Mechanism of the beneficial effect of melatonin in experimental Parkinson's disease. Neurochem Int. 2014;79:1–11. doi: 10.1016/j.neuint.2014.09.005. doi: 10.1016/j.neuint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Hua ZX, Cai LJ. Melatonin free-radical effect and its mechanism. Chin Pharmacol. 1997;15:24–7. [Google Scholar]


