ABSTRACT
Acinetobacter baumannii has become an important concern for human health due to rapid development and wide spread of antimicrobial-resistant strains and high mortality associated with the infection. Passive immunizations with antisera targeting outer membrane proteins (OMPs) have shown encouraging results in protecting mice from A. baumannii infection, but monoclonal anti-OMP antibodies have not been developed, and their potential therapeutic properties have not been explored. The goal of this report is to evaluate the antibacterial activity of monoclonal antibodies (MAbs) targeting outer membrane protein A (OmpA) of A. baumannii. Five anti-OmpA MAbs were developed using hybridoma technology and showed strong binding to strain ATCC 19606. However, low antibody binding was observed when they were tested against six clinical isolates, which included extensively drug-resistant strains. In contrast, high binding to an isogenic K1 capsule-negative mutant (AB307.30) was shown, suggesting that capsular polysaccharide mediated the inhibition of MAb binding to OmpA. Anti-OmpA MAbs increased the macrophage-mediated bactericidal activity of AB307.30 but failed to increase phagocytic killing of capsule-positive strains. Capsular polysaccharide was also protective against complement-mediated bactericidal activity in human ascites in the presence and absence of opsonization. Lastly, passive immunization with anti-OmpA MAbs did not confer protection against challenge with AB307-0294, the encapsulated parent strain of AB307.30, in a mouse sepsis infection model. These results reveal the important role of capsule polysaccharide in shielding OmpA and thereby inhibiting anti-OmpA MAb binding to clinical isolates. This property of capsule hindered the therapeutic utility of anti-OmpA MAbs, and it may apply to other conserved epitopes in A. baumannii.
KEYWORDS: Acinetobacter baumannii, outer membrane protein A, capsule polysaccharide, passive immunization, monoclonal antibody
INTRODUCTION
Acinetobacter baumannii is listed by the World Health Organization as one of the three top antibiotic-resistant priority pathogens. A. baumannii infections, including ventilator-associated pneumonia (VAP), bacteremia, skin and wound infections, urinary tract infections, and meningitis related to neurosurgical procedures, have been acquired mainly in health care facilities, especially in intensive care units (ICUs) (1). Approximately 45,900 and 1,000,000 cases are reported annually in the United States and globally (2). Importantly, mortality associated with A. baumannii infections is high, ranging from 40% to 70% in VAP patients and 34% to 49% with bacteremia (3). Furthermore, the antibiotic resistance of A. baumannii has increased ∼15-fold in the past 10 years (4). Approximately 50% of A. baumannii isolates from ICUs are extensively drug resistant (XDR) (i.e., resistant to all antimicrobials except polymyxins or tigecycline) in the United States, and cases of pandrug resistance (i.e., resistance to all antimicrobials) are increasing (5–7). Therefore, treatment of A. baumannii infections has become challenging.
The role of antibody in defense against microbial infections is undisputed. Passive immunization was successfully applied for prophylaxis and treatment of bacterial infections in the preantibiotic era in the form of serum therapy (8). Recent advances in monoclonal antibody (MAb) technology have driven the development of antibacterial MAbs. Three MAbs (i.e., raxibacumab, obitoxaximab, and bezlotoxumab) have been marketed to prevent and treat Bacillus anthracis and Clostridium difficile infections since 2012. Passive immunization with antibodies targeting outer membrane proteins (OMPs) of A. baumannii has been considered as a potential therapeutic approach for XDR A. baumannii infections either alone or as an adjunctive therapy to antimicrobials. This is due to the conservation of OMPs among clinical isolates; target specificity without cross-reactivity to human epitopes (9); decreased selective pressure for cross-resistance; less disturbance of the normal flora; and the long half-life of antibodies, thereby enabling less frequent dosing. Recently, active immunization with outer membrane protein A (OmpA) and passive immunization with polyclonal anti-OmpA sera have shown promising protection against multidrug-resistant (MDR) and XDR A. baumannii clinical isolates in a mouse bacteremia model (9, 10). Further, passive immunization with polyclonal antisera that target other OMPs, such as Omp22 and outer membrane complexes, also conferred protection against MDR A. baumannii in a mouse sepsis model (11, 12). However, treatment with polyclonal antisera has inevitable drawbacks, including “serum sickness” or immune complex hypersensitivity that can occur in 10 to 50% of patients, lot-to-lot variation in efficacy, low content of specific antibodies, and potential hazards in the transmission of infectious diseases (13–16). In contrast, monoclonal antibodies are potentially advantageous due to higher specific activity, homogeneity, consistency, safety, and reduced immunogenicity with humanized MAbs, but MAbs targeting OMPs of A. baumannii have not been reported. One concern for the use of anti-OMP MAbs is a report that surface polysaccharides shield these conserved targets, decreasing antibody binding and mitigating the effects of opsonization on complement- and phagocyte-mediated bactericidal activity (17–20).
In this study, we tested the hypothesis that MAbs directed against OmpA of A. baumannii could be used to enhance macrophage-mediated bactericidal activity and complement-dependent bacterial killing in vitro and to protect against infection in vivo. However, capsule polysaccharide in A. baumannii was found to impede the binding of anti-OmpA MAbs, which in turn inhibited anti-OmpA MAb-mediated macrophage and complement-dependent bactericidal activity. Passive immunization with the anti-OmpA MAb did not provide protection against encapsulated A. baumannii AB307-0294 in a mouse sepsis infection model. These results raise the concern that OMPs may not be viable targets in A. baumannii for passive and perhaps active immunization.
RESULTS
Development of MAbs directed against OmpA from A. baumannii.
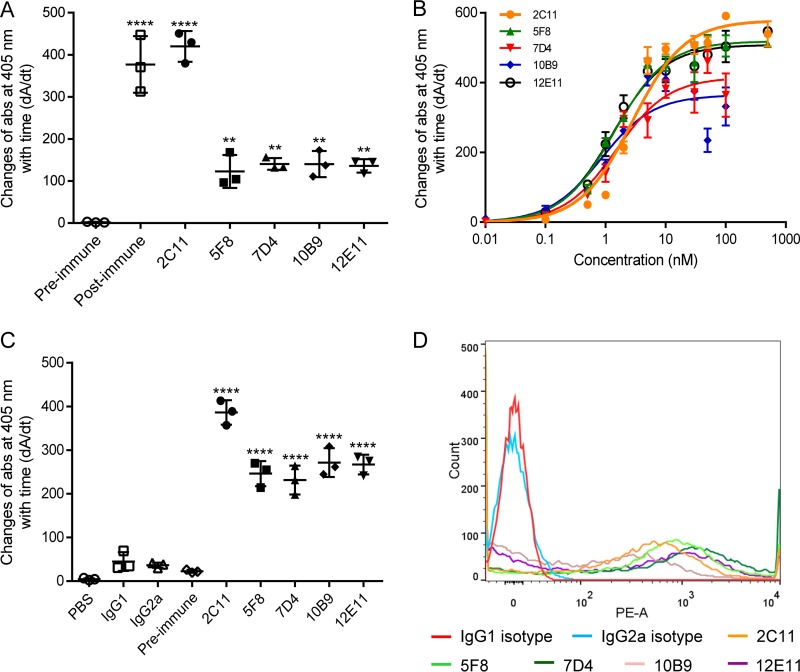
The anti-OmpA MAbs used in this study were developed via hybridoma technology. The mouse that yielded the highest antiserum titer after immunization was chosen for hybridoma development. To ensure generation of monoclones, hybridomas were cloned at least twice until all randomly picked cells produced positive anti-OmpA antibodies. Five anti-OmpA MAbs (2C11, 5F8, 7D4, 10B9, and 12E11) that showed positive binding to recombinant OmpA (rOmpA) based on enzyme-linked immunosorbent assay (ELISA) (Fig. 1A) and Western blotting of whole-cell lysates (Fig. 3) were generated. The ELISA values of the MAbs were 60- to 200-fold higher than those of the preimmune serum, and a single binding band was observed at 38 kDa (the molecular mass of OmpA) via Western blot analysis, establishing specificity for OmpA (Fig. 3). Among the five MAbs, 2C11 is IgG2a(κ), and the others are IgG1(κ). The equilibrium dissociation constants of the MAbs were estimated to be 1.10 to 2.79 nM using rOmpA-based ELISA (Fig. 1B and Table 1), and all five MAbs reached maximum binding at concentrations of ≥100 nM.
FIG 1.
Binding of anti-OmpA MAbs to rOmpA and A. baumannii ATCC 19606. (A) Purified anti-OmpA MAbs showed positive binding to rOmpA compared to preimmune serum based on ELISA. Postimmune serum was used as a positive control. (B) Michaelis-Menten profiles of binding of anti-OmpA MAbs to rOmpA. (C and D) Anti-OmpA MAbs showed strong binding to formalin-killed ATCC 19606 using cell-based ELISA (C) and to live ATCC 19606 via flow cytometry (D) compared to antibody isotype controls (2C11 versus IgG2a; 5F8, 7D4, 10B9, and 12E11 versus IgG1). n = 3. **, P < 0.01; ****, P < 0.0001 (one-way ANOVA followed by Dunnett's multiple comparisons). The data are presented as means ± standard deviations. PE-A, phycoerythrin area.
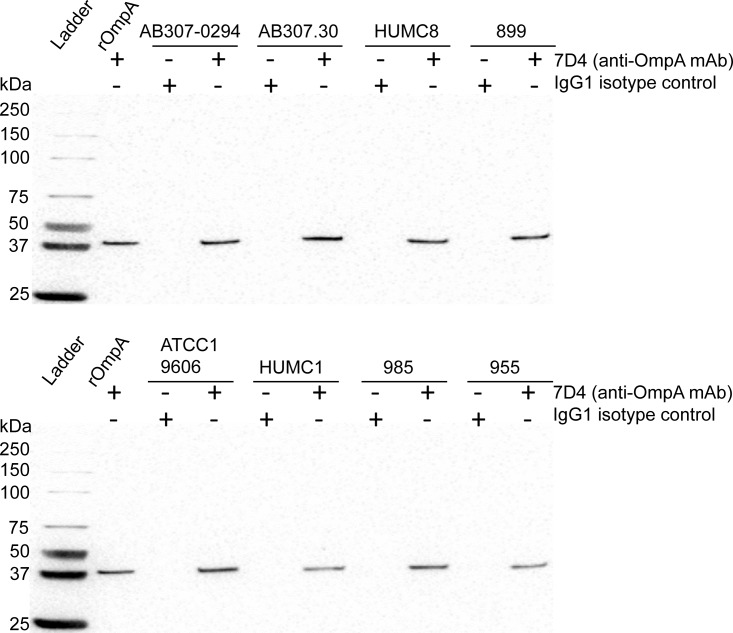
FIG 3.
Western blot analysis of A. baumannii cell lysates. OMPs were separated using 4 to 15% polyacrylamide gel electrophoresis with 50 μg total proteins loaded in each well. Expression of OmpA was detected on polyvinylidene difluoride (PVDF) membranes using anti-OmpA MAb 7D4 and anti-mouse IgG horseradish peroxidase conjugates (Millipore Sigma) as primary and secondary antibodies, respectively. Proteins were visualized using enhanced chemiluminescence substrates (Thermo Fisher Scientific). Recombinant OmpA (100 ng/well) was used as a positive control. OmpA was detected in all tested A. baumannii strains, shown as a positive binding band at 38 kDa.
TABLE 1.
Isotypes and equilibrium dissociation constants of anti-OmpA MAbs
| Anti-OmpA MAb | Isotype | Kd (nM) (%)a | Bmax (dA/dt) (%)a |
|---|---|---|---|
| 2C11 | IgG2a(κ) | 2.79 (15.1) | 578 (3.11) |
| 5F8 | IgG1(κ) | 1.40 (12.1) | 518 (2.32) |
| 7D4 | IgG1(κ) | 1.50 (23.3) | 415 (4.82) |
| 10B9 | IgG1(κ) | 1.10 (28.2) | 368 (5.43) |
| 12E11 | IgG1(κ) | 1.32 (14.4) | 508 (2.56) |
The values in parentheses are coefficients of variation (CV%).
The binding of anti-OmpA MAbs to clinical isolates of A. baumannii was significantly lower than that observed for ATCC 19606.
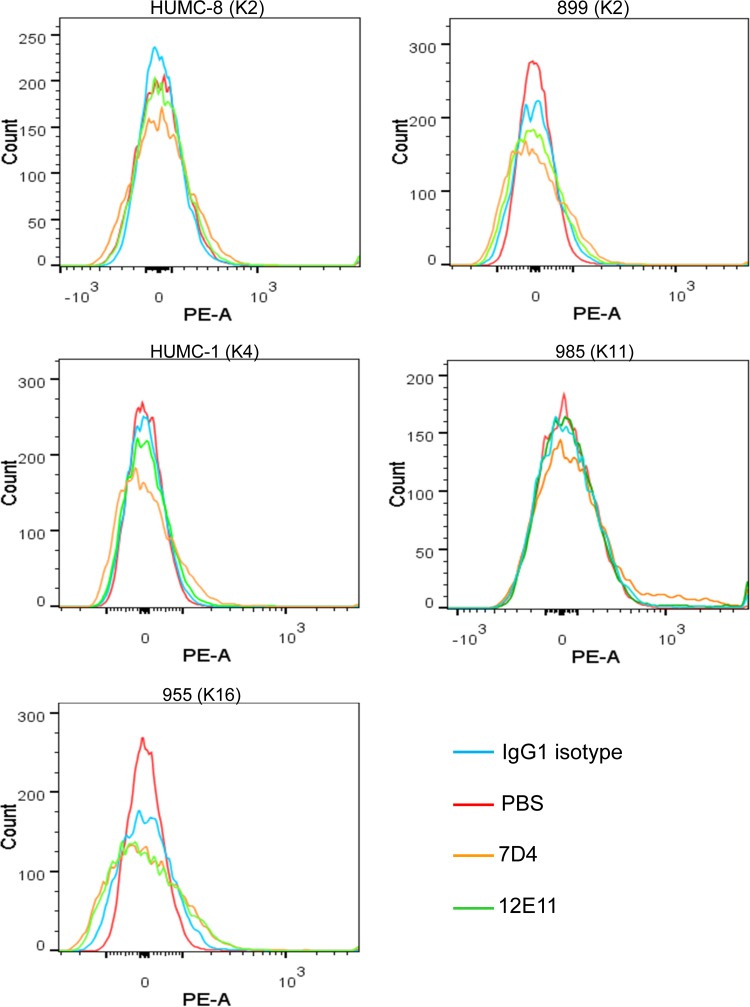
Binding of antibodies to bacteria is a necessary step for subsequent antibody-mediated immune responses, such as antibody-dependent phagocytosis and the classical pathway of complement-mediated bactericidal activity. Binding of the MAbs to OmpA in situ was initially assessed using ATCC 19606 via cell-based ELISA and flow cytometry. All five MAbs showed strong binding to ATCC 19606 (K4 capsular serotype, or KL3 in the classification system designed by Kenyon and Hall [21]) compared to antibody isotype controls (Fig. 1C and D). Although the mean fluorescence values of anti-OmpA MAbs shifted ∼1,000-fold compared to isotype controls, percentages of detectable antibody-bound bacteria (i.e., populations that did not overlap the isotype controls) ranged from 58.1% to 74.4% at a predetermined saturation antibody concentration (200 nM) (Fig. 1D and Table 2). To confirm this finding in clinical isolates, binding to carbapenem-resistant (i.e., XDR) strains and various capsular serotypes, including HUMC8 and 899 (K2 capsular serotype), HUMC1 (K4 capsular serotype), 985 (K11 capsular serotype), and 955 (K16 capsular serotype), was assessed using flow cytometry (Fig. 2). Surprisingly and in contrast to strong binding to ATCC 19606, binding to these strains was low (Fig. 2 and Table 2). Importantly, however, OmpA expression was confirmed in all tested strains using Western blot analysis of bacterial cell lysates, which was demonstrated as positive binding bands at 38 kDa (Fig. 3).
TABLE 2.
Binding of anti-OmpA MAbs to A. baumannii assessed via flow cytometry
| MAb | % detectable antibody-bound bacteriaa |
|||||||
|---|---|---|---|---|---|---|---|---|
| ATCC 19606 | AB307 | AB307.30 | HUMC8 | 899 | HUMC1 | 985 | 955 | |
| 2C11 | 71.0 | 8.00 | 77.9 | NA | NA | NA | NA | NA |
| 5F8 | 74.4 | 5.30 | 78.1 | NA | NA | NA | NA | NA |
| 7D4 | 70.3 | 14.3 | 95.0 | 2.97 | 4.47 | 2.68 | 5.56 | 3.88 |
| 10B9 | 48.2 | 5.80 | 24.2 | NA | NA | NA | NA | NA |
| 12E11 | 58.1 | 17.0 | 89.4 | 2.01 | 2.88 | 1.46 | 2.49 | 3.96 |
NA, not available; the sample was not tested.
FIG 2.
A negligible degree of binding of anti-OmpA MAbs was observed with all A. baumannii clinical isolates tested. Negligible binding of anti-OmpA MAbs (7D4 and 12E11) against all tested encapsulated clinical isolates, including HUMC8 and 899 (K2 capsular serotype), HUMC1 (K4 capsular serotype), 985 (K11 capsular serotype), and 955 (K16 capsular serotype), was found. Binding reactions were assessed by flow cytometry. Mouse IgG1 and IgG2a were used as negative controls. PE-A, phycoerythrin area.
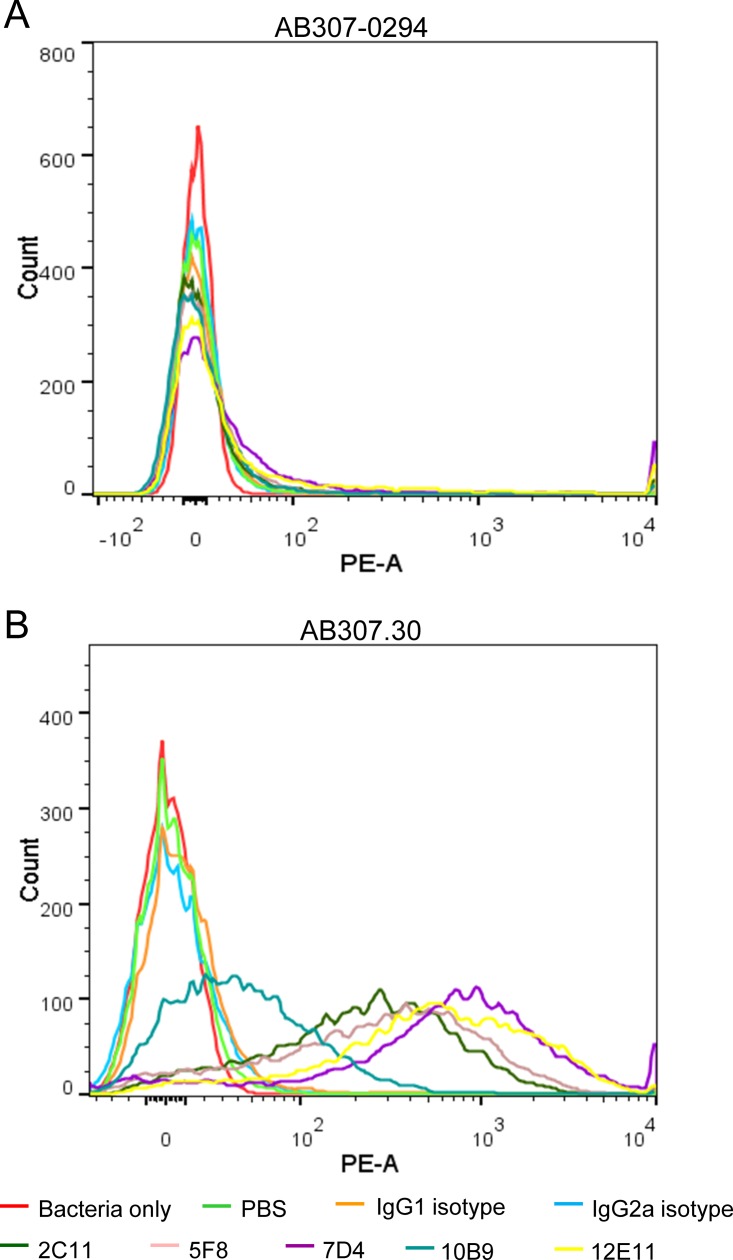
The K1 capsule polysaccharide of A. baumannii impeded binding of anti-OmpA MAbs.
Since the surface polysaccharide capsule and the O antigen moiety of lipopolysaccharide (LPS) have been shown to impede antibody binding in several bacteria (17–20) and A. baumannii likely does not express O antigen, as demonstrated by Kenyon and Hall (21), we hypothesized that the capsular polysaccharide produced by clinical isolates might be responsible for the observed decrease in binding of MAbs to OmpA. To test this hypothesis, MAb binding to AB307.30, an isogenic capsule-negative derivative of AB307-0294 (K1 capsular serotype, or KL1 in the classification system designed by Kenyon and Hall [21]), was assessed by flow cytometry (22). In contrast to the lack of binding observed for AB307-0294, significant binding of the MAbs was observed for AB307.30 (Fig. 4 and Table 2). Percentages of detectable antibody-bound bacteria were high, ranging from 78% to 95% (except for 10B9 at 24.2%), with mean fluorescence values shifted 450-fold to 1.72 × 103-fold. Anti-OmpA MAb 7D4 consistently showed the greatest degree of binding to OmpA as presented in live A. baumannii. Hence, this MAb was chosen for subsequent bactericidal studies in vitro and in vivo.
FIG 4.
Capsule polysaccharide impeded the binding of anti-OmpA MAbs to A. baumannii clinical isolates. Anti-OmpA MAbs showed low binding to wild-type AB307-0294 (K1 capsular serotype) (A) but strong binding to the capsule-negative derivative AB307.30 (B). PE-A, phycoerythrin area.
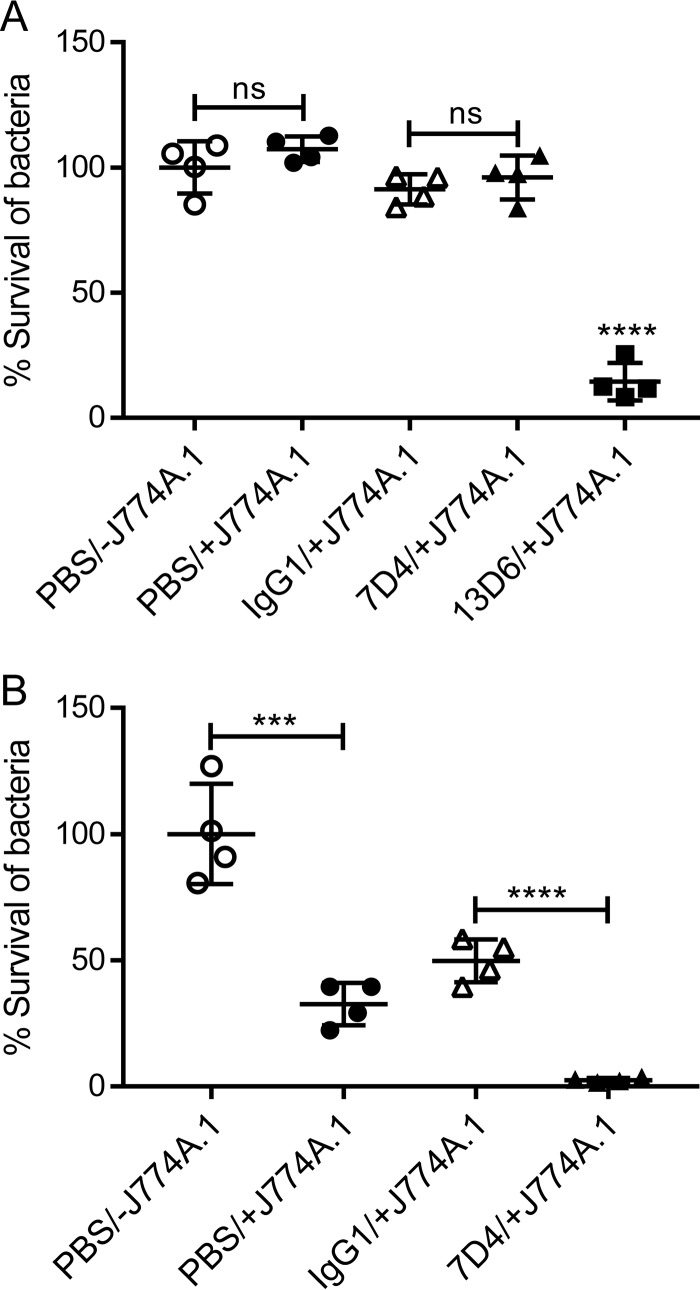
The inhibition of MAb binding to OmpA by capsular polysaccharide impeded macrophage-mediated bactericidal activity.
We hypothesized that inhibition of antibody binding would adversely affect macrophage-mediated bactericidal activity. Preincubation of AB307-0294 (wild type [wt]; K1 capsule positive) with 7D4, followed by incubation with murine macrophage J774A.1 cells, did not affect bacterial survival compared to AB307-0294 not preincubated with 7D4 or incubated in the absence of macrophages (Fig. 5A). In contrast, 67.4% of AB307.30 bacteria (K1 capsule negative) were killed by macrophages over 60 min (Fig. 5B), which demonstrates the role of capsule polysaccharide in conferring protection from macrophage-mediated bactericidal activity. In addition, and as expected, opsonization of AB307.30 with 7D4 further increased the killing of AB307.30 (97.5% reduction of bacterial CFU) (Fig. 5B). To determine if opsonization of AB307-0294 could increase macrophage-mediated bactericidal activity, AB307-0294 was opsonized with an anti-K1 capsule MAb, 13D6 (23), and subsequently incubated with murine macrophage J774A.1 cells. A significant decrease in the viability of AB307-0294 (85.5% reduction in the bacterial count) was observed (Fig. 5A). These data demonstrate the critical role that capsule plays in mediating protection in the absence of antibody opsonization. The data also demonstrate that capsule-mediated inhibition of antibody binding protects bacteria from opsonophagocytic killing. Last, and importantly, if an antibody is able to bind to the bacterium (e.g., the anti-K1 capsule MAb 13D6), then macrophage-mediated bactericidal activity is increased. These data also support capsular polysaccharide as an antigenic target for active or passive immunization.
FIG 5.
K1 capsule polysaccharide protected wild-type AB307-0294 from murine macrophage-mediated phagocytic killing. (A) Wild-type strain AB307-0294. (B) Capsule-negative strain AB307.30. The presence of macrophages (+J774A.1) did not affect the viability of AB307-0294 (A) but significantly increased bactericidal activity against the capsule-negative strain AB307.30 (PBS/−J774A.1 versus PBS/+J774A.1) (B). Preincubation with anti-OmpA MAb 7D4 did not enhance the phagocytic killing of AB307-0294 by macrophages (A) but resulted in dramatic killing of capsule-negative AB307.30 (IgG1/+J774A.1 versus 7D4/+J774A.1) (B). An anti-K1 capsule MAb, 13D6, used as a positive control, significantly increased bactericidal activity against the capsule-positive strain AB307-0294 (A). The results are expressed as percent survival compared to bacterial samples in the absence of J774A.1 (PBS/−J774A.1). n = 4. ***, P < 0.001; ****, P < 0.0001; ns, not significant (two-tailed unpaired t test). The data are presented as means ± standard deviations.
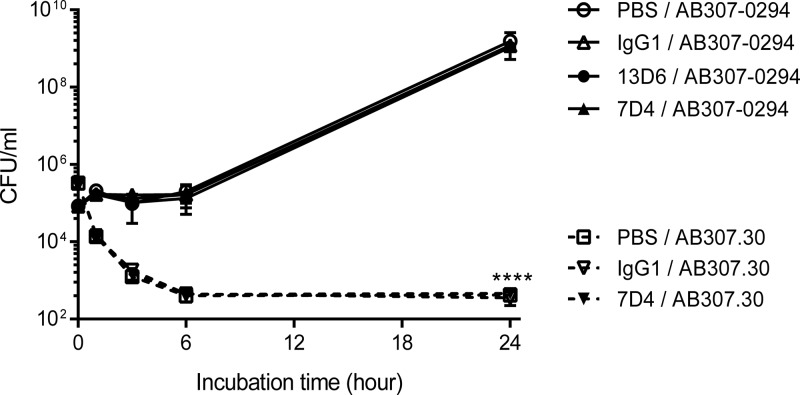
Capsular polysaccharide was protective against complement-mediated bactericidal activity in human ascites in the presence and absence of opsonization.
We hypothesized next that inhibition of antibody binding would prevent an increase in complement-mediated bactericidal activity, which was assessed in human ascites fluid because it contains active complement (22, 24). AB307-0294 was able to grow in human ascites and reached a plateau density of ∼1 × 109 CFU/ml after incubation for 24 h. Preincubation of AB307-0294 (wt; K1 capsule positive) with 7D4, anti-K1 capsule MAb 13D6, or an IgG1 isotype control did not affect the growth or survival of AB307-0294 in human ascites (Fig. 6). In contrast, AB307.30 (capsule negative) was killed so rapidly in the absence of anti-OmpA MAbs (95.8% and 99.9% of AB307.30 bacteria were killed in 1 and 3 h) that its role in enhancing complement-mediated bactericidal activity could not be assessed (Fig. 6). These results demonstrate the critical role of capsular polysaccharide in protecting against complement-mediated bactericidal activity. Further, the data also demonstrate that complement-mediated bactericidal activity is not increased when anti-OmpA MAb binding is inhibited by capsular polysaccharide. However, even when opsonization occurred using the anti-K1 MAb 13D6, capsule still protected against complement-mediated bactericidal activity.
FIG 6.
K1 capsule polysaccharide protected A. baumannii from complement-mediated bacterial killing in human ascites. AB307.30 (capsule negative) was rapidly killed in 100% human ascites fluid compared to its wild-type parent, AB307-0294. Preincubation with anti-OmpA MAb 7D4 did not enhance complement-mediated bactericidal activity against both AB307-0294 (wt; K1 capsule positive) and AB307.30 (capsule negative) in 100% human ascites fluid. Opsonization with anti-K1 capsule MAb 13D6 also did not augment the bactericidal activity of human ascites. n = 3. ****, P < 0.0001 (two-way ANOVA). The data are presented as means ± standard deviations.
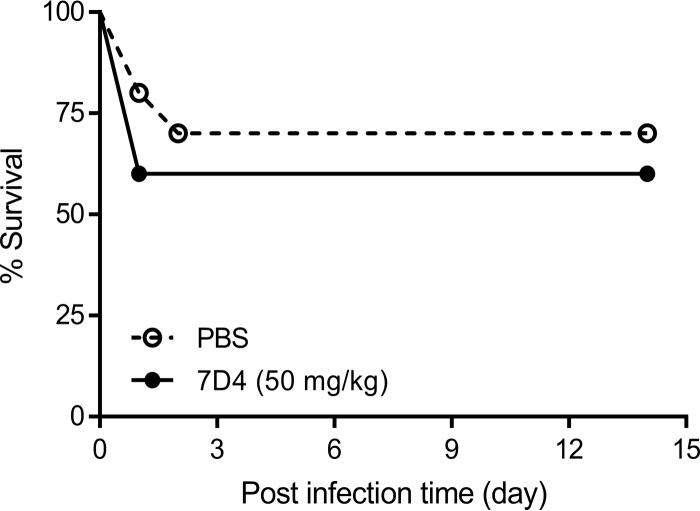
The anti-OmpA MAb 7D4 did not confer protection against challenge with AB307-0294 in the mouse subcutaneous infection model.
Passive immunization has been proposed as a potential therapeutic option in the management of A. baumannii infection. As proof of principal, potential protection of anti-OmpA MAbs against A. baumannii infection was investigated using a mouse subcutaneous infection model. The antibody dose was calculated, based on the binding affinity (Kd = 1.50 nM) (Table 1), to yield a plasma 7D4 concentration of >1,000 times the Kd value and hence ensure saturation of target binding. However, passive immunization with 50 mg 7D4/kg of body weight did not improve survival of AB307-0294-infected mice compared to the phosphate-buffered saline (PBS)-treated control group (Fig. 7). This was consistent with the lack of effect of 7D4 in enhancing macrophage- and complement-mediated bactericidal activity in vitro.
FIG 7.
Passive immunization with anti-OmpA MAb 7D4 did not provide protection against AB307-0294 (wt; K1 capsule positive) in a mouse sepsis infection model. BALB/c mice (n = 10 per group) were infected with 2.1 × 109 CFU AB307-0294 via subcutaneous injection and immediately treated intraperitoneally with 50 mg/kg 7D4 or 200 μl PBS. Survival data were analyzed using a log rank (Mantel-Cox) test.
DISCUSSION
A. baumannii has emerged as perhaps the most troubling pathogen globally due to the increasing prevalence of XDR strains and the paucity of antimicrobials active against A. baumannii in the drug development pipeline (25, 26). Passive immunizations with polyclonal antisera targeting OMPs of A. baumannii, such as OmpA, Omp22, and OMP complexes, have shown protection against clinical isolates in mouse sepsis models (9, 11, 12). These promising animal study results suggested a new therapeutic modality for the treatment of A. baumannii infections. However, anti-OMP MAbs, a clinically more applicable formulation than polyclonal antisera, have not been developed, and their potential antibacterial activities have not been investigated. In the present study, five anti-OmpA MAbs were generated and showed strong binding to rOmpA and A. baumannii ATCC 19606 (Fig. 1). However, negligible binding to clinical isolates, including carbapenem-resistant strains, was observed (Fig. 2 and Table 2). We demonstrated that the mechanism responsible for binding inhibition was due to capsular polysaccharide, since all the MAbs could bind to the isogenic capsule-negative derivative AB307.30 (Fig. 4B). Additionally, OmpA expression was determined in all studied A. baumannii strains, which excluded the possibility that lack of OmpA expression was responsible. Further, this inhibition had an important functional effect on a critical host innate defense mechanism. The inhibition of MAb binding to OmpA by capsular polysaccharide impeded macrophage-mediated bactericidal activity (Fig. 5). Interestingly, opsonization with MAbs in the presence of capsular polysaccharide did not affect complement-mediated bactericidal activity. This result is consistent with the work of Luo et al. showing that anti-OmpA sera could not overcome the innate resistance of A. baumannii strain HUMC1 to complement-mediated killing (9). Lastly, passive immunization with anti-OmpA MAbs did not confer protection against challenge with AB307-0294 in a mouse sepsis model.
The reason our results differ from those of previously published work is not completely clear; however, several plausible explanations exist. First, it is possible that the polyclonal anti-OmpA sera used for these studies contained antibodies directed to other epitopes. For example, it is possible that anti-capsular-polysaccharide antibodies were present. Capsule is an accessible target, and our work demonstrated that the anti-K1 capsule MAb 13D6 was able to enhance the phagocytic killing of AB307-0294 significantly (23). Second, polyclonal antibodies bound to different targets or OmpA epitopes may have resulted in synergistic protection. Preliminary data from our laboratory showed that anti-capsule MAb enhanced the binding of anti-OmpA MAb to AB307-0294. The synergism on binding may translate to synergistic bactericidal activities. In addition, chemically induced immunocompromised mice were used by both Luo et al. and Zhang et al. (9, 10), where much lower bacterial inocula were used for infection, and this may exaggerate the effect of antibodies. Further, strain differences may also be important. ATCC strain 17978 has been shown to produce a less dense and/or shorter capsule polysaccharide (27). This altered capsule structure may affect its ability to shield OmpA and inhibit antibody binding. A similar observation was made with ATCC 19606 in this study (Fig. 2A and B). Such observations emphasize the importance of using clinically relevant isolates for such studies (28).
The capsular polysaccharides produced by other pathogens have been previously shown to shield Gram-negative bacteria from host immune attack (29). For example, capsules of Streptococcus pneumoniae and extraintestinal pathogenic Escherichia coli have been shown to inhibit the binding of antisera, which were harvested from mice immunized with capsule-negative mutant stains, to their wild-type parent strains. As a result, antibody-dependent phagocytosis and complement-mediated killing of S. pneumoniae and E. coli were also inhibited by the capsule (17, 18). This is the first study that we are aware of that examined this role of capsule polysaccharide in A. baumannii. It remains unclear which other epitopes may be shielded by the capsular polysaccharide of A. baumannii. However, one of the appeals of OmpA as an immunization target was its high degree of conservation. Perhaps, in retrospect, antigenically conserved targets may suggest that they are being shielded and are not under selective pressure to develop antigenic variation, although support for this speculation awaits future studies. These data also support the notion that surface-exposed targets, such as the K1 capsule, should be considered as potential therapeutic targets for passive immunization. For empirical treatment, polyvalent antibody formulations that contain MAbs targeting the most prevalent capsular polysaccharides will be required to maximize the chances of efficacy. Alternatively, a capsule-specific MAb could be used if methods are developed to rapidly identify the capsular serotype of the infecting strain.
In summary, anti-OmpA MAbs were developed and showed binding to ATCC 19606 and the capsule-negative derivative AB307.30. However, the capsular polysaccharide of wild-type A. baumannii clinical isolates impeded binding of anti-OmpA MAbs, and as a result, macrophage-mediated bactericidal activity was not enhanced. In addition, anti-OmpA MAb alone was not able to confer protection against AB307-0294 infection in mice. These results suggest that OmpA, and possibly other OMPs of A. baumannii, is masked by capsule polysaccharide and hence may not be a viable therapeutic target for passive immunization.
MATERIALS AND METHODS
Bacterial strains and cell culture.
A. baumannii strain ATCC 19606 was provided by Brian Tsuji of the University at Buffalo. Strain AB307-0294 was isolated from a patient hospitalized at Erie County Medical Center (Buffalo, NY) (24), and its capsule-negative derivative, AB307.30, was developed as described previously (22). Clinical isolates 899, 985, and 955 were obtained from Walter Reed Medical Center (Bethesda, MD) and were provided by D. Craft; HUMC1 and HUMC8 were obtained from Harbor-UCLA Medical Center (Los Angeles, CA) and were provided by B. Spellberg (9). Capsule serotypes in A. baumannii have not been completely defined (21). Thus, capsular serotypes in the strains were designated based on the wzc classification developed in our laboratory (unpublished data), where capsular serotypes in AB307-0294, HUMC8 and 899, HUMC1 and ATCC 19606, 985, and 955 were designated K1, K2, K4, K11, and K16, respectively. Wild-type clinical isolates were grown in lysogeny broth (LB), and AB307.30 was grown in LB medium with 40 μg/ml of kanamycin. The procedure for obtaining human ascites was reviewed and approved by the Western New York Veterans Administration or the University at Buffalo-SUNY Institutional Review Board. Ascites was collected from deidentified patients who were undergoing therapeutic paracentesis for symptoms due to abdominal distension. These individuals were not being treated with antimicrobials. The ascites was cultured to confirm sterility, divided into aliquots, and stored at −80°C. Murine macrophage J774A.1 (ATCC TIB-67) cells were grown in Dulbecco's modified Eagle's medium (ATCC 30-2002) supplemented with 10% heat-inactivated fetal bovine serum for opsonophagocytosis assay.
Generation of anti-OmpA MAbs.
All animal studies were approved by and conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University at Buffalo. Monoclonal antibodies that bound specifically to OmpA of A. baumannii were developed as described previously (30). Briefly, female BALB/c mice were immunized with 3 μg rOmpA (MyBioSource) and Freund's incomplete adjuvant via subcutaneous injection every 3 weeks. Mouse splenocytes were isolated after the third immunization and fused with SP2/0-Ag14 (ATCC CRL-1581) cells to obtain hybridomas. Binding of antibodies to rOmpA was initially assessed using Western blotting and ELISA (31). In brief, 1 μg/ml of rOmpA in 20 mM Na2HPO4 was used as the coating agent, followed by incubation with hybridoma supernatants. Preimmune and postimmune mouse plasmas were used as negative and positive controls. Binding of antibodies to rOmpA was detected by 1,000×-diluted Fc-specific goat anti-mouse IgG-alkaline phosphatase conjugates (Thermo Fisher Scientific) and 4 mg/ml of the substrate p-nitrophenyl phosphate (Thermo Fisher Scientific). Changes of absorbance at 405 nm with time (dA/dt) were monitored using SpectraMax 340PC (Molecular Devices). Hybridomas that produced antibodies bound to rOmpA were cloned under a microscope and grown in serum-free medium. The MAbs were then purified using a protein G affinity column. Isotypes of the MAbs were determined using Pierce rapid isotyping kits (Thermo Fisher Scientific).
Equilibrium dissociation constants (Kd) of anti-OmpA MAbs.
Binding affinities of anti-OmpA MAbs were measured using rOmpA-based ELISA as described above. A wide range of anti-OmpA MAb concentrations, 0.001 to 1,000 nM, were used to generate complete binding profiles. To estimate Kd values, using GraphPad Prism, the results were fitted to a Michaelis-Menten function as follows: R = (Bmax × C)/(Kd + C), where Bmax is the binding capacity, C is the MAb concentration, and R is the ELISA value.
Cell-based ELISA for the detection of MAbs binding to A. baumannii.
A. baumannii strain ATCC 19606 was washed with PBS and then treated with 0.5% buffered formal saline overnight at 4°C. The bacteria were washed and resuspended at 107 CFU/ml in 20 mM Na2HPO4, which served as the coating agent in ELISA plates. Purified anti-OmpA MAbs, at a concentration of 200 nM, were then applied for binding assessment.
Flow cytometry.
Binding of anti-OmpA MAbs to live A. baumannii bacteria was assessed using flow cytometry as described previously (23). Briefly, 1 × 106 CFU of A. baumannii was incubated with 200 nM anti-OmpA MAbs in 100 μl of PBS at 37°C for 60 min. Samples were then washed with 1 ml of PBS. Binding of the MAbs to the bacteria was detected using 200×-diluted goat anti-mouse IgG–R-phycoerythrin (RPE) conjugates (Thermo Fisher Scientific) via incubation at 37°C for 30 min. Samples were washed and resuspended in 0.5 ml of PBS for assessment of binding using flow cytometry. Mouse IgG1 and IgG2a MAbs that bind Treponema denticola (developed in our laboratory) were used as isotype controls.
Extraction of outer membrane proteins.
OMPs were extracted from A. baumannii using a method described previously with modifications (32, 33). Briefly, A. baumannii was harvested from a 10-ml overnight culture via centrifugation at 7,000 × g for 15 min and washed with 10 mM HEPES, pH 7.4. The bacteria were resuspended in 2.0 ml of 10 mM HEPES containing 1.8 mg/ml lysozyme and incubated at 37°C for 60 min. The bacterial cells were then further disrupted by sonication (Vibra-Cell; Sonics & Materials, Inc.). The cells were pelleted and resuspended in 10 mM HEPES with 1% N-lauroylsarcosine and incubated for 60 min at room temperature. Insoluble cell debris was spun down, and the total protein concentration in the supernatant was determined by measuring the absorbance at 280 nm using a NanoDrop.
Opsonophagocytosis assay.
Antibody-dependent phagocytosis was performed as described previously with modifications (23). A. baumannii was opsonized with 100 nM anti-OmpA MAb 7D4, IgG1 isotype control, anti-K1 capsule MAb 13D6 (25), or equal volumes of PBS at 37°C for 60 min. The opsonized bacteria were then added to 24-well plates containing murine macrophage J774A.1 (5 × 105 cells/well) to give a multiplicity of infection (MOI) of 1:5 (bacteria to macrophages). The plates were centrifuged at 250 × g for 10 min and incubated at 37°C for 60 min. The macrophages were lysed with 0.5 mg/ml of saponin to release live intracellular bacteria at the end of the experiment. Total bacteria (i.e., bacteria in the supernatant, bacteria bound to the macrophage surface, and released intracellular bacteria) in each well were enumerated by CFU counting on LB agar plates. The results were expressed as percent survival of bacteria compared to the bacterial control in the absence of murine macrophages.
Growth/survival in human ascites.
Growth of strains AB307-0294 and AB307.30 in cell-free human ascites fluid were performed as described previously (22, 24). In brief, ∼1 × 105 CFU bacteria was incubated in 1 ml of 100% human ascites at 37°C. Aliquots (50 μl) of the samples were collected at 1, 3, 6, and 24 h, and the bacteria were enumerated by serial 10-fold dilutions and plating on LB agar plates. The classical pathway of complement-mediated bacterial killing was assessed by addition of 100 nM anti-OmpA MAb 7D4, anti-K1 capsule MAb 13D6, IgG1 isotype control, or equal volumes of PBS to human ascites fluid.
Mouse subcutaneous infection model.
Animal studies were reviewed and approved by the University at Buffalo and Veterans Administration IACUC, in compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals. Male BALB/c mice (8 weeks old) were anesthetized with isoflurane and subcutaneously challenged with ∼1 × 109 CFU AB307-0294 or 200 μl PBS. 7D4 (50 mg/kg) or 200 μl PBS was injected intraperitoneally immediately following bacterial inoculation. Survival of mice was monitored daily for 14 days.
Statistical analyses.
Flow cytometry data were analyzed using FlowJo (FlowJo, LLC). All other graphs and data were analyzed using GraphPad Prism 7.00 (GraphPad Software, Inc.), and the data are presented as means ± standard deviations. The results of opsonophagocytosis assays were analyzed using Student's t test. One-way analysis of variance (ANOVA) followed by Dunnett's multiple-comparison test was performed to analyze ELISA results, and two-way ANOVA was used for the analysis of bacterial survival in human ascites. Survival of mice was analyzed using a log rank (Mantel-Cox) test.
ACKNOWLEDGMENTS
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (T.A.R.), and through funding provided by the Center of Protein Therapeutics (J.P.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Michalopoulos A, Falagas ME. 2010. Treatment of Acinetobacter infections. Expert Opin Pharmacother 11:779–788. doi: 10.1517/14656561003596350. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Rex JH. 2013. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov 12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.March GA, Bratos MA. 2015. A meta-analysis of in vitro antibiotic synergy against Acinetobacter baumannii. J Microbiol Methods 119:31–36. doi: 10.1016/j.mimet.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Manchanda V, Sanchaita S, Singh N. 2010. Multidrug resistant acinetobacter. J Glob Infect Dis 2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlaes DM, Sahm D, Opiela C, Spellberg B. 2013. The FDA reboot of antibiotic development. Antimicrob Agents Chemother 57:4605–4607. doi: 10.1128/AAC.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard A, O'Donoghue M, Feeney A, Sleator RD. 2012. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Park WH. 1931. Serum therapy. Bull N Y Acad Med 7:401–411. [PMC free article] [PubMed] [Google Scholar]
- 9.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Doi Y, Adams MD, Russo TA, Spellberg B. 2012. Active and passive immunization protects against lethal, extreme drug-resistant Acinetobacter baumannii infection. PLoS One 7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Yang T, Cao J, Sun J, Dai W, Zhang L. 2016. Mucosal immunization with purified OmpA elicited protective immunity against infections caused by multidrug-resistant Acinetobacter baumannii. Microb Pathog 96:20–25. doi: 10.1016/j.micpath.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Yao Y, Wang S, Xia Y, Yang X, Long Q, Sun W, Liu C, Li Y, Chu X, Bai H, Yao Y, Ma Y. 2016. Immunization with a 22-kDa outer membrane protein elicits protective immunity to multidrug-resistant Acinetobacter baumannii. Sci Rep 6:20724. doi: 10.1038/srep20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell MJ, Dominguez-Herrera J, Smani Y, Lopez-Rojas R, Docobo-Perez F, Pachon J. 2011. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect Immun 79:518–526. doi: 10.1128/IAI.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall A. 1996. Antibody-based therapies for emerging infectious diseases. Emerg Infect Dis 2:200–208. doi: 10.3201/eid0203.960306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felton LD. 1928. The units of protective antibody in antipneumococcus serum and antibody solution. J Infect Dis 43:531–542. doi: 10.1093/infdis/43.6.531. [DOI] [Google Scholar]
- 15.Weisman LE, Cruess DF, Fischer GW. 1994. Opsonic activity of commercially available standard intravenous immunoglobulin preparations. 13:1122–1125. [DOI] [PubMed] [Google Scholar]
- 16.Slade HB. 1994. Human immunoglobulins for intravenous use and hepatitis C viral transmission. Clin Diagn Lab Immunol 1:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun 78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo TA, Beanan JM, Olson R, MacDonald U, Cope JJ. 2009. Capsular polysaccharide and the O-specific antigen impede antibody binding: a potential obstacle for the successful development of an extraintestinal pathogenic Escherichia coli vaccine. Vaccine 27:388–395. doi: 10.1016/j.vaccine.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 19.Pluschke G, Mayden J, Achtman M, Levine RP. 1983. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun 42:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Ley P, Kuipers O, Tommassen J, Lugtenberg B. 1986. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb Pathog 1:43–49. doi: 10.1016/0882-4010(86)90030-6. [DOI] [PubMed] [Google Scholar]
- 21.Kenyon JJ, Hall RM. 2013. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS One 8:e62160. doi: 10.1371/journal.pone.0062160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun 78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo TA, Beanan JM, Olson R, MacDonald U, Cox AD, St Michael F, Vinogradov EV, Spellberg B, Luke-Marshall NR, Campagnari AA. 2013. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun 81:915–922. doi: 10.1128/IAI.01184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo TA, MacDonald U, Beanan JM, Olson R, MacDonald IJ, Sauberan SL, Luke NR, Schultz LW, Umland TC. 2009. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J Infect Dis 199:513–521. doi: 10.1086/596317. [DOI] [PubMed] [Google Scholar]
- 25.Hesterkamp T. 2016. Antibiotics clinical development and pipeline. Curr Top Microbiol Immunol 398:447–474. doi: 10.1007/82_2015_451. [DOI] [PubMed] [Google Scholar]
- 26.Butler MS, Blaskovich MA, Cooper MA. 2017. Antibiotics in the clinical pipeline at the end of 2015. J Antibiot 70:3–24. doi: 10.1038/ja.2016.72. [DOI] [PubMed] [Google Scholar]
- 27.Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwodo UU, Green E, Okoh AI. 2012. Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13:14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennett RH. 1979. Cell fusion. Methods Enzymol 58:345–359. doi: 10.1016/S0076-6879(79)58149-X. [DOI] [PubMed] [Google Scholar]
- 31.Tayab ZR, Balthasar JP. 2004. Development and validation of enzyme-linked immunosorbent assays for quantification of anti-methotrexate IgG and Fab in mouse and rat plasma. J Immunoassay Immunochem 25:335–344. doi: 10.1081/IAS-200033830. [DOI] [PubMed] [Google Scholar]
- 32.Cuenca FF, Pascual A, Martinez Marinez L, Conejo MC, Perea EJ. 2003. Evaluation of SDS-polyacrylamide gel systems for the study of outer membrane protein profiles of clinical strains of Acinetobacter baumannii. J Basic Microbiol 43:194–201. doi: 10.1002/jobm.200390022. [DOI] [PubMed] [Google Scholar]
- 33.Hobb RI, Fields JA, Burns CM, Thompson SA. 2009. Evaluation of procedures for outer membrane isolation from Campylobacter jejuni. Microbiology 155:979–988. doi: 10.1099/mic.0.024539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]