ABSTRACT
Epidemiological observations have linked increased host iron with malaria susceptibility, and perturbed iron handling has been hypothesized to contribute to the potentially life-threatening anemia that may accompany blood-stage malaria infection. To improve our understanding of these relationships, we examined the pathways involved in regulation of the master controller of iron metabolism, the hormone hepcidin, in malaria infection. We show that hepcidin upregulation in Plasmodium berghei murine malaria infection was accompanied by changes in expression of bone morphogenetic protein (BMP)/sons of mothers against decapentaplegic (SMAD) pathway target genes, a key pathway involved in hepcidin regulation. We therefore investigated known agonists of the BMP/SMAD pathway and found that Bmp gene expression was not increased in infection. In contrast, activin B, which can signal through the BMP/SMAD pathway and has been associated with increased hepcidin during inflammation, was upregulated in the livers of Plasmodium berghei-infected mice; hepatic activin B was also upregulated at peak parasitemia during infection with Plasmodium chabaudi. Concentrations of the closely related protein activin A increased in parallel with hepcidin in serum from malaria-naive volunteers infected in controlled human malaria infection (CHMI) clinical trials. However, antibody-mediated neutralization of activin activity during murine malaria infection did not affect hepcidin expression, suggesting that these proteins do not stimulate hepcidin upregulation directly. In conclusion, we present evidence that the BMP/SMAD signaling pathway is perturbed in malaria infection but that activins, although raised in malaria infection, may not have a critical role in hepcidin upregulation in this setting.
KEYWORDS: hepcidin, iron, malaria, innate immunity
INTRODUCTION
Malaria is one of the world's deadliest and most geographically widespread human infectious diseases, causing hundreds of thousands of deaths per year (1). Malaria infections contribute significantly to the worldwide burden of anemia (2), and measures taken to decrease malaria at the population level frequently decrease anemia prevalence (3).
The mechanisms involved in the pathogenesis of malarial anemia include increased clearance of infected and uninfected red blood cells (iRBC and uRBC, respectively), dyserythropoiesis as a consequence of cytokine upregulation, and inadequate absorption and utilization of iron, for which the iron-regulatory hormone hepcidin has been implicated (reviewed in references 4–7). Hepcidin controls iron stores and localization by causing internalization and degradation of the iron export protein ferroportin (8), which mediates iron release into the circulation from erythrophagocytic macrophages and across the basolateral membrane of enterocytes (9–11). Raised hepcidin therefore inhibits both recycling of red cell iron through macrophages and iron absorption from the diet.
Hepcidin is upregulated during many bacterial, fungal, and viral infections (12–15) and also during symptomatic and asymptomatic natural human malaria infections (16–18), in volunteers undergoing controlled human malaria infection (CHMI) in clinical trials (19), and in malaria-infected mice (20, 21). Raised hepcidin during asymptomatic malaria infection is associated with poor iron absorption (22), and, in children with postmalarial anemia, with diminished erythrocyte incorporation of orally administered iron (23). Hepcidin renders mice infected with blood-stage malaria resistant to secondary sporozoite infection by decreasing the amount of iron in hepatocytes (20), and hepcidin-mediated macrophage iron sequestration has been proposed as a mechanism contributing to the increased growth of macrophage-tropic bacteria in malaria-infected hosts (24).
Hepcidin levels increase homeostatically under high-iron conditions (7) and in response to inflammation and infection (25) via the BMP/SMAD and interleukin-6 (IL-6)/STAT3 pathways, respectively. Appropriate regulation of hepcidin levels in response to fluctuations in iron is complex and requires many proteins, including Bmp6, HJV, Bmp type I and type II receptors, Hfe, and TfR2. These molecules combine to sense iron and to modulate transcription of hepcidin via the BMP/SMAD signaling pathway (26). In contrast, during anemia and under conditions of erythroid demand, hepcidin suppression occurs to facilitate iron release to plasma for erythropoiesis. A recently identified bone marrow-derived erythropoietin-induced hormone named erythroferrone likely plays a key role in hepcidin suppression in this context (27), which also appears to act via BMP/SMAD signaling (28).
The SMAD pathway also may be involved in the hepcidin response to inflammation, since the transforming growth factor β (TGFβ) superfamily member, activin B, is upregulated by inflammation in mice, associating it with hepcidin upregulation, independently of the IL-6 pathway (29). Activin B, a homodimer of two inhibin βB subunits, is known to be stimulated by inflammatory and infectious stimuli (30) and to contribute to hepcidin upregulation in vitro (29). However, it is unclear whether activin B is an essential component of the hepcidin response to inflammatory stimuli. One study noted that hepcidin upregulation in response to LPS was preserved in activin B knockout mice (Inhbb−/−) (31), suggesting that activin B is not required for hepcidin upregulation in this context, while a second noted that the activin binding protein follistatin blunted the hepcidin increase to lipopolysaccharide (LPS) in a murine model (32). Additionally, the roles of related proteins activin A (a homodimer of two inhibin βA subunits) and activin AB (a heterodimer of inhibin βB and inhibin βA subunits) are less defined with regard to hepcidin upregulation, with some studies (33) showing that activin A upregulates hepcidin but others demonstrating no effect of activin A or activin AB (32, 34).
Here, we study molecular regulation of hepcidin expression in the context of murine malaria infections and CHMI studies. We find evidence that the BMP/SMAD pathway is involved in hepcidin upregulation but that although activin B and activin A are increased in malaria, these molecules are unlikely to play a major role in controlling hepcidin expression.
RESULTS
Hepcidin upregulation during murine Plasmodium berghei infection is associated with increased BMP/SMAD pathway activity.
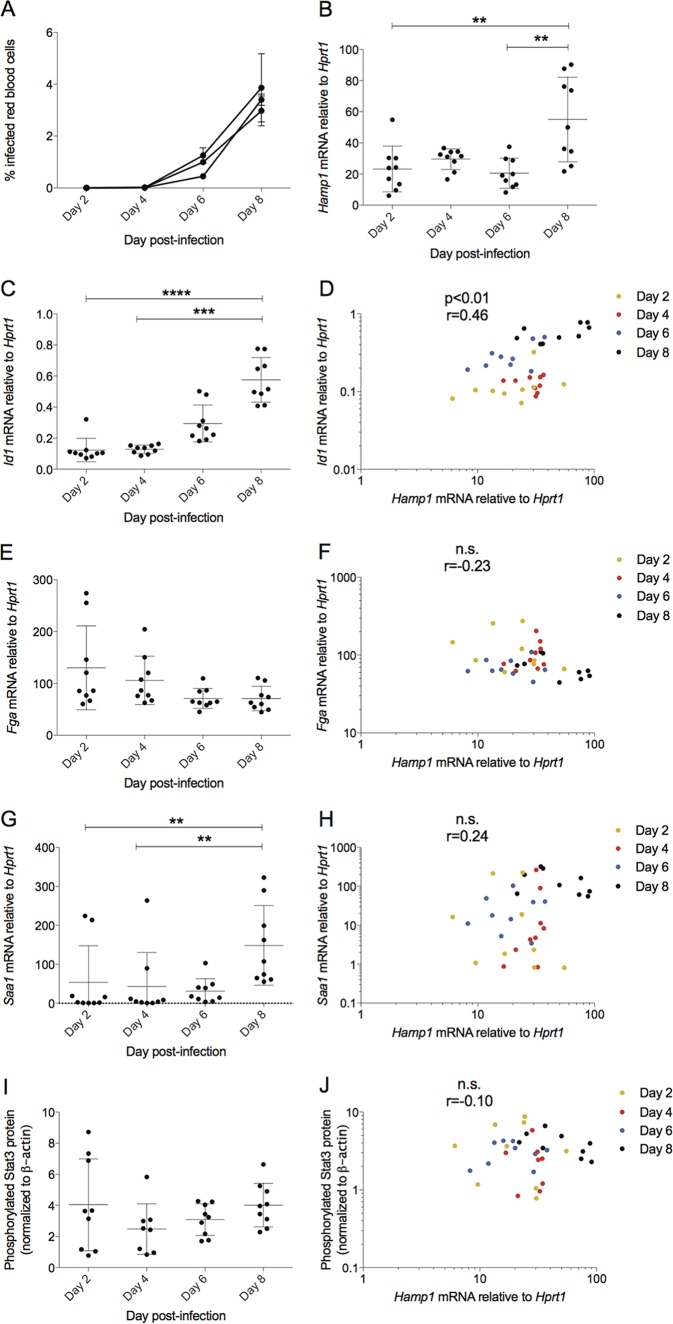
We infected male BALB/c mice with 103 P. berghei ANKA sporozoites and harvested tissues from infected and control mice 2, 4, 6, or 8 days postinfection. Blood-stage parasitemia increased to 2 to 4% by 8 days postinfection (Fig. 1A). Hepatic hepcidin (Hamp1) mRNA expression was significantly increased on day 8 postinfection relative to day 2 (undetectable parasitemia) (Fig. 1B), consistent with previous studies showing elevated hepcidin mRNA only when parasitemia rises above a certain threshold (20, 21).
FIG 1.
Hepcidin expression in Plasmodium berghei-infected BALB/c mice correlates with BMP pathway indicator gene Id1, not inflammatory pathway indicators. Mice were infected with P. berghei ANKA sporozoites, and groups were sacrificed at 2-day intervals postinfection. Data in all graphs are combined from 3 independent experiments (n = 3 mice/day/experiment, n = 9 total). (A) Mouse parasitemia as percent infected red blood cells, monitored by thin smear. (B) Hamp1 mRNA in the liver increases on day 8 postinfection relative to day 2 (no parasitemia). BMP-responsive gene Id1 increases on day 8 postinfection (C) and correlates significantly with hepcidin message (D). Acute-phase gene Fga message does not increase on day 8 postinfection (E) and does not correlate with hepcidin (F). Acute-phase gene Saa-1 increases significantly on day 8 postinfection (G) but does not correlate with hepcidin (H). STAT3 phosphorylation does not increase significantly on day 8 postinfection (I) and does not correlate with hepcidin (J). All genes are shown as normalized to endogenous control gene Hprt1. Statistical analyses in dot plots are Dunn's multiple-comparison tests after Kruskal-Wallis test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. In all correlation graphs, each symbol denotes a single mouse, and the color of the symbol indicates the day of sacrifice. All correlations are from Spearman's correlation tests. P values and r values are stated. ns, P > 0.05.
We then examined whether hepcidin expression was associated with the expression of genes indicative of activity of two well-characterized hepcidin regulatory pathways: the BMP/SMAD and IL-6/STAT3 pathways. We quantified hepatic expression of the BMP-responsive gene, inhibitor of DNA-binding 1 (Id1) (35), and the IL-6/STAT3-responsive acute-phase genes fibrinogen alpha (Fga) (12) and serum amyloid alpha-1 (Saa-1). Id1 was significantly upregulated on day 8 postinfection relative to days 2 and 4 (Fig. 1C) and associated positively with Hamp1 expression (Fig. 1D). This association remained significant when considering only the 9 mice from day 8 in the analysis (P = 0.05, r = 0.68) (Fig. 1D, black symbols). We also analyzed hepatic expression of three other BMP target genes, Atoh8, Smad6, and Smad7. Atoh8 expression correlated with Hamp1 expression overall (see Fig. S1 in the supplemental material) and when analyses were limited to day 8 (P < 0.01, r = 0.78); gene expression of Smad6 and Smad7 on day 8 also correlated with Hamp1 (P < 0.01 for both, r = 0.73 and 0.75, respectively), although this correlation was not significant when including the earlier time points with lower parasitemia (Fig. S1).
Conversely, Fga expression was not increased on day 8 postinfection (Fig. 1E) and did not correlate with hepcidin (Fig. 1F). Saa-1 increased on day 8 postinfection relative to days 2 and 4 (Fig. 1G) but also was not significantly correlated with hepcidin (Fig. 1H). We used quantitative Western blot detection to measure phosphorylated STAT3 (pSTAT3) directly: pSTAT3 was not significantly upregulated at day 8 postinfection relative to any time points (Fig. 1I, blots from representative experiment shown in Fig. S2) and did not correlate with hepcidin (Fig. 1J). When limiting analysis to day 8 samples (black symbols in all correlation graphs), there still was no significant association between Hamp1 and Fga, Hamp1 and Saa-1, or Hamp1 and pSTAT3. These data suggest that in this blood-stage malaria model, increased BMP signaling parallels, and so may contribute to, Hamp1 upregulation.
Expression of activin B, not Bmp genes, increases in P. berghei infection.
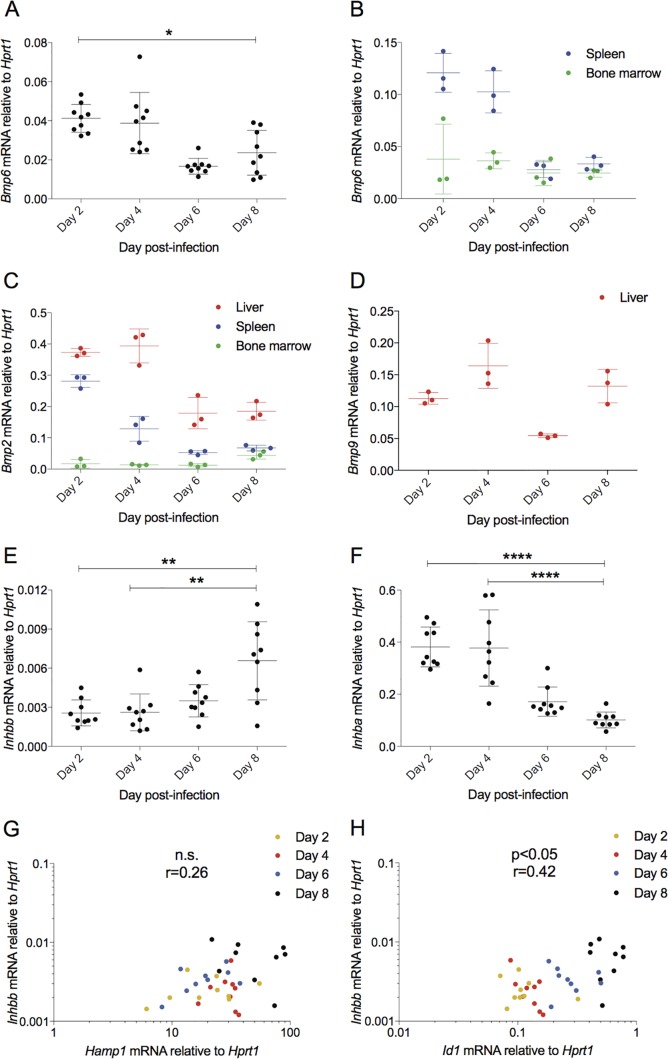
Bmp6 knockout mice exhibit severe iron overload (36), and blocking Bmp6 in vivo also decreases hepcidin and increases serum iron (37). However, we found that hepatic Bmp6 mRNA was downregulated as parasitemia increased (Fig. 2A). Other Bmp proteins are capable of stimulating hepcidin transcription in vitro, and Bmp2 has recently been shown to be a key hepatic regulator of hepcidin expression in vivo (38–41). We therefore examined whether Bmp genes were upregulated in the liver, bone marrow, and spleen samples. No significant increases in Bmp6 (Fig. 2B) or Bmp2 (Fig. 2C) mRNA were observed in any tissue on day 8 postinfection. Bmp9 mRNA was undetectable in bone marrow and spleen and showed no increase on day 8 in the liver (Fig. 2D). Therefore, hepcidin and Id1 upregulation during malaria infection was not accompanied by changes in expression of Bmp genes.
FIG 2.
Bmp gene expression is not increased, but activin B (Inhbb) gene expression increases with hepcidin gene expression in Plasmodium berghei-infected BALB/c mice. (A) Liver Bmp6 mRNA expression decreases on day 8 of infection (n = 3 mice per day per experiment, n = 9 total). (B to D) A representative experiment (n = 3 mice per day) was examined further to see if other Bmp genes increase in other candidate tissues. (B) Bmp6 mRNA did not show any trend toward upregulation in spleen or bone marrow. (C) Bmp2 did not increase in liver, spleen, or bone marrow. (D) Bmp9 mRNA was undetectable in spleen and bone marrow and did not increase in liver. Hepatic activin B message (Inhbb) is increased on day 8 postinfection (E), while hepatic activin A mRNA (Inhba) is decreased (F). Inhbb expression shows a trend toward correlation with Hamp1 (G) and correlates with Id1 (H). Statistical analyses in dot plots are Dunn's multiple-comparison tests after Kruskal-Wallis test. *, P < 0.05; **, P < 0.01; ****, P < 0.0001. All correlations are from Spearman's correlation tests.
Recent reports have provided evidence that increased hepatic activin B expression during inflammation contributes to hepcidin induction, involving the BMP/SMAD pathway (29, 32). Activin A, closely related to activin B, is increased in animal and human sera following similar stimuli (30, 42). We found that hepatic activin B mRNA (Inhbb) was increased significantly on day 8 postinfection relative to days 2 and 4 (Fig. 2E), although activin A mRNA (Inhba) was significantly decreased (Fig. 2F). Activin B expression showed high intermouse variability but correlated significantly with the BMP response gene Id1 (Fig. 2H), although it did not show significant correlation with Hamp1 (Fig. 2G). Based on these results, we hypothesized that activin B contributes to hepcidin upregulation in murine blood-stage malaria infection.
Activin B expression also increases at peak parasitemia during Plasmodium chabaudi infection.
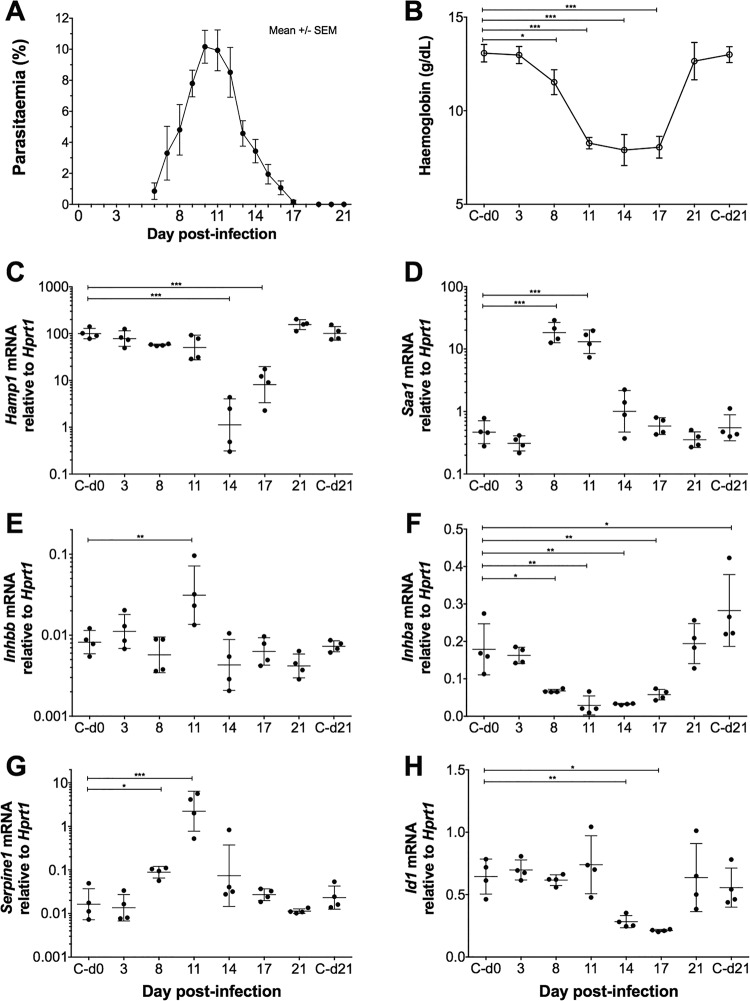
To investigate whether these data were more broadly applicable, we examined C57BL/6 mice infected with a second malaria parasite species, Plasmodium chabaudi chabaudi AS (PccAS), widely used as a self-resolving model of severe malarial anemia (43). In this experiment, C57BL/6 mice were injected intravenously with 105 PccAS-infected erythrocytes, and groups of mice were culled at intervals following injection. Infected mice developed parasitemias that peaked around day 11 and then resolved (Fig. 3A). Severe anemia, demonstrated by marked reductions in hemoglobin concentration as parasitemia developed, reached a nadir concurrently with peak parasitemia and persisted for a further week before returning to normal (Fig. 3B). Regulation of hepcidin in this context is likely complicated due to conflicting signals during the concurrent inflammation and anemia, which enhance and suppress hepcidin expression, respectively. We did not observe increased liver Hamp1 mRNA expression in the earlier phases of infection (Fig. 3C) despite evidence of inflammation (Fig. 3D, increased Saa1), likely because of signals that suppress hepcidin arising as anemia develops (for example, erythroferrone); indeed, as anemia becomes more severe, Hamp1 expression is strongly decreased. Changes in activin gene expression similar to those observed in P. berghei infection were observed during escalation of this parasitemia: there was a significant upregulation of liver Inhbb mRNA expression at peak parasitemia (Fig. 3E), with a concomitant smaller decrease in liver Inhba expression (Fig. 3F). Consistent with activin signaling, there was increased expression of Serpine1 (known to be induced by activin signaling) (Fig. 3G) as the parasitemia escalated to its peak. Paralleling Hamp1 expression, hepatic Id1 was not upregulated in early infection but was decreased as anemia became more severe (Fig. 3H), consistent with erythroid-mediated suppression of hepcidin (as a result of anemia) requiring a decrease in Smad signaling (28).
FIG 3.
Changes in activin gene expression during the course of Plasmodium chabaudi infection of C57BL/6 mice. Groups of mice infected with PccAS were culled at intervals following infection (n = 4/group). (A) Mouse parasitemia as percent infected red blood cells (means ± standard errors of the means [SEM]), monitored by thin smear (films were made from each mouse each day until they were culled, and data are plotted from day 6). (B) Hemoglobin concentrations during PccAS infection (data are missing from one mouse on day 11 due to sample clotting); C-d0 and C-d21 represent data from uninfected control mice culled on day 0 and day 21. (C to G) Hepatic gene expression analysis by qRT-PCR, plotting expression relative to the endogenous control gene, Hprt1, yielding the following results: no detectable increase in Hamp1 mRNA expression during parasitemia development but marked suppression during severe PccAS-associated anemia (C); upregulation of the acute-phase response gene Saa1 as parasitemia increases (D); increase in Inhbb mRNA expression on day 11 postinfection (E); downregulation of Inhba around peak parasitemia (F); upregulation of activin-responsive gene Serpine1 around peak parasitemia (G); BMP signaling indicator gene Id1 expression decreases at peak parasitemia (H). Statistical analyses were by one-way ANOVA with Dunnett's multiple-comparison test (relative to controls on day 0), and posttests were used. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Plots depict means ± standard deviations, except where y axes are plotted on log scales, in which case statistical analyses are performed on log-transformed data and plots show geometric means ± geometric standard deviations.
Hepcidin and activin A peptide are upregulated in human volunteers experimentally infected with malaria.
Given these observed changes in activin expression in murine malaria infection, we sought to assess the applicability of our findings to human infection. We measured serum concentrations of hepcidin, activin A, C-reactive protein (CRP), and ferritin, besides transferrin saturation, in control subjects (n = 18) from three CHMI trials with P. falciparum. At the time of experimentation, we were unable to obtain serum assays for activin B. Although in the murine model hepatic activin A mRNA expression was suppressed at day 8, previous studies have suggested that hepatic activin A mRNA does not associate with serum activin A concentrations (30, 42).
Parasitemia data for each trial are shown Fig. S3A to C. Between trials, there were no differences in times from challenge (C) to day of diagnosis (DoD), from C to the first quantifiable quantitative PCR (qPCR) measurement postinfection (termed PCR patency), or in parasitemia measured by qPCR at DoD (Fig. S3D to F). In subsequent analyses, we therefore combined data from the three trials to increase power.
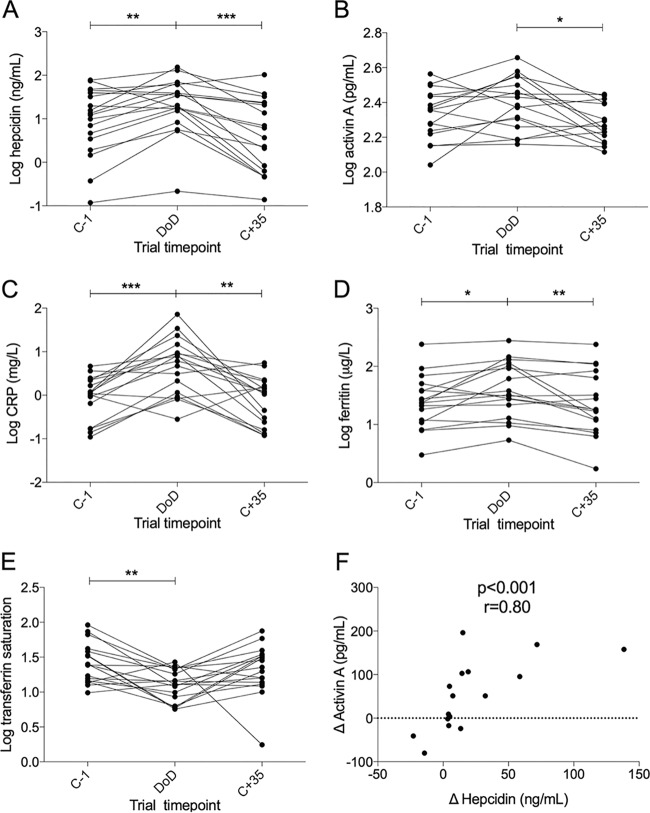
Serum samples were taken 1 day prior to challenge (C−1), at DoD, and 35 days postinfection, when the infection had resolved (C+35). Hepcidin concentrations were significantly increased at DoD compared to those at both C−1 and C+35 (Fig. 4A). Activin A showed a trend toward an increase on DoD over C−1 and was significantly lower at the resolution of infection at C+35 (Fig. 4B). CRP (Fig. 4C) and ferritin (Fig. 4D) were elevated at DoD. Accordingly, transferrin saturation was decreased at DoD compared to that at C−1, indicative of inflammatory hypoferremia (Fig. 4E).
FIG 4.
Changes in serum hepcidin, activin A C-reactive protein (CRP), ferritin, and transferrin saturation during CHMI trials. (A) Serum hepcidin increased on DoD compared to that at either the C−1 or C+35 time point. Hepcidin was measured in n = 18 volunteers. (B) Activin A was significantly upregulated at DoD versus the level at C+35. CRP (C) and ferritin (D) increased at DoD over other time points. (E) Transferrin saturation was reduced on DoD compared to that at C−1 only. (F) Δ serum hepcidin and Δ activin A were significantly correlated (Spearman's correlation test); each symbol denotes a single individual. Δ protein increases during infection were calculated by the value at DoD minus the mean of values at C−1 and C+35. Data were missing for iron and activin A measurements due to insufficient sample volume available (n = 17 in panels C, D, and E and n = 16 in panels B and F). (A to E) All data are log transformed, and statistical analyses are on log-transformed data. Statistical comparisons in before-after dot plots are Dunnett's multiple-comparison tests after ANOVA test. Spearman correlation is shown in panel F, and data are not log transformed. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
A strong correlation was evident between Δ hepcidin and Δ activin A protein in infection (where Δ is the value at DoD minus the average of values at C−1 and C+35). Those volunteers who demonstrated the most pronounced hepcidin increases at DoD also showed the greatest activin A increases (Fig. 4F). Full data for each volunteer are shown in Fig. S4.
In two published studies that compared activin A tissue mRNA levels with circulating protein, one tested only liver and the other examined multiple tissues, including spleen and bone marrow; neither found significant correlations (30, 42). Further work has suggested that stored activin A protein can be produced and released into the circulation by hematopoietic cells, and activin A can also be produced de novo from circulating white blood cells (44, 45). We previously demonstrated that hepcidin mRNA was upregulated in peripheral blood mononuclear cells (PBMC) from healthy malaria-naive donors cocultured with P. falciparum iRBC (46). We quantified activin A mRNA (INHBA) on samples from four donors from this study and found that both hepcidin (HAMP) and activin A mRNA were significantly upregulated in PBMC cocultured with iRBC but not uRBC (Fig. S5A and B). Thus, serum activin A induction during malaria infection may at least in part be produced by PBMC exposed to iRBC.
Activin proteins upregulate hepcidin.
Previous studies have demonstrated hepcidin induction by recombinant activin B protein in primary hepatocytes or hepatoma cell lines (29, 32). Likewise, we found that activin B and, to a lesser extent, activin A, induced HAMP mRNA in HepG2 hepatoma cells at 4 h posttreatment (Fig. S6A) but did not significantly induce ID1 mRNA (Fig. S6B). Hepcidin induction by both activin proteins was most pronounced between 1 and 8 h posttreatment (Fig. S6C and D). Pretreatment of HepG2 cells with the BMP type 1 receptor inhibitor molecule LDN-193189 (LDN) (47) prior to activin administration reduced the basal expression of both HAMP (Fig. S6E) and ID1 (Fig. S6F) but did not decrease the proportional increase in response to activin treatment. These data are consistent with recent reports suggesting that activin A/B-mediated hepcidin upregulation proteins occur through the LDN-sensitive BMP type 1 receptor Alk2 or Alk3 (29, 32), but that this pathway is resistant to LDN inhibition as a result of Alk2 complex formation with ActRIIA (34).
Anti-activin A/B antibodies do not decrease hepcidin expression in a malaria-infected mouse model.
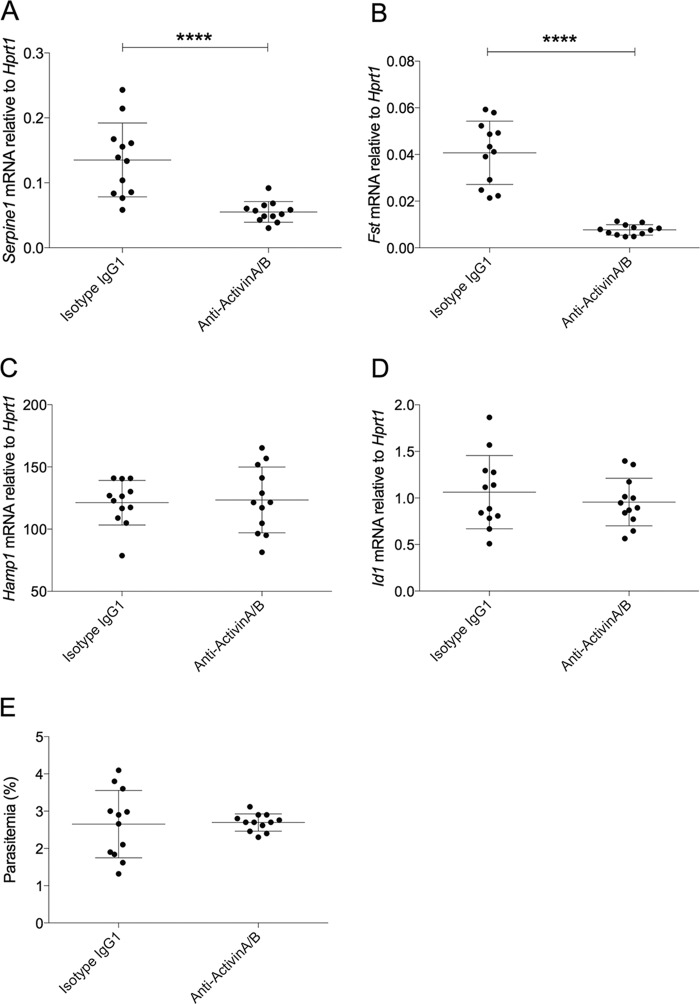
We finally investigated whether inhibiting activin activity affected hepcidin expression during murine blood-stage P. berghei infection. Sporozoite-infected mice were treated with anti-activin A/B or isotype control antibodies on days 6 and 7 postinfection (30 and 6 h prior to sacrifice on day 8, respectively). Expression of Serpine1 (encoding plasminogen activator inhibitor 1 [PAI-1]), which is responsive to activins (48) and the activin-regulatory gene encoding follistatin (Fst), were suppressed in mice treated with anti-activin antibodies, indicative of effective inhibition of liver activin signaling (Fig. 5A and B). However, Hamp1 (Fig. 5C) and Id1 expression (Fig. 5D) were not significantly different between malaria-infected mice treated with anti-activin A/B and isotype control antibodies. There was no difference in parasitemia between the two groups (Fig. 5E).
FIG 5.
Hepcidin expression in murine P. berghei infection was not decreased by administration of anti-activin A and anti-activin B antibodies. Mice were infected with P. berghei ANKA sporozoites as described in the text and sacrificed at day 8 postinfection. Mice were injected i.p. twice with a cocktail of anti-activin A and anti-activin B antibodies (containing 100 μg of each, in 400 μl) at 6 and 30 h prior to sacrifice; controls were given isotype IgG control (200 μg, in 400 μl) only. Data in all graphs are combined from 2 independent experiments (n = 6 mice per treatment per experiment, n = 12 total). Gene expression is shown relative to housekeeping gene Hprt1. mRNA of activin-responsive genes Serpine1 (A) and Fst (follistatin) (B) were significantly decreased in anti-activin treated mice, indicating antibody efficacy. Hamp1 (C) and Id1 (D) mRNA were not significantly altered between infected mice treated with anti-activin A/B and isotype IgG control. (E) Parasitemia on day 8 (day of sacrifice) was unchanged between groups. Statistical analyses are Mann-Whitney tests. ****, P < 0.0001.
DISCUSSION
Iron is required for Plasmodium growth, and host iron-handling proteins can influence outcome of infection; for example, lipocalin-2, which can sequester iron, controls the severity of Plasmodium yoelii infection in mice (49). Here, we focused on regulation of the iron-regulatory hormone hepcidin during malaria. Hepcidin is raised in uncomplicated malaria infection in mice and humans (18–20). Hepcidin redistributes iron into the macrophage compartment and away from the serum and so may contribute to dyserythropoiesis and the development of malarial anemia. In established severe malarial anemia, hepcidin then is suppressed (50) through a pathway that likely involves the erythroid progenitor-derived factor erythroferrone (51). The initial hepcidin upregulation blocks oral iron absorption during and after infection (22, 23) and plays a role in determining host susceptibility to malaria superinfection (20) and, possibly, coinfections with other pathogens (24). Understanding the mechanisms of hepcidin induction during uncomplicated malaria therefore is important.
IL-6 upregulates hepcidin and this cytokine increases in malaria infection, and in some studies, IL-6 has been correlated with hepcidin in human serum (50, 52, 53); however, in one study of infected children, urinary IL-6 and hepcidin were not associated (16). Murine studies have also shown different outcomes: one study found a close correlation between pSTAT3 levels and Hamp1 (21), but another noted only minor IL-6 upregulation, without pSTAT3 increase, in infected mice (51). Hepcidin induction in primary hepatocytes treated with serum from infected mice was shown to be abrogated by the BMP pathway inhibitor dorsomorphin, while IL-6 neutralizing antibodies were less effective (20). Finally, PBMC, when cocultured with P. falciparum-infected red blood cells, upregulated hepcidin expression without appreciable IL-6 increases (46). The role of IL-6 in hepcidin upregulation in malaria infection is unclear.
During blood-stage P. berghei ANKA malaria infection in mice, we found that hepcidin expression correlated most closely with hepatic expression of the BMP/SMAD response genes Id1 and Atoh8 and less so with IL-6/STAT3 pathway response gene Fga or Saa-1 or STAT3 phosphorylation. These findings indicate that hepcidin upregulation in this model co-occurs with BMP/SMAD pathway signaling. The expression of Bmp genes in infected mice was unchanged or moderately downregulated as parasitemia increased. Of relevance, previous work demonstrated that Bmp gene expression was not elevated in mice injected with LPS despite evidence of BMP/SMAD pathway activity occurring concurrently with Hamp1 elevation (29). Hepatic activin B mRNA (Inhbb) was significantly raised post-LPS injection, suggesting that activins play a role in hepcidin upregulation through the BMP/SMAD pathway in inflammatory contexts (29, 30, 34). A previous study noted a marginal increase in hepatic message levels of activin B, and a decrease in Bmp6, in a murine model of malaria infection (51).
Activin proteins were initially discovered as reproductive factors, but increasing evidence has demonstrated a role in the acute host response to infectious and inflammatory stimuli, as well as shaping the subsequent immune response (54). Activin A increases in serum of septic human patients (55, 56) and in animals following LPS injection (30, 34, 42, 57). Notably, although activin A protein levels increase in sera post-LPS injection, hepatic expression of Inhba mRNA decreases (30, 42). This disconnect between activin A serum protein and liver expression have led researchers to hypothesize that activin A is at least partially regulated at the posttranscriptional level and/or produced by tissues other than the liver, such as bone marrow-derived cells (42, 58), peripheral blood monocytes (45), or dendritic cells (44). Activin B previously has been thought to be functionally similar to activin A, possibly with slightly weaker effects (59–61), although studies of knockout mice have identified some differences in the roles of the two proteins (62–64). The effect of activin A and activin B on hepcidin regulation has been debated (32–34). In our in vitro system, we found some effects of activin A on hepcidin upregulation, although it was less potent than activin B.
We found increased hepatic Inhbb expression in P. berghei-infected mice, occurring concurrently with hepcidin and Id1 expression increase. Hepatic activin A (Inhba) mRNA was decreased (as described in reference 29) as Hamp1, Inhbb, and parasitemia increased. Similarly, increased hepatic Inhbb expression and decreased Inhba expression were observed during the peak parasitemia of Plasmodium chabaudi infection of C57BL/6 mice, together with upregulation of Serpine1 (consistent with activin signaling).
Importantly, an increase in Inhbb expression does not formally indicate an increase in activin B protein, as activin βB subunits can also combine with activin βA subunits to form activin AB protein. However, given the message decrease in Inhba in the liver, decreasing the local concentration of activin βA subunits, it is likely that the Inhbb increase we observed did result in an increase in circulating activin B and not activin AB.
An enzyme-linked immunosorbent assay (ELISA) for human activin B protein has been reported (65) but was not available at the time of experimentation. However, given the known independence of activin A liver mRNA and serum protein levels in animal models (30, 42), a possible role of activin A in hepcidin upregulation, and several studies that indicate that serum levels of activin A and B are coupregulated in different inflammatory and infectious states (66–68), we next chose to extend our studies by quantifying serum activin A and hepcidin concentrations from humans infected with P. falciparum as part of CHMI clinical trials. We found that hepcidin was increased during untreated blood-stage parasite infection. A previous CHMI study also reported increased hepcidin (19), although importantly this prior study only noted hepcidin increases subsequent to the initiation of antimalarial treatment, which likely induces transient inflammation due to release of parasite-derived material into the bloodstream. The increase in hepcidin we observed was accompanied by increases in acute-phase markers, decreased transferrin saturation, and increased serum activin A. Moreover, changes in activin A and hepcidin during infection were correlated, with the volunteers who exhibited the greatest hepcidin induction also demonstrating the most marked increases in activin A concentration. To our knowledge, this is the first report of activin A induction in the context of malaria infection and also the first to directly compare hepcidin and activin A levels in human serum. The moderate response we observed in volunteers infected in CHMI trials may be more pronounced in naturally infected individuals in the field, in whom parasitemia levels can greatly exceed the low parasitemia that is allowed in CHMI. We also showed that human PBMC, when cocultured with iRBC, upregulated activin A mRNA, providing one plausible physiological source for increased activin A serum levels during infection.
Finally, we treated P. berghei-infected mice with a combination of anti-activin A and anti-activin B antibodies to test whether activin neutralization could abrogate the hepcidin response. The antibody treatment was efficacious in blocking activin signaling, as shown by significant decreases in activin-regulated genes Serpine1 and Fst, but there was no observed change in hepcidin or Id1 expression. These data suggest activins are not required for hepcidin upregulation in this murine model of Plasmodium infection, although they do not rule out that activins could play a role in hepcidin regulation in malaria in humans. Our findings are consistent with a recent study demonstrating that Inhbb−/− mice continued to display hepcidin upregulation in response to LPS challenge (31) but contrast with another study that demonstrated abrogation of hepcidin upregulation after LPS challenge by use of the activin-binding protein follistatin (32).
In conclusion, we provide evidence that hepcidin upregulation in uncomplicated blood-stage Plasmodium infection is correlated with BMP/SMAD pathway activity in the liver. However, despite showing perturbations to expression of activins A and B in mouse and human malaria and confirming that activins induce hepcidin expression, neutralization of activins during murine malaria infection did not affect hepcidin. This suggests other factors are responsible for hepcidin expression in this context.
MATERIALS AND METHODS
Plasmodium berghei sporozoite infections of BALB/c mice.
Six- to 8-week-old male BALB/c mice (Harlan, United Kingdom) were housed under specific-pathogen-free conditions with ad libitum access to standard chow (2018SX; Fe2+ content of ∼200 ppm; Harlan-Teklad). Mice were infected intravenously (i.v.; via tail vein) with 103 Plasmodium berghei ANKA sporozoites (obtained from Anopheles stephensi mosquito salivary glands 21 days after feeding on blood containing infectious gametocytes) in 200 μl RPMI as previously described (69). Control mice were injected with 200 μl RPMI i.v. Mice were culled and samples harvested at 2, 4, 6, or 8 days postinfection. For activin neutralization experiments, mice were injected intraperitoneally (i.p.) on days 7 and 8 postinfection (30 h and 6 h prior to culling, respectively) with 100 μg each of anti-activin A (MAB3381; Bio-Techne, Abingdon, United Kingdom) and anti-activin B (MAB659; Bio-Techne) antibodies, or 200 μg isotype control IgG1 (MAB002; Bio-Techne), in 400 μl Dulbecco's phosphate-buffered saline (PBS) (Gibco) vehicle.
Plasmodium chabaudi infections of C57BL/6 mice.
Six-week-old female C57BL/6 mice were infected intravenously with 105 Plasmodium chabaudi chabaudi AS (PccAS)-infected red blood cells in Krebs glucose solution (PBS plus 1% glucose), harvested during the escalation of parasitemia from donor C57BL/6 mice infected from a frozen parasite stock (serially blood passaged rather than recently mosquito transmitted). Groups of 4 mice were culled at days 3, 8, 11, 14, 17, and 21 postinfection; 2 groups of 4 uninfected mice were culled as controls on day 0 and day 21. Parasitemia was assessed by counting Giemsa-stained thin films.
Mouse sample harvest and storage.
Mice were given a lethal anesthetic injection and blood was extracted via cardiac puncture; serum was isolated using BD SST Microtainers (Bunzl Healthcare, London, United Kingdom), and spleens, livers, and right hind legs were collected. For Plasmodium chabaudi infections, blood was also taken into BD EDTA Microtainers for assessment of anemia.
Livers and spleen explants (approximately 2 mm3) were preserved in RNAlater (Qiagen, Crawley, United Kingdom) for RNA extraction. Liver was snap-frozen in liquid nitrogen for Western blot analysis. Bone marrow was aspirated from tibias and immediately lysed in 350 μl RLT buffer (Qiagen), homogenized using QIAshredders (Qiagen), and stored at −20°C for later RNA extraction.
Mouse serum iron measurements.
Serum iron and unsaturated iron binding capacity (UIBC) of mouse sera were measured using the iron/total iron binding capacity (TIBC) reagent set (Pointe Scientific), scaling the recommended protocol to 96-well-plate format (6% volume) and reading absorbances (560 nm) on an Infinite M200 Pro Tecan microplate reader. TIBC was calculated as serum iron plus UIBC. Transferrin saturation (percent) was calculated by serum iron divided by TIBC times 100.
Measurement of hemoglobin concentrations in mice.
Hemoglobin concentrations were measured in EDTA-blood using an ABX Pentra60 benchtop analyzer (Horiba).
Hepatoma cell culture and activin protein treatment.
All in vitro experiments were performed in biological duplicate. HepG2 human hepatoma cells (ECACC) were cultured in minimal essential medium (MEM-alpha modification; Sigma) supplemented with 10% fetal calf serum (FCS; PAA Laboratories), 2 mM glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin (all Sigma). Cells were plated in a 12-well plate at 2 × 105 cells/ml, 1 ml/well, allowed to adhere overnight, and starved for 5 h in MEM-alpha with 0.1% FCS prior to activin treatment. Cells were treated with recombinant activin A (50 ng/ml), activin B (50 ng/ml), or BMP9 (100 ng/ml; all Bio-Techne) for 4 h unless otherwise stated. LDN-193189 (LDN; 100 nM; Axon Medchem), when used, was added 30 min before administration of activins/BMP9.
Human PBMC culture and treatment.
PBMC had been isolated using a Ficoll (GE Healthcare) gradient from the heparinized blood of consenting healthy adult donors according to the Weatherall Institute of Molecular Medicine local procedures, as described previously (12, 46). Cells (5 × 106 cells/ml, 1 ml/well, 12-well plate) plated in RPMI 1640 media (10% FCS supplemented with 2 mM glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; all Sigma) were cocultured with 107 P. falciparum A4 strain schizont iRBC or an equivalent number of control uRBC for 3 h as previously reported (46). Gene expression data from these experiments previously were published elsewhere (46); here, we investigated changes in activin expression in the same samples.
RNA extraction, cDNA synthesis, and quantitative real-time PCR (qRT-PCR).
RNA was extracted using RNeasy minikits (Qiagen) according to the manufacturer's protocol. Mouse spleen and liver samples from RNAlater were homogenized using a TissueRuptor (Qiagen). Cells cultured in vitro were homogenized using QIAshredders (Qiagen).
RNA concentrations were quantified using a NanoDrop ND-1000 spectrophotometer (Wilmington, DE, USA). cDNA was synthesized using the high-capacity RNA-to-cDNA kit (Applied Biosystems). Gene expression was quantified relative to endogenous control genes by qRT-PCR using TaqMan gene expression master mix and inventoried TaqMan assays (all Applied Biosystems) in 10-μl reaction mixtures in technical duplicate on a 7500Fast or QuantStudio7 instrument (Applied Biosystems), as previously described (12). Details of the inventoried TaqMan gene expression assays used are shown in Fig. S7 in the supplemental material.
Western blotting.
Approximately ∼10-mg frozen murine liver explants were lysed on wet ice using a TissueRuptor in lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 5 mM EDTA, pH 8, 1% NP-40, and inhibitors of proteases and phosphatases [all Sigma]) as in previous studies (70). Protein concentration was assessed using the Thermo Scientific Pierce protein assay (Fischer Scientific); lysates were diluted to 20 μg protein/10 μl solution with 1/3-volume bromophenol blue loading buffer, run through 12% SDS separating gel, and blotted onto activated polyvinylidene difluoride (PVDF) membranes. Size comparison was provided by a Bio-Rad Precision Plus Protein all blue ladder (Bio-Rad).
Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences) for 1 h and incubated overnight with a combination of two primary antibodies: mouse anti-β-actin (1:10,000; antibody AC15; Sigma-Aldrich) and either rabbit anti-phosphorylated STAT3 (pSTAT3; 1:1,000; D3A7; Cell Signaling) or rabbit anti-STAT3 (1:3,000; 79D7; Cell Signaling). Membranes were washed 3× in PBS–Tween (0.1%) and then incubated for 1 h with two secondary antibodies (donkey anti-mouse red 680 [1:20,000] and goat anti-rabbit green 800 [1:15,000]; LI-COR Biosciences) in 50% Odyssey–50% PBS buffer with 0.01% SDS and 0.1% Tween. Membranes were washed 3× in PBS–Tween (0.1%) and 1× in PBS only and then dried prior to examination with a LI-COR Biosciences instrument. Band intensities were quantified using LI-COR software. Each pSTAT3 or STAT3 band was normalized to its internal β-actin control prior to comparison with the mean of normalized band intensities of 3 uninfected age- and sex-matched mice sacrificed simultaneously and run on the same gel.
CHMI studies.
Serum samples were obtained from eighteen 18- to 50-year-old, malaria-naive, unvaccinated volunteers from three separate United Kingdom CHMI clinical trials conducted to assess the efficacy of novel vaccines: NCT01623557, NCT00890760, and NCT01142765 (also termed VAC045, MAL034B, and VAC039, respectively), all registered with ClinicalTrials.gov (71–73). All volunteers gave written informed consent to participate and for their samples to be stored and used for further investigations to assess immunity to malaria. The samples analyzed presently were from the six nonvaccinated volunteers who formed the infectivity control group in each of the three CHMI studies.
As detailed elsewhere (71), five Anopheles stephensi mosquitoes infected with P. falciparum 3D7 clone sporozoites were allowed to bite each volunteer. The day of infection is termed the day of challenge (C). From day 6.5 postchallenge until 21 days postchallenge, volunteers were assessed once to twice daily by Giemsa-stained thick smear for the presence of parasites, and samples were collected for qRT-PCR analysis of P. falciparum parasitemia. Upon meeting the criteria for diagnosis (71–73), treatment with artemether-lumefantrine (Riamet) or atovaquone-proguanil (Malarone) was initiated. This time point was termed day of diagnosis (DoD) and also was typically the point of maximal parasitemia. Larger blood samples were collected the day before CHMI (C−1), at DoD, and after resolution of infection (C+35), and as such samples from these time points were available for investigation here.
Measurement of human serum analytes.
Serum iron, UIBC, CRP, and ferritin in human samples were measured using an Abbott Architect cSystem Analyzer as described previously (14). TIBC and transferrin saturation were calculated as described above.
Serum hepcidin was quantified using the hepcidin-25 (human) enzyme immunoassay kit (EIA; Bachem), with the protocol modified as previously described (14, 74). Samples were initially measured at 1:8 dilution. Concentrations were determined using a 2-fold dilution curve (25 ng/ml to 0.05 ng/ml) as previously described (14). Duplicate concentrations with coefficients of variation of >15% were rerun. Samples with hepcidin concentrations falling outside the linear portion of the standard curve were rerun at appropriate dilutions. For those in which hepcidin was not detectable at the lowest possible dilution (typically 1:1), a value of 50% of the limit of detection (LOD; 0.118 ng/ml), multiplied by the dilution at which they were run, was assigned.
Serum activin A levels were measured by solid-phase sandwich ELISA (R&D Systems). Samples and standards were run in duplicate; samples with high coefficients of variation (>15%) were rerun.
Statistical analysis.
Data processing was performed using Microsoft Excel. Statistical analyses were performed and graphs generated using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). All analyses on untransformed data were nonparametric. In mouse experiments, Kruskal-Wallis tests with Dunn's multiple-comparison posttests were used to specifically compare the group of interest with the others. In experiments with only two groups, Mann-Whitney tests were used. In human data with paired samples, data were log transformed and analysis of variance (ANOVA) with Dunnett's multiple-comparison tests were used. In grouped analyses shown in Fig. S5, ANOVA was used. All correlations are Spearman's correlations. The significance level was set at P = 0.05 throughout.
Ethics.
All murine malaria infection experiments were performed in accordance with the terms of the United Kingdom Animals (Scientific Procedures) Act Project License (PPL 30/2889) and were approved by the University of Oxford Animal Welfare and Ethical Review Body.
All human CHMI trials were conducted in accordance with good clinical practices (GCP) and the principles of the Declaration of Helsinki. Trials were approved by the Oxfordshire Research Ethics Committee. The results of each trial and details of all necessary ethical and regulatory approvals for CHMI trials are reported elsewhere (71–73).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the trial volunteers and blood donors. We thank Jodie Babitt, Kimberly Bullough, and Alan Schneyer for helpful advice on in vitro and in vivo activin experiments; Craig Webster, Kamaljit Chatha, and Pamela Sturges (Department of Biochemistry, Birmingham Heartlands Hospital, United Kingdom) for human serum iron and acute-phase marker measurements; Tracey Rouault and Gennadiy Kovtunovych for advice on usage of the Pierce Scientific serum iron kit; and Lucy Eddowes, Sant-Rayn Pasricha, Kinda Al-Hourani, Alain Townsend, Pramila Rijal, Timothy Powell, Lisa Schimanski, Andrew Prentice, Silvia Portugal, Tom Ganz, Céline Besson-Fournier, Marie-Paule Roth, and Hélène Coppin for providing invaluable advice and suggestions. We also thank Adrian Hill, Geraldine O'Hara, Christopher Duncan, Anita Gola, Marta Ulaszewska, and the clinical trials team at the Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, for access to CHMI trial samples (funded by the PATH Malaria Vaccine Initiative, European Commission and European Malaria Vaccine Development Association).
N.S. was funded by the NIH-Oxford Cambridge Scholars Program. S.J.D. holds a Wellcome Trust Senior Fellowship (grant number 106917/Z/15/Z) and is a Jenner Investigator and Lister Institute Research Prize Fellow. S.H.H. held a Wellcome Trust Research Training Fellowship (097940/Z/11/Z). D.L. was supported by the Rhodes Trust. P.D. was supported in part by the Intramural Research Program of the NIH, NIAID. H.D. is supported by the UK Medical Research Council (grant MC_UU_12010/1), the Bill and Melinda Gates Foundation, and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Hematology Theme at Oxford University Hospitals NHS Trust and University of Oxford.
The funders played no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00191-17.
REFERENCES
- 1.WHO. 2015. World malaria report 2015. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ, Murray CJ. 2014. A systematic analysis of global anemia burden from 1990 to 2010. Blood 123:615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meremikwu MM, Donegan S, Sinclair D, Esu E, Oringanje C. 2012. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev 2:CD003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nweneka CV, Doherty CP, Cox S, Prentice A. 2010. Iron delocalisation in the pathogenesis of malarial anaemia. Trans R Soc Trop Med Hyg 104:175–184. doi: 10.1016/j.trstmh.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. 2000. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480:147–150. doi: 10.1016/S0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 6.Park CH, Valore EV, Waring AJ, Ganz T. 2001. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 7.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. 2001. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 9.Abboud S, Haile DJ. 2000. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 10.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. 2000. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5:299–309. doi: 10.1016/S1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 12.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. 2011. Hepcidin regulation by innate immune and infectious stimuli. Blood 118:4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- 13.Darton TC, Blohmke CJ, Giannoulatou E, Waddington CS, Jones C, Sturges P, Webster C, Drakesmith H, Pollard AJ, Armitage AE. 2015. Rapidly escalating hepcidin and associated serum iron starvation are features of the acute response to typhoid infection in humans. PLoS Negl Trop Dis 9:e0004029. doi: 10.1371/journal.pntd.0004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, Smith NM, Huang X, Xu X, Pasricha SR, Li N, Wu H, Webster C, Prentice AM, Pellegrino P, Williams I, Norris PJ, Drakesmith H, Borrow P. 2014. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci U S A 111:12187–12192. doi: 10.1073/pnas.1402351111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, Gulig PA, Ganz T, Nemeth E, Bulut Y. 2015. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe 17:47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, Silalye S, Verhoef H, Sauerwein RW, Swinkels DW, van der Ven AJ. 2009. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis 199:253–262. doi: 10.1086/595790. [DOI] [PubMed] [Google Scholar]
- 17.Howard CT, McKakpo US, Quakyi IA, Bosompem KM, Addison EA, Sun K, Sullivan D, Semba RD. 2007. Relationship of hepcidin with parasitemia and anemia among patients with uncomplicated Plasmodium falciparum malaria in Ghana. Am J Trop Med Hyg 77:623–626. [PubMed] [Google Scholar]
- 18.de Mast Q, Syafruddin D, Keijmel S, Riekerink TO, Deky O, Asih PB, Swinkels DW, van der Ven AJ. 2010. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 95:1068–1074. doi: 10.3324/haematol.2009.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Mast Q, van Dongen-Lases EC, Swinkels DW, Nieman AE, Roestenberg M, Druilhe P, Arens TA, Luty AJ, Hermsen CC, Sauerwein RW, van der Ven AJ. 2009. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol 145:657–664. doi: 10.1111/j.1365-2141.2009.07664.x. [DOI] [PubMed] [Google Scholar]
- 20.Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H, Mota MM. 2011. Host-mediated regulation of superinfection in malaria. Nat Med 17:732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HZ, He YX, Yang CJ, Zhou W, Zou CG. 2011. Hepcidin is regulated during blood-stage malaria and plays a protective role in malaria infection. J Immunol 187:6410–6416. doi: 10.4049/jimmunol.1101436. [DOI] [PubMed] [Google Scholar]
- 22.Cercamondi CI, Egli IM, Ahouandjinou E, Dossa R, Zeder C, Salami L, Tjalsma H, Wiegerinck E, Tanno T, Hurrell RF, Hounhouigan J, Zimmermann MB. 2010. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr 92:1385–1392. doi: 10.3945/ajcn.2010.30051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, Armitage AE, Drakesmith H. 2012. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood 119:1922–1928. doi: 10.1182/blood-2011-11-391219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Santen S, de Mast Q, Swinkels DW, van der Ven AJ. 2013. The iron link between malaria and invasive non-typhoid Salmonella infections. Trends Parasitol 29:220–227. doi: 10.1016/j.pt.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. 2002. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Investig 110:1037–1044. doi: 10.1172/JCI0215686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muckenthaler MU, Rivella S, Hentze MW, Galy B. 2017. A red carpet for iron metabolism. Cell 168:344–361. doi: 10.1016/j.cell.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. 2014. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Core AB, Canali S, Zumbrennen-Bullough KB, Ozer S, Umans L, Zwijsen A, Babitt JL. 2017. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood 130:73–83. doi: 10.1182/blood-2016-12-759423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, Coppin H. 2012. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood 120:431–439. doi: 10.1182/blood-2012-02-411470. [DOI] [PubMed] [Google Scholar]
- 30.Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, Phillips DJ. 2007. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A 104:16239–16244. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Besson-Fournier C, Gineste A, Latour C, Gourbeyre O, Meynard D, Martin P, Oswald E, Coppin H, Roth MP. 2017. Hepcidin upregulation by inflammation is independent of Smad1/5/8 signaling by activin B. Blood 129:533–536. doi: 10.1182/blood-2016-10-748541. [DOI] [PubMed] [Google Scholar]
- 32.Canali S, Core AB, Zumbrennen-Bullough KB, Merkulova M, Wang CY, Schneyer AL, Pietrangelo A, Babitt JL. 2016. Activin B induces noncanonical SMAD1/5/8 signaling via BMP type I receptors in hepatocytes: evidence for a role in hepcidin induction by inflammation in male mice. Endocrinology 157:1146–1162. doi: 10.1210/en.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. 2007. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Investig 117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanamori Y, Sugiyama M, Hashimoto O, Murakami M, Matsui T, Funaba M. 2016. Regulation of hepcidin expression by inflammation-induced activin B. Sci Rep 6:38702. doi: 10.1038/srep38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. 1999. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- 36.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. 2009. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 37.Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. 2009. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, Paulson RF, Miller JL. 2009. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood 114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grcevic D, Kusec R, Kovacic N, Lukic A, Lukic IK, Ivcevic S, Nemet D, Seiwerth RS, Ostojic SK, Croucher PI, Marusic A. 2010. Bone morphogenetic proteins and receptors are over-expressed in bone-marrow cells of multiple myeloma patients and support myeloma cells by inducing ID genes. Leuk Res 34:742–751. doi: 10.1016/j.leukres.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys L, Rivera S, Vanderkerken K, Lichtenstein A, Ganz T. 2010. In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood 116:3635–3644. doi: 10.1182/blood-2010-03-274571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch PS, Olsavszky V, Ulbrich F, Sticht C, Demory A, Leibing T, Henzler T, Meyer M, Zierow J, Schneider S, Breitkopf-Heinlein K, Gaitantzi H, Spencer-Dene B, Arnold B, Klapproth K, Schledzewski K, Goerdt S, Geraud C. 2017. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood 129:415–419. doi: 10.1182/blood-2016-07-729822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Chen Y, Winnall WR, Phillips DJ, Hedger MP. 2012. Acute regulation of activin A and its binding protein, follistatin, in serum and tissues following lipopolysaccharide treatment of adult male mice. Am J Physiol Regul Integr Comp Physiol 303:R665–R675. doi: 10.1152/ajpregu.00478.2011. [DOI] [PubMed] [Google Scholar]
- 43.Chang KH, Stevenson MM. 2004. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int J Parasitol 34:1501–1516. doi: 10.1016/j.ijpara.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Robson NC, Phillips DJ, McAlpine T, Shin A, Svobodova S, Toy T, Pillay V, Kirkpatrick N, Zanker D, Wilson K, Helling I, Wei H, Chen W, Cebon J, Maraskovsky E. 2008. Activin-A: a novel dendritic cell-derived cytokine that potently attenuates CD40 ligand-specific cytokine and chemokine production. Blood 111:2733–2743. doi: 10.1182/blood-2007-03-080994. [DOI] [PubMed] [Google Scholar]
- 45.Eramaa M, Hurme M, Stenman UH, Ritvos O. 1992. Activin A/erythroid differentiation factor is induced during human monocyte activation. J Exp Med 176:1449–1452. doi: 10.1084/jem.176.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armitage AE, Pinches R, Eddowes LA, Newbold CI, Drakesmith H. 2009. Plasmodium falciparum infected erythrocytes induce hepcidin (HAMP) mRNA synthesis by peripheral blood mononuclear cells. Br J Haematol 147:769–771. doi: 10.1111/j.1365-2141.2009.07880.x. [DOI] [PubMed] [Google Scholar]
- 47.Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. 2008. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett 18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murakami M, Ikeda T, Saito T, Ogawa K, Nishino Y, Nakaya K, Funaba M. 2006. Transcriptional regulation of plasminogen activator inhibitor-1 by transforming growth factor-beta, activin A and microphthalmia-associated transcription factor. Cell Signal 18:256–265. doi: 10.1016/j.cellsig.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Zhao H, Konishi A, Fujita Y, Yagi M, Ohata K, Aoshi T, Itagaki S, Sato S, Narita H, Abdelgelil NH, Inoue M, Culleton R, Kaneko O, Nakagawa A, Horii T, Akira S, Ishii KJ, Coban C. 2012. Lipocalin 2 bolsters innate and adaptive immune responses to blood-stage malaria infection by reinforcing host iron metabolism. Cell Host Microbe 12:705–716. doi: 10.1016/j.chom.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Casals-Pascual C, Huang H, Lakhal-Littleton S, Thezenas ML, Kai O, Newton CR, Roberts DJ. 2012. Hepcidin demonstrates a biphasic association with anemia in acute Plasmodium falciparum malaria. Haematologica 97:1695–1698. doi: 10.3324/haematol.2012.065854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latour C, Wlodarczyk MF, Jung G, Gineste A, Blanchard N, Ganz T, Roth MP, Coppin H, Kautz L. 2017. Erythroferrone contributes to hepcidin repression in a mouse model of malarial anemia. Haematologica 102:60–68. doi: 10.3324/haematol.2016.150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonker FA, Calis JC, van Hensbroek MB, Phiri K, Geskus RB, Brabin BJ, Leenstra T. 2012. Iron status predicts malaria risk in Malawian preschool children. PLoS One 7:e42670. doi: 10.1371/journal.pone.0042670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burte F, Brown BJ, Orimadegun AE, Ajetunmobi WA, Afolabi NK, Akinkunmi F, Kowobari O, Omokhodion S, Osinusi K, Akinbami FO, Shokunbi WA, Sodeinde O, Fernandez-Reyes D. 2013. Circulatory hepcidin is associated with the anti-inflammatory response but not with iron or anemic status in childhood malaria. Blood 121:3016–3022. doi: 10.1182/blood-2012-10-461418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locci M, Wu JE, Arumemi F, Mikulski Z, Dahlberg C, Miller AT, Crotty S. 2016. Activin A programs the differentiation of human TFH cells. Nat Immunol 17:976–984. doi: 10.1038/ni.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michel U, Ebert S, Phillips D, Nau R. 2003. Serum concentrations of activin and follistatin are elevated and run in parallel in patients with septicemia. Eur J Endocrinol 148:559–564. doi: 10.1530/eje.0.1480559. [DOI] [PubMed] [Google Scholar]
- 56.Petrakou E, Fotopoulos S, Anagnostakou M, Anatolitou F, Samitas K, Semitekolou M, Xanthou G, Xanthou M. 2013. Activin-A exerts a crucial anti-inflammatory role in neonatal infections. Pediatr Res 74:675–681. doi: 10.1038/pr.2013.159. [DOI] [PubMed] [Google Scholar]
- 57.Jones KL, de Kretser DM, Clarke IJ, Scheerlinck JP, Phillips DJ. 2004. Characterisation of the rapid release of activin A following acute lipopolysaccharide challenge in the ewe. J Endocrinol 182:69–80. doi: 10.1677/joe.0.1820069. [DOI] [PubMed] [Google Scholar]
- 58.Wu H, Chen Y, Winnall WR, Phillips DJ, Hedger MP. 2013. Regulation of activin A release from murine bone marrow-derived neutrophil precursors by tumour necrosis factor-alpha and insulin. Cytokine 61:199–204. doi: 10.1016/j.cyto.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. 2011. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm 85:255–297. doi: 10.1016/B978-0-12-385961-7.00013-5. [DOI] [PubMed] [Google Scholar]
- 60.Mason AJ, Berkemeier LM, Schmelzer CH, Schwall RH. 1989. Activin B: precursor sequences, genomic structure and in vitro activities. Mol Endocrinol 3:1352–1358. doi: 10.1210/mend-3-9-1352. [DOI] [PubMed] [Google Scholar]
- 61.Phillips DJ, Brauman JN, Mason AJ, de Kretser DM, Hedger MP. 1999. A sensitive and specific in vitro bioassay for activin using a mouse plasmacytoma cell line, MPC-11. J Endocrinol 162:111–116. doi: 10.1677/joe.0.1620111. [DOI] [PubMed] [Google Scholar]
- 62.Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. 2000. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25:453–457. doi: 10.1038/78161. [DOI] [PubMed] [Google Scholar]
- 63.Brown CW, Li L, Houston-Hawkins DE, Matzuk MM. 2003. Activins are critical modulators of growth and survival. Mol Endocrinol 17:2404–2417. doi: 10.1210/me.2003-0051. [DOI] [PubMed] [Google Scholar]
- 64.Bonomi L, Brown M, Ungerleider N, Muse M, Matzuk MM, Schneyer A. 2012. Activin B regulates islet composition and islet mass but not whole body glucose homeostasis or insulin sensitivity. Am J Physiol Endocrinol Metab 303:E587–E596. doi: 10.1152/ajpendo.00177.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludlow H, Phillips DJ, Myers M, McLachlan RI, de Kretser DM, Allan CA, Anderson RA, Groome NP, Hyvonen M, Duncan WC, Muttukrishna S. 2009. A new “total” activin B enzyme-linked immunosorbent assay (ELISA): development and validation for human samples. Clin Endocrinol (Oxf) 71:867–873. doi: 10.1111/j.1365-2265.2009.03567.x. [DOI] [PubMed] [Google Scholar]
- 66.de Kretser DM, Bensley JG, Pettila V, Linko R, Hedger MP, Hayward S, Allan CA, McLachlan RI, Ludlow H, Phillips DJ. 2013. Serum activin A and B levels predict outcome in patients with acute respiratory failure: a prospective cohort study. Crit Care 17:R263. doi: 10.1186/cc13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linko R, Hedger MP, Pettila V, Ruokonen E, Ala-Kokko T, Ludlow H, de Kretser DM. 2014. Serum activin A and B, and follistatin in critically ill patients with influenza A(H1N1) infection. BMC Infect Dis 14:253. doi: 10.1186/1471-2334-14-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Kretser DM, Bensley JG, Phillips DJ, Levvey BJ, Snell GI, Lin E, Hedger MP, O'Hehir RE. 2016. Substantial increases occur in serum activins and follistatin during lung transplantation. PLoS One 11:e0140948. doi: 10.1371/journal.pone.0140948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodman AL, Forbes EK, Williams AR, Douglas AD, de Cassan SC, Bauza K, Biswas S, Dicks MD, Llewellyn D, Moore AC, Janse CJ, Franke-Fayard BM, Gilbert SC, Hill AV, Pleass RJ, Draper SJ. 2013. The utility of Plasmodium berghei as a rodent model for anti-merozoite malaria vaccine assessment. Sci Rep 3:1706. doi: 10.1038/srep01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kautz L, Besson-Fournier C, Meynard D, Latour C, Roth MP, Coppin H. 2011. Iron overload induces BMP6 expression in the liver but not in the duodenum. Haematologica 96:199–203. doi: 10.3324/haematol.2010.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ewer KJ, O'Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, Longley RJ, Rowland R, Poulton ID, Draper SJ, Blagborough AM, Berrie E, Moyle S, Williams N, Siani L, Folgori A, Colloca S, Sinden RE, Lawrie AM, Cortese R, Gilbert SC, Nicosia A, Hill AV. 2013. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun 4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, Collins KA, Edwards NJ, Douglas AD, Anagnostou NA, Ewer KJ, Havelock T, Mahungu T, Bliss CM, Miura K, Poulton ID, Lillie PJ, Antrobus RD, Berrie E, Moyle S, Gantlett K, Colloca S, Cortese R, Long CA, Sinden RE, Gilbert SC, Lawrie AM, Doherty T, Faust SN, Nicosia A, Hill AV, Draper SJ. 2012. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther 20:2355–2368. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Longley R, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AV. 2015. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis 211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atkinson SH, Armitage AE, Khandwala S, Mwangi TW, Uyoga S, Bejon PA, Williams TN, Prentice AM, Drakesmith H. 2014. Combinatorial effects of malaria season, iron deficiency and inflammation determine plasma hepcidin concentration in African children. Blood 123:3221–3229. doi: 10.1182/blood-2013-10-533000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.