Abstract
Background
Postsurgical peritoneal adhesions (PPAs) are pathologic fibrous bands within the peritoneal cavity. The aim of this study was to investigate the protective effect of hydrogen-rich saline (HRS) on PPAs formation in mice.
Material/Methods
Adhesions were induced in mice using the cecum rubbing model. The mice were allocated into 4 groups: control sham group without cecum rubbing; PPA group with saline applied intraperitoneally (i.p.) daily after cecum rubbing; PPA+HRS (5) group with 5 ml/kg of HRS applied i.p. daily after cecum rubbing; and PPA+HRS (10) group with 10 ml/kg of HRS applied i.p. daily after cecum rubbing. On the 1st, 3rd, and 7th days after the operation, mice were killed and pathological adhesion bands were quantified to detect the effect of HRS on PPAs formation.
Results
HRS did not affect PPAs formation on the 1st day, but did make a significant reduction on the 3rd and 7th days. A significant increase of t-PA and decrease of TGF-β1 and PAI-1 in the peritoneal fluids were observed in the HRS-treated groups. The levels of MDA and MPO in the HRS-treated groups were significantly lower than those in the PPA group. TNF-α and IL-6 levels in HRS-treated groups significantly decreased compared with those in the PPA group on postoperative day 3 and 7. Moreover, HRS decreased the mRNA levels of pro-inflammatory cytokines and TGF-β1 expression in the postsurgical adhesion bands.
Conclusions
These results showed that HRS had therapeutic potential for preventing PPAs formation, possibly through balancing the expression of TGF-β1, t-PA, and PAI-1, and inhibiting oxidative stress and inflammation.
MeSH Keywords: Hydrogen, Inflammation, Oxidative Stress, Tissue Adhesions
Background
Postsurgical peritoneal adhesions (PPAs) are pathological fibrous bands formed within the peritoneal cavity, which can join the viscera together or adhere to the abdominal wall. PPAs develop in 67–93% of patients who undergo a laparotomy and 97% of patients who undergo open gynecological surgery [1–3]. The symptoms of PPAs include, but are not limited to, ileus, stomach ache, sterility in women, and difficulties in re-operation. Great efforts have been made by researchers and many strategies have been applied to prevent and alleviate PPAs, such as anti-inflammatory agents, anti-oxidative stress drugs, fibrinolytic drugs, and solid barriers. Although application of physical solid barriers is most frequently used in clinical practice, it has undeniable shortcomings [4–9]. New complementary methods, which can be easily and conveniently used for the prevention of PPAs, are urgently needed.
The mechanism of PPAs formation is complicated and unclear. The pathology of PPAs formation involves the mesothelial cells, basal membrane, and sub-endothelial connective tissue. Inflammation, oxidative stress, and fibrinolytic-fibroblast imbalance are the key factors in the PPAs formation process [10–13]. Excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS) can be induced by hypoxia, physical stretch, or necrosis during surgery. Moreover, oxidative damage induced by ROS and RNS can directly make cells, proteins, and DNAs injury to promote the PPAs formation [14,15]. Inflammation and oxidative stress induced by the operation can cause coagulation or fibrin formation to increase the thickness of PPAs [11,16,17]. The adhesions formation depends on the comprehensive results of fibrinolysis, peritoneal repair, and fibroblast proliferation [18].
Hydrogen, the lightest chemical element in nature, has remarkable curative effects in many diseases through its efficiency in selectively scavenging hydroxyl radicals and ROS and inhibition of inflammation [19,20]. Moreover, hydrogen can also protect organs from several other types of diseases, such as metabolic syndrome, sepsis, and ischemia-reperfusion injury [21]. In regard to its effect in fibrogenesis, several previous studies found that hydrogen could protect against pulmonary fibrosis induced by irradiation and chronic paraquat intoxication [22]. Koyama et al. [23] found that hydrogen could protect against liver fibrogenesis by scavenging hydroxyl radicals. Bo et al. [24] also found that hydrogen-rich saline (HRS) improved unilateral ureteral obstruction-induced renal interstitial fibrosis in rats. Hence, we speculated that HRS might also have possible effects on PPAs progression.
Material and Methods
Experimental animals and HRS preparation
Male C57BL/6 mice (4–5 weeks old, 21–26 g) (Animal Feeding Center of Xi’an Jiaotong University Health Science Center) were used in the study. They were fed a standard mice diet and water with 12-h light-dark cycles. The study was approved by and animals were cared for in accordance with the Ethics Committee, Xi’an Jiaotong University Health Science Center.
Hydrogen-rich saline (HRS) was produced and provided by Huoli Qingyuan Co. Ltd., Beijing, China, and it was used in other studies as well [25,26]. In general, molecular hydrogen was dissolved in saline under high pressure using a gas compressor. The HRS was kept at 4°C under atmospheric pressure in an aluminum bag. Gas chromatography method was used to detect the hydrogen content, as previously described [27]. The hydrogen content of the HRS in the present study was 0.89–1.03 mmol/L.
Experimental design
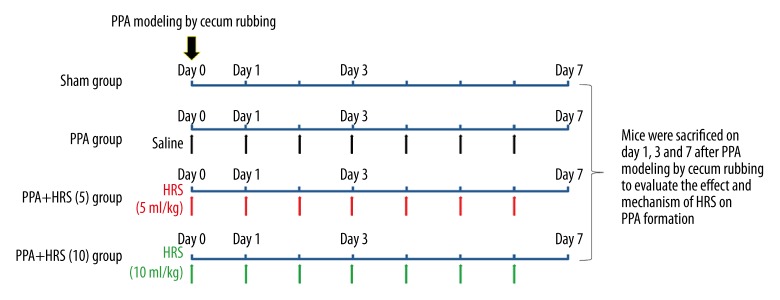
PPAs were induced by laparotomy with cecum rubbing, which was validated by previous studies [28,29]. In general, mice were anesthetized using chloral hydrate before laparotomy was performed, after which the cecum was identified and pulled out. The cecum was rubbed softly with 2 pieces of dry gauze both at ventral and dorsal surfaces until the placenta percreta lost their shine and hemorrhagic points arose. Then, the cecum was returned and the abdomen was closed. Mice were randomly assigned to the following groups: Group 1 (sham group), after laparotomy, the cecum was isolated from the surrounding tissues and put back gently without rubbing; Group 2 (PPA group), after cecum rubbing, saline was administrated intraperitoneally (i.p.) daily until killing; Group 3 (PPA+HRS (5) group), after cecum rubbing, 5 ml/kg of HRS was administrated i.p. daily until killing; and Group 4 (PPA+HRS (10) group), after cecum rubbing, 10 ml/kg of HRS was administrated i.p. daily until killing. Mice (n=10 per group) in the experimental groups were killed on day 1, 3, or 7 after cecum rubbing to determine the degree of PPAs formation. Blood samples, peritoneal tissues, and peritoneal fluid were collected for further detection. Serum samples were separated from blood samples by centrifugation at 4°C and 3000×g for 15 min. A detailed schematic diagram of experiment design was presented in Figure 1.
Figure 1.
Schematic diagram of the present study.
Adhesion bands grading
Degrees of PPAs formation were measured on day 1, 3, and 7 after the operation according to methods described by Kennedy et al. [29] because scoring of adhesions on day 7, 14, and 28 indicated similar results [30]. The adhesion bands grading system was shown in Figure 2 and the inflammation scoring system was presented in Figure 3.
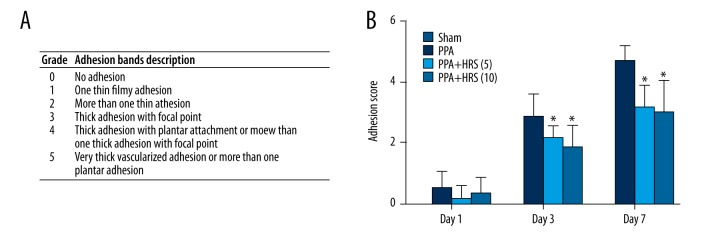
Figure 2.
Hydrogen-rich saline (HRS) decreased the postsurgical adhesion grades in mice. The intraperitoneal administration of HRS (5 and 10 ml/kg) significantly decreased peritoneal adhesions formation compared with saline treatment. (A) The grade system of adhesion bands. (B) The adhesion scores in the sham, PPA, PPA+HRS (5) and PPA+HRS (10) groups. All data were expressed as mean ±SD. * P<0.05 vs. PPA group.
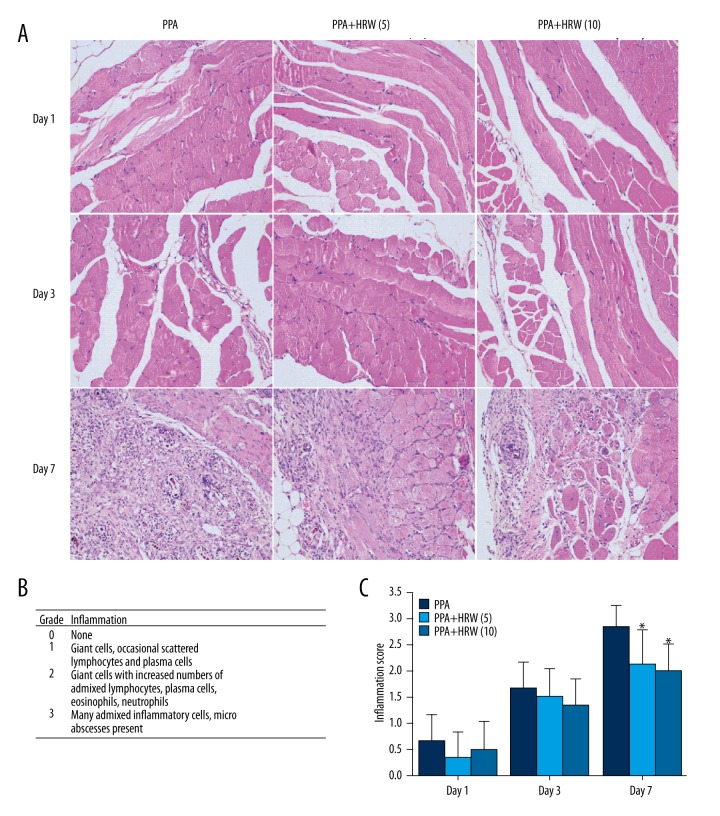
Figure 3.
Hydrogen-rich saline (HRS) improved the postsurgical peritoneal tissue histology in mice. (A) Representative images were derived from PPA, PPA+HRS (5) and PPA+HRS (10) groups (200×). The numerical scoring of inflammation based on histology (B) was shown in panel (C). All data were expressed as mean ± SD. * P<0.05 vs. PPA group.
Enzyme-linked immunosorbent assays (ELISA)
Commercially available ELISA kits were used to detect the serum tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels and the peritoneal fluid concentrations of tissue plasminogen activator (t-PA), plasminogen activator inhibitor (PAI-1), and transforming growth factor-β1 (TGF-β1), following the manufacturers’ instructions.
Measurement of peritoneal oxidative stress
The peritoneal tissue myeloperoxidase (MPO) and malonaldehyde (MDA) activities were detected by use of activity assay kits purchased from NanJing JianCheng Bioengineering Institute.
Histological and morphological examination
Cecum and surrounding peritoneal tissues were separated after bands grading. Samples were fixed in 10% formalin and immersed in paraffin. Ten sections of 5-μm thickness from each group were obtained and stained with hematoxylin and eosin (H&E) to evaluate the morphology and the degree of inflammation.
Immunohistochemical (IHC) examination
For TGF-β1 protein localization, serial paraffin-embedded tissue sections of 5-μm thickness were mounted onto saline-coated slides, dewaxed, and rehydrated in a graded series of alcohol and rinsed in distilled water. A mouse polyclonal antibody of TGF-β1 (Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) was used at a working dilution of 1: 200.
For semi-quantitation of TGF-β1 expression, the results of IHC were obtained by 2 researchers under blinded conditions. Representative images of TGF-β1 staining were acquired using a Nikon TE2000-s microscope. The integral optical density (IOD) of immunoreactivity was calculated using Image J software. In each image, all cells, including the positively and negatively stained cells, in the adhesive tissues were counted. Six fields in each specimen were randomly selected for each subject of the groups, and the percentage of positively stained cells was counted at 200× magnification. The intensity of IHC staining was scored as 0 for negative, 1for weak, 2 for moderately strong, and 3 for strong. The extent of staining was assessed based on the percentage of positive cells: 0 for negative, 1 for 1–25%, 2 for 26–50%, 3 for 51–75%, and 4 for 76–100%. The final staining score for each sample was the mean of the sum of the intensity and extent scoring from 5 fields which ranged from 0 to 12, as previously described in detail elsewhere [31].
Transcriptional level quantification
Total RNAs from PPAs samples were isolated by RNAfast200 Kit (Fastagen Biotech, Shanghai, China) according to the instructions. PrimeScript RT reagent Kit (TaKaRa Biotechnology, Dalian, China) was used to perform the reverse transcription. mRNA expression was detected in triplicate and normalized to the 18S mRNA expression by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The relative transcriptional level quantification was calculated by the comparative-Ct method (ΔΔCt method). The following primers were used to amplify vascular cell adhesion molecule-1(VCAM-1): 5′-TGCCGAGCTAAATTACACATTG-3′ (sense) and 5′-CCTTGTGGAGGGATGTACAGA-3′ (antisense); intercellular adhesion molecule-1(ICAM-1): 5′-TGCCTCTGAAGCTCGGATATAC-3′ (sense) and 5′-TCTGTC GAACTCCTCAGTCAC-3′ (antisense); monocyte chemotactic protein-1(MCP-1): 5′-TAAAAACCTGGATCGGAACCAAA-3′ (sense) and 5′-GCATTAGCTTCAGATTTACGGGT-3′ (antisense); E-selectin: 5′-GGCAAATTCAACGGCACAGT-3′ (sense) and 5′-GGGTCTCGCTCCTGGAAGAT-3′ (antisense); E-cadherin: 5′-TCGGAAGACTCCCGATTCAAA-3′ (sense) and 5′-CGGACGAGGAAACTGGTCTC-3′ (antisense); matrix metalloproteinase-9 (MMP-9): 5′-GCGTCGTGATCCCCACTTAC-3′ (sense) and 5′-CAGGCCGAATAGGAGCGTC-3′ (antisense); 18S: 5′-AAACGGCTACCACATCCAAG-3′ (sense) and 5′-CCTCCA ATGGATCCTCGTTA-3′ (antisense).
Statistical analysis
GraphPad Prism 6.0 software (version 6.0, GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. All the variables were expressed as mean±standard deviation (mean ±SD). Student’s t-test (2 groups) and (or) one-way ANOVA (multiple groups) were used to detect the difference. Significant differences were determined by P<0.05.
Results
Effect of HRS on grade of adhesion bands
The average adhesion score based on the system of Kennedy et al. for mice (10 mice/group) was presented in Figure 2. There were no PPAs formation in the sham group on day 1, 3, and 7. The mean values of PPAs scoring in the PPA group were 0.5000±0.5477 on day 1, 2.8330±0.7528 on day 3, and 4.6670±0.5164 on day 7. The mean values of adhesion scoring in PPA+HRS (5) group were 0.1667±0.4082 on day 1, 2.1670±0.4082 on day 3, and 3.1670±0.7528 on day 7. The mean values of adhesion scoring in PPA+HRS (10) group were 0.3333±0.5164 on day 1, 1.8330±0.7528 on day 3, and 3.0000±1.0950 on day 7. There was no significant difference in the adhesion scoring among the PPA, PPA+HRS (5), and PPA+HRS (10) groups on day 1, but the adhesion scoring in the PPA+HRS (5) and PPA+HRS (10) groups were much lower compared with the PPA group on day 3 and 7 (P<0.05). The efficacy of different doses of hydrogen (5 and 10 ml/kg) was evaluated, and the hydrogen dose of 5 mg/kg was not different from the 10 mg/kg dose, which yielded a valid protective effect in preventing PPAs formation. The results showed that HRS inhibited PPAs formation to a significantly greater extent than saline did.
Effect of HRS on histological change
H&E staining was conducted to detect the histological change in the peritoneal tissues (Figure 3A). The detailed inflammation score system was shown in Figure 3B. The inflammation scoring, evaluated by 2 assessors based on the histologic data, were presented in Figure 3C. In the PPA, PPA+HRS (5), and PPA+HRS (10) groups, few inflammatory cells could be observed on day 1 and 3 after cecum rubbing. However, massive inflammatory cells were recruited to peritoneal tissues and some micro-abscesses could be observed in the PPA group on day 7. In contrast to the PPA group, the count of inflammatory cells decreased significantly and few micro-abscesses could be observed in the PPA+HRS (5) and PPA+HRS (10) groups (Figure 3A). In conclusion, a significant decrease of the inflammation score was observed in the HRS-treated groups (Figure 3C), but there was no difference between PPA+HRS (5) and PPA+HRS (10) groups. These results clearly showed a dramatic inhibitory effect of hydrogen on migration of inflammatory cells to the peritoneal cavity in response to injury.
Effect of HRS on peritoneal t-PA, PAI-1 and TGF-β1
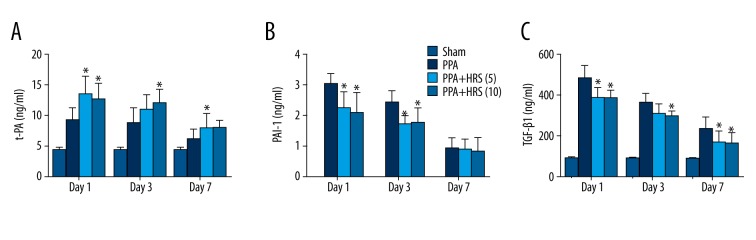
Levels of t-PA, PAI-1, and TGF-β1 in peritoneal fluids were measured by ELISA in all groups on day 1, 3 and 7 (Figure 4). t-PA in the groups which received 5 and 10 ml/kg doses of HRS showed a remarkable increase compared to the PPA group (P<0.05), but no significant difference was found between PPA+HRS (5) and PPA+HRS (10) groups on day 1. The level of t-PA in the PPA+HRS (10) group was much higher than that in the PPA group (day 3) and t-PA in the PPA+HRS (5) group was significantly higher than that in the PPA group (day 7). The level of PAI-1 in each group was also measured. There was a significant difference in PAI-1 levels in peritoneal fluids among the PPA, PPA+HRS (5), and PPA+HRS (10) groups on day 1 and 3 but not on day 7. Figure 4 showed the significant difference in TGF-β1 levels in peritoneal fluids between the PPA group and PPA+HRS [(5) and (10)] groups on day 1 and 7. The concentration of TGF-β1 in the PPA+HRS (10) group was much higher compared with the PPA group on day 3, but no difference was found between PPA+HRS (5) and PPA+HRS (10) groups on day 1, 3, and 7 (Figure 4).
Figure 4.
Hydrogen-rich saline (HRS) increased postsurgical t-PA and decreased TGF-β1 and PAI-1 levels. The concentrations of (A) t-PA, (B) PAI-1, and (C) TGF-β1 in the peritoneal fluid in all groups were measured by enzyme-linked immunosorbent assays. All data were expressed as mean ± SD. * P<0.05 vs PPA group.
Effect of HRS on peritoneal oxidative stress
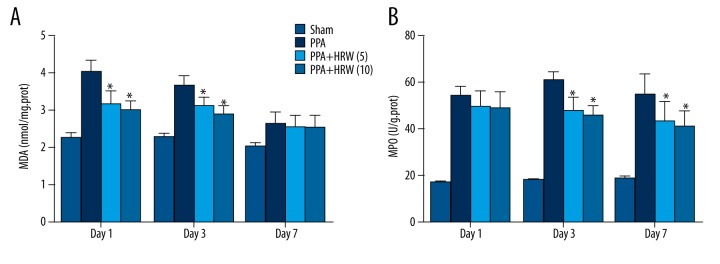
The activities of MDA and MPO in peritoneal tissues of each group were also evaluated on day 1, 3, and 7 (Figure 5). The levels of MDA in the PPA+HRS (5) and PPA+HRS (10) groups were much lower compared with the PPA group (P<0.05) on day 1 and 3 after the operation. Moreover, the levels of MPO in the PPA+HRS (5) and PPA+HRS (10) groups were also much lower in comparison to the PPA group (P<0.05) on day 3 and 7.
Figure 5.
Hydrogen-rich saline (HRS) decreased oxidative stress levels in postsurgical peritoneal tissues. The levels of (A) malonaldehyde (MDA) and (B) myeloperoxidase (MPO) in the peritoneal tissue in all groups were measured. All data were expressed as mean ±SD. * P<0.05 vs. PPA group.
Effect of HRS on serum cytokines
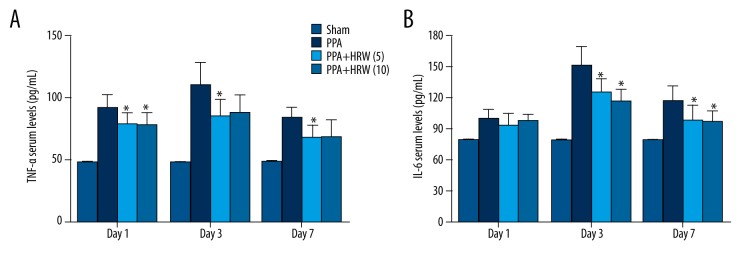
We measured serum TNF-α and IL-6 levels (Figure 6). TNF-α levels of PPA+HRS (5) and PPA+HRS (10) groups decreased significantly in contrast to that in the PPA group on day 1 (P<0.05). The TNF-α level in PPA+HRS (5) group decreased in comparison to the PPA group on day 3 and 7. Serum IL-6 level also decreased significantly in the PPA+HRS (5) and PPA+HRS (10) groups in comparison to the PPA group on day 3 (P<0.05). No significant difference was detected among PPA, PPA+HRS (5), and PPA+HRS (10) groups on day 1. The level of IL-6 in the PPA+HRS (10) group was much lower compared with PPA group on day 7. However, no significant differences were found in TNF-α and IL-6 levels between the PPA+HRS (5) and PPA+HRS (10) groups.
Figure 6.
Hydrogen-rich saline (HRS) decreased serum cytokine levels. The levels of (A) TNF-α and (B) IL-6 in the serum in all groups were measured by enzyme-linked immunosorbent assays. All data were expressed as mean ±SD. * P<0.05 vs. PPA group.
Effect of HRS on peritoneal mRNA levels of pro-inflammatory cytokines
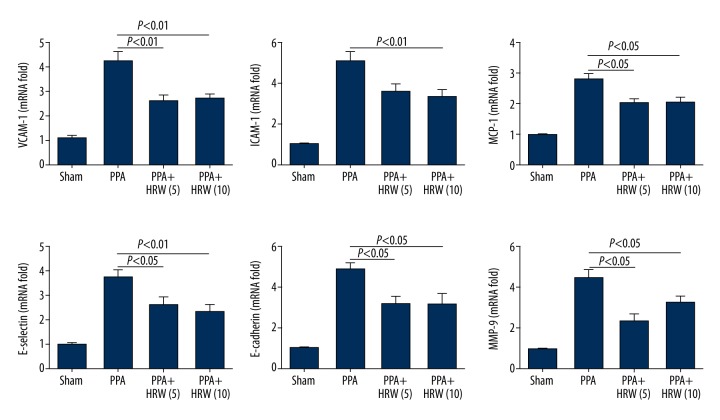
Peritoneal adhesion bands and adjacent tissues were removed from all mice on the 7th day after surgery and the effectiveness of HRS on the transcriptional levels of pro-inflammatory cytokines were evaluated by qRT-PCR. The mRNA of VCAM-1, ICAM-1, MCP-1, E-selectin, E-cadherin, and MMP-9 increased significantly in the peritoneal tissues in the PPA group, and HRS treatment effectively decreased their expressions. However, there was no difference between the PPA +HRS (5) group and PPA+HRS (10) group (Figure 7).
Figure 7.
Hydrogen-rich saline (HRS) decreased peritoneal mRNA levels of pro-inflammatory cytokines. Vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1(ICAM-1), monocyte chemotactic protein-1(MCP-1), E-selectin, E-cadherin, matrix metalloproteinase-9 (MMP-9) in peritoneal adhesion bands in sham, PPA, PPA+HRS (5), and PPA+HRS (10) groups were detected by quantitative reverse transcription-polymerase chain reaction (qRT–PCR). All data were expressed as mean ±SD.
Effect of HRS on IHC of TGF-β1
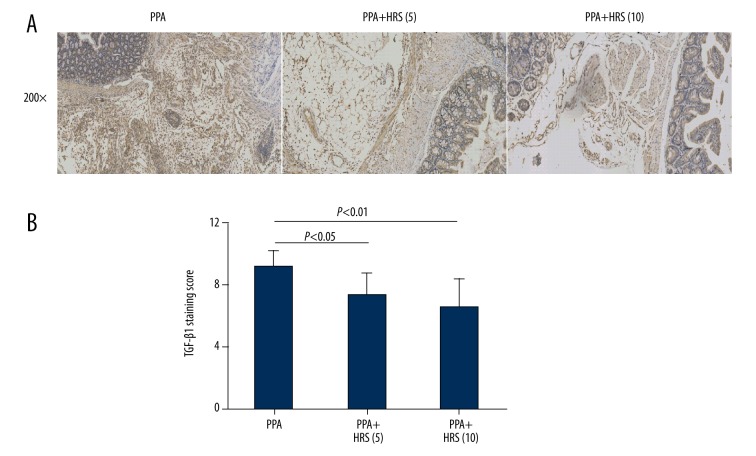
TGF-β1 is an important regulatory molecule in the PPAs formation and it is a classical marker of PPAs as well. Immunohistochemistry was performed to localize and semi-quantitate TGF-β1 expression in tissue sections from 7 days after PPAs formation in PPA, PPA+HRS (5), and PPA+HRS (10) groups (Figure 8). Peritoneum and intestinal sections from the PPA group showed a significant TGF-β1 positive staining in the intestinal sub-mesothelial connective tissues and peritoneum interstitial tissues when compared with PPA + HRS (5) and PPA+HRS (10) groups (Figure 8A). The semi-quantitation of TGF-β1 staining showed the same results (Figure 8B).
Figure 8.
Hydrogen-rich saline (HRS) decreased TGF-β1 expression in postsurgical peritoneal tissues. (A) Immunohistochemistry was performed to localize and semi-quantitate TGF-β1 expression. The intraperitoneal administration of HRS (5 and 10 ml/kg) significantly decreased TGF-β1 expression in the intestinal sub-mesothelial connective tissues and peritoneum interstitial tissues when compared with saline controls. (B) The results were confirmed by the semi-quantitation calculation. All data were expressed as mean ±SD.
Discussion
In this study, we found intraperitoneal injection of hydrogen-rich saline (HRS) could reduce postsurgical adhesion bands (PPAs) formation. This was demonstrated by comparing HRS with saline treatment groups using the Kennedy scoring method and pathological alteration in a mouse model. The mechanism of HRS which attenuated the abdominal adhesions might be mediated through: (1) attenuating the severity of inflammation; (2) reducing the expression of cytokines which promoted fibrin production; (3) separation of abdominal viscera and the peritoneal wall for its liquid property; (4) promotion of t-PA that increased the fibrinolysis; and (5) inhibition of TGF-β1 generation that facilitated fibrogenesis (Figure 9). No difference was found between treatment with HRS at 5 and 10 ml/kg. We concluded that treatment of HRS at 5 and 10 ml/kg had the same effects in decreasing the formation of PPAs. However, we suggested that 5 ml/kg was better for HRS infusion because of the lower liquid burden and this dose was also consistent with previous studies.
Figure 9.
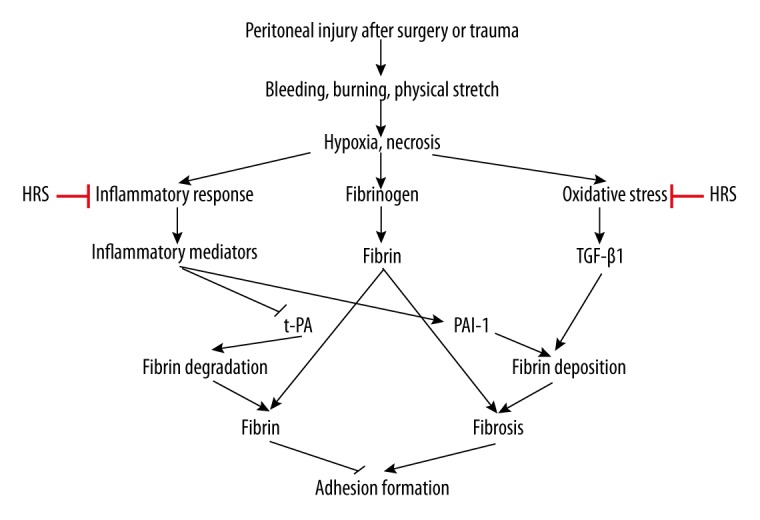
Schematic of hydrogen-rich saline (HRS) in the treatment of postoperative intra-abdominal adhesion bands formation. Bleeding, burning, and physical stretch were caused and induced by peritoneal injury after surgery or trauma. The pathophysiological processes, including hypoxia and necrosis, resulted in the peritoneal inflammation, oxidative stress, and fibrinogen, which all promoted fibrin formation. The balance between fibrin deposition and fibrin degradation was mainly regulated by the PAI-1 and t-PA and partly by TGF-β1. HRS had therapeutic potential for preventing postsurgical adhesion bands formation, mainly by inhibiting of inflammation and oxidative stress.
PPAs formation was a severe surgical complication which was ignored to some extent by surgeons. Although it was a common complication after laparotomy, the detailed mechanism responsible for PPAs formation remained unclear. Tissue injury, inflammation, and foreign material produced during the surgery could intervene in PPAs formation [32–34]. The formation of PPAs could be described as occurring in 4 phases: post-injury inflammatory phase; fibrin dissolution phase; fibrous phase; and remodeling and phagocytic phase [35]. For example, when the peritoneum was injured during the operation, blood and an increase of fluid rich in fibrinogen could be released from the impaired serosa [36,37]. Then, local inflammation occurs, which could recruit macrophages, leukocytes, and fibroblasts, leading to release of cytokines in this area [38]. If this local inflammation was not controlled, large-scale inflammation could develop in the whole peritoneal cavity. However, these processes might cause maturity and adhesion of fibers [18]. The impaired fibrinolytic-fibroblast imbalance caused by this pathological process might be associated with excess cellular growth, fibrosis, and deposition of collagen-rich extracellular matrix, which resulted in to pathological adhesion bands formation.
Oxidative stress was also important in PPAs formation [15]. The peritoneum was exposed and damaged by massive ROS and RNS after the operation, which could facilitate the formation of PPAs by breaking the fibrinolytic-fibroblast balance in the peritoneal cavity [39]. Previous studies indicated that antioxidants could reduce adhesion formation [29,40,41]. In tissues, MDA was a lipid peroxidation indicator and also acted as an indicator of tissue injury, and MPO was a classical marker of neutrophil infiltration and was also an oxidative stress marker. We demonstrated that MDA and MPO levels in tissues were highly increased in the PAA group but were decreased by using HRS. The mechanism of the decrease in MDA and MPO levels in the HRS group was through the powerful anti-oxidative effect of HRS, which could effectually inhibit the lipid peroxidation and neutrophil infiltration. The antioxidant effect of HRS had been widely studied [42]. In the previous studies, HRS had been proven to have tremendous capacity in decreasing the activities of tissue MDA and MPO [43,44].
We also found that the serum TNF-α and IL-6 levels were decreased during HRS infusion with the decrease in PPAs formation. The pro-inflammatory cytokines such as TNF-α and IL-6, which were mostly derived from activated macrophages, had immuno-suppressive ability after trauma. The inflammatory reaction and relevant cytokines were associated with the PPAs formation [13,45]. However, HRS could decrease the inflammation to reduce PPAs formation. We used qRT-PCR to detect the transcriptional level of cytokines in the peritoneal adhesion tissues, and the results showed that HRS could inhibit the transcription. In fact, HRS was found to suppress the inflammatory response in many diseases, including organic ischemia-reperfusion injury, sepsis, and drug-induced hepatotoxicity, mainly by decreasing the polymorphonuclear cells infiltration and pro-inflammatory cytokines production [46–50]. Inhibiting the reaction of inflammatory via drugs had been also widely studied to decrease PPAs formation [51–53]. Our study provided a new way to decrease PPAs formation based on the anti-inflammation effect of HRS.
Previous studies showed that t-PA, PAI-1, and TGF-β1 were the 3 major molecules which participated in the formation of PPAs. Elevated TGF-β1 and a breaking balance of t-PA and PAI-1 were the 2 key steps for the PPAs formation [54–56]. In our study, we also detected the effect of HRS on the peritoneal levels oft-PA, PAI-1, and TGF-β1. The results showed that HRS could reduce abdominal adhesions, possibly due to the decreased TGF-β1 in peritoneal fluid. HRS could also affect the peritoneal concentrations of t-PA and PAI-1. t-PA could convert plasminogen to plasmin and degrade fibrin. Moreover, PAI-1 was an important indicator of developing adhesions in tissues [57]. Thus, we suggested that HRS could promote fibrinolytic cascade in plasma and peritoneal tissues, and it also could balance the level of t-PA and PAI-1 and inhibit TGF-β1 expression. As a result, HRS could reduce PPAs formation.
Conclusions
The present study suggested that HRS administration could decrease PPAs formation after operations. The 5 ml/kg and 10 ml/kg doses of HRS had similar effects. This protective role possibly acted through reducing oxidative stress and inflammation and the balancing of TGF-β1, t-PA, and PAI-1. Further studies are needed to understand the detailed molecular pathways of HRS in reducing PPAs. Moreover, prospective clinical studies are also needed to evaluate its effects and safety.
Footnotes
Compliance with ethics guidelines
Zhong Liu, Sanfang Cheng, Changwei Gu, Honghong Pei, and Xin Hong declared that they had no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed.
Source of support: Departmental sources
References
- 1.Van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007;9(s2):25–34. doi: 10.1111/j.1463-1318.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 2.Liakakos T, Thomakos N, Fine PM, et al. Peritoneal adhesions: Etiology, pathophysiology, and clinical significance. Dig Surg. 2001;18(4):260–73. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 3.ten Broek RP, Bakkum EA, Laarhoven CJ, van Goor H. Epidemiology and prevention of postsurgical adhesions revisited. Ann Surg. 2016;263(1):12–19. doi: 10.1097/SLA.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 4.Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: A prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183(4):297–306. [PubMed] [Google Scholar]
- 5.Takeuchi H, Kitade M, Kikuchi I, et al. A novel instrument and technique for using seprafilm hyaluronic acid/carboxymethylcellulose membrane during laparoscopic myomectomy. J Laparoendosc Adv Surg Tech A. 2006;16(5):497–502. doi: 10.1089/lap.2006.16.497. [DOI] [PubMed] [Google Scholar]
- 6.Fenton BW, Fanning J. Laparoscopic application of hyaluronate/carboxymethylcellulose slurry: An adhesion barrier in a slurry formulation goes where the available sheets cannot. Am J Obstet Gynecol. 2008;199(3):325.e1. doi: 10.1016/j.ajog.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Lim R, Morrill JM, Lynch RC, et al. Practical limitations of bioresorbable membranes in the prevention of intra-abdominal adhesions. J Gastrointest Surg. 2009;13(1):35–42. doi: 10.1007/s11605-008-0724-3. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi S, Takayama T, Masuda H, et al. Bioresorbable membrane to reduce postoperative small bowel obstruction in patients with gastric cancer: A randomized clinical trial. Ann Surg. 2008;247(5):766–70. doi: 10.1097/SLA.0b013e3181656d4e. [DOI] [PubMed] [Google Scholar]
- 9.Van der Wal J, Iordens G, Vrijland W, et al. Adhesion prevention during laparotomy: Long-term follow-up of a randomized clinical trial. Ann Surg. 2011;253(6):1118–21. doi: 10.1097/SLA.0b013e318217e99c. [DOI] [PubMed] [Google Scholar]
- 10.diZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001;7(6):547–55. doi: 10.1093/humupd/7.6.547. [DOI] [PubMed] [Google Scholar]
- 11.Braun KM, Diamond MP. The biology of adhesion formation in the peritoneal cavity. Semin Pediatr Surg. 2014;23(6):336–43. doi: 10.1053/j.sempedsurg.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Isik A, Soyturk M, Süleyman S, et al. Correlation of bowel wall thickening seen using computerized tomography with colonoscopies: A preliminary study. Surg Laparosc Endosc Percutan Tech. 2017 doi: 10.1097/SLE.0000000000000389. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Isik A, Gursul C, Peker K, et al. Metalloproteinases and their inhibitors in patients with inguinal hernia. World J Surg. 2017;41(5):1259–66. doi: 10.1007/s00268-016-3858-6. [DOI] [PubMed] [Google Scholar]
- 14.Alpay Z, Saed GM, Diamond MP. Female infertility and free radicals: Potential role in adhesions and endometriosis. J Soc Gynecol Investig. 2006;13(6):390–98. doi: 10.1016/j.jsgi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Awonuga AO, Belotte J, Abuanzeh S, et al. Advances in the pathogenesis of adhesion development: The role of oxidative stress. Reprod Sci. 2014;21(7):823–36. doi: 10.1177/1933719114522550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attard JA, MacLean AR. Adhesive small bowel obstruction: Epidemiology, biology and prevention. Can J Surg. 2007;50(4):291–300. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Li X, Zhang Q, et al. Berberine hydrochloride prevents postsurgery intestinal adhesion and inflammation in rats. J Pharmacol Exp Ther. 2014;349(3):417–26. doi: 10.1124/jpet.114.212795. [DOI] [PubMed] [Google Scholar]
- 18.Cheong YC, Laird SM, Li TC, et al. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7(6):556–66. doi: 10.1093/humupd/7.6.556. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JY, Liu C, Zhou L, et al. A review of hydrogen as a new medical therapy. Hepatogastroenterology. 2012;59(116):1026–32. doi: 10.5754/hge11883. [DOI] [PubMed] [Google Scholar]
- 20.Ichihara M, Sobue S, Ito M, et al. Beneficial biological effects and the underlying mechanisms of molecular hydrogen – comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostojic SM. Molecular hydrogen: An inert gas turns clinically effective. 2015;47(4):301–4. doi: 10.3109/07853890.2015.1034765. [DOI] [PubMed] [Google Scholar]
- 22.Sato C, Kamijo Y, Yoshimura K, et al. Effects of hydrogen water on paraquat-induced pulmonary fibrosis in mice. Kitasato Med J. 2015;45(1):9–16. [Google Scholar]
- 23.Koyama Y, Taura K, Hatano E, et al. Effects of oral intake of hydrogen water on liver fibrogenesis in mice. Hepatol Res. 2014;44(6):663–77. doi: 10.1111/hepr.12165. [DOI] [PubMed] [Google Scholar]
- 24.Xu B, Zhang YB, Li ZZ, et al. Hydrogen-rich saline ameliorates renal injury induced by unilateral ureteral obstruction in rats. Int Immunopharmacol. 2013;17(2):447–52. doi: 10.1016/j.intimp.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Kang Z, Liu K, et al. Neuroprotective effects of hydrogen saline in neonatal hypoxia-ischemia rat model. Brain Res. 2009;1256:129–37. doi: 10.1016/j.brainres.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 26.Tian R, Hou Z, Hao S, et al. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 2016;1637:1–13. doi: 10.1016/j.brainres.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–94. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 28.Dinarvand P, Hassanian SM, Weiler H, Rezaie AR. Intraperitoneal administration of activated protein C prevents postsurgical adhesion band formation. Blood. 2015;125(8):1339–48. doi: 10.1182/blood-2014-10-609339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy R, Costain DJ, McAlister VC, Lee TD. Prevention of experimental postoperative peritoneal adhesions by N, O-carboxymethyl chitosan. Surgery. 1996;120(5):866–70. doi: 10.1016/s0039-6060(96)80096-1. [DOI] [PubMed] [Google Scholar]
- 30.Molinas CR, Mynbaev O, Pauwels A, et al. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76(3):560–67. doi: 10.1016/s0015-0282(01)01964-1. [DOI] [PubMed] [Google Scholar]
- 31.Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89(12):2637–45. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17(41):4545–53. doi: 10.3748/wjg.v17.i41.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monk BJ, Berman ML, Montz F. Adhesions after extensive gynecologic surgery: Clinical significance, etiology, and prevention. Am J Obstet Gynecol. 1994;170(5):1396–403. doi: 10.1016/s0002-9378(94)70170-9. [DOI] [PubMed] [Google Scholar]
- 34.Schnüriger B, Barmparas G, Branco BC, et al. Prevention of postoperative peritoneal adhesions: A review of the literature. Am J Surg. 2011;201(1):111–21. doi: 10.1016/j.amjsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Holmdahl L, Kotseos K, Bergström M, et al. Overproduction of transforming growth factor-β1 (TGF-β1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery. 2001;129(5):626–32. doi: 10.1067/msy.2001.113039. [DOI] [PubMed] [Google Scholar]
- 36.Reed KL, Fruin AB, Bishop-Bartolomei KK, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. J Surg Res. 2002;108(1):165–72. doi: 10.1006/jsre.2002.6533. [DOI] [PubMed] [Google Scholar]
- 37.Tulandi T. Introduction – prevention of adhesion formation: The journey continues. Hum Reprod Update. 2001;7(6):545–46. doi: 10.1093/humupd/7.6.545. [DOI] [PubMed] [Google Scholar]
- 38.Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165(11):1012–19. doi: 10.1080/110241599750007810. [DOI] [PubMed] [Google Scholar]
- 39.Gotloib L, Wajsbrot V, Cuperman Y, Shostak A. Acute oxidative stress induces peritoneal hyperpermeability, mesothelial loss, and fibrosis. J Lab Clin Med. 2004;143(1):31–40. doi: 10.1016/j.lab.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Heydrick SJ, Reed KL, Cohen PA, et al. Intraperitoneal administration of methylene blue attenuates oxidative stress, increases peritoneal fibrinolysis, and inhibits intraabdominal adhesion formation. J Surg Res. 2007;143(2):311–19. doi: 10.1016/j.jss.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Chu DI, Lim R, Heydrick S, et al. N-acetyl-l-cysteine decreases intra-abdominal adhesion formation through the upregulation of peritoneal fibrinolytic activity and antioxidant defenses. Surgery. 2011;149(6):801–12. doi: 10.1016/j.surg.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Ohta S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JY, Song SD, Pang Q, et al. Hydrogen-rich water protects against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol. 2015;21(14):4195–209. doi: 10.3748/wjg.v21.i14.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Wu Q, Song S, et al. Effect of hydrogen-rich water on acute peritonitis of rat models. Int Immunopharmaco. 2014;21(1):94–101. doi: 10.1016/j.intimp.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Sayar I, Bicer S, Gursul C, et al. Protective effects of ellagic acid and ozone on rat ovaries with an ischemia/reperfusion injury. J Obstet Gynaecol Res. 2016;42(1):52–58. doi: 10.1111/jog.12858. [DOI] [PubMed] [Google Scholar]
- 46.Sun Q, Kang Z, Cai J, et al. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med (Maywood) 2009;234(10):1212–19. doi: 10.3181/0812-RM-349. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda K, Asoh S, Ishikawa M, et al. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361(3):670–74. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 48.Wang F, Yu G, Liu SY, et al. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167(2):e339–44. doi: 10.1016/j.jss.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Wu Q, Zhang J, Wan Y, et al. Hydrogen water alleviates lung injury induced by one-lung ventilation. J Surg Res. 2015;199(2):664–70. doi: 10.1016/j.jss.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Zhu D. Molecular hydrogen therapy ameliorates organ damage induced by sepsis. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/5806057. 5806057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montz F, Monk BJ, Lacy SM, Fowler JM. Ketorolac tromethamine, a nonsteroidal anti-inflammatory drug: Ability to inhibit post-radical pelvic surgery adhesions in a porcine model. Gynecol Oncol. 1993;48(1):76–79. doi: 10.1006/gyno.1993.1012. [DOI] [PubMed] [Google Scholar]
- 52.Guvenal T, Cetin A, Ozdemir H, et al. Prevention of postoperative adhesion formation in rat uterine horn model by nimesulide: A selective COX-2 inhibitor. Hum Reprod. 2001;16(8):1732–35. doi: 10.1093/humrep/16.8.1732. [DOI] [PubMed] [Google Scholar]
- 53.Greene AK, Alwayn IP, Nose V, et al. Prevention of intra-abdominal adhesions using the antiangiogenic COX-2 inhibitor celecoxib. Ann Surg. 2005;242(1):140–46. doi: 10.1097/01.sla.0000167847.53159.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ersoy R, Celik A, Yilmaz O, et al. The effects of irbesartan and spironolactone in prevention of peritoneal fibrosis in rats. Perit Dial Int. 2007;27(4):424–31. [PubMed] [Google Scholar]
- 55.Ghellai AM, Stucchi AF, Chegini N, et al. Role of transforming growth factor beta-1 in peritonitis-induced adhesions. J Gastrointest Surg. 2000;4(3):316–23. doi: 10.1016/s1091-255x(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 56.Chegini N, Kotseos K, Zhao Y, et al. Differential expression of TGF-beta1 and TGF-beta3 in serosal tissues of human intraperitoneal organs and peritoneal adhesions. Hum Reprod. 2001;16(6):1291–300. doi: 10.1093/humrep/16.6.1291. [DOI] [PubMed] [Google Scholar]
- 57.Falk K, Björquist P, Strömqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg. 2001;88(2):286–89. doi: 10.1046/j.1365-2168.2001.01647.x. [DOI] [PubMed] [Google Scholar]