ABSTRACT
In multicellular organisms, autophagy is induced as an innate defense mechanism. Notably, the obligate intracellular bacterium Ehrlichia chaffeensis resides in early endosome-like vacuoles and circumvents lysosomal fusion through an unknown mechanism, thereby avoiding destruction in the autophagolysosome. In this report, we reveal that Wnt signaling plays a crucial role in inhibition of lysosomal fusion and autolysosomal destruction of ehrlichiae. During early infection, autophagosomes fuse with ehrlichial vacuoles to form an amphisome indicated by the presence of autophagy markers such as LC3 (microtubule-associated protein 1 light chain 3), Beclin-1, and p62. LC3 colocalized with ehrlichial morulae on days 1, 2, and 3 postinfection, and increased LC3II levels were detected during infection, reaching a maximal level on day 3. Ehrlichial vacuoles did not colocalize with the lysosomal marker LAMP2, and lysosomes were redistributed and dramatically reduced in level in the infected cells. An inhibitor specific for the Wnt receptor signaling component Dishevelled induced lysosomal fusion with ehrlichial inclusions corresponding to p62 degradation and promoted transcription factor EB (TFEB) nuclear localization. E. chaffeensis infection activated the phosphatidylinositol 3-kinase (PI3K)–Akt–mTOR (mechanistic target of rapamycin) pathway, and activation was induced by three ehrlichial tandem repeat protein (TRP) effectors, with TRP120 inducing the strongest activation. Moreover, induction of glycogen synthase kinase-3 (GSK3) performed using a Wnt inhibitor and small interfering RNA (siRNA) knockdown of critical components of PI3K-GSK3-mTOR signaling decreased ehrlichial survival. This report reveals Ehrlichia exploitation of the evolutionarily conserved Wnt pathway to inhibit autolysosome generation, thereby leading to evasion of this important innate immune defense mechanism.
KEYWORDS: Ehrlichia chaffeensis, Wnt, autophagy, lysosome
INTRODUCTION
Ehrlichia chaffeensis is an obligately intracellular Gram-negative bacterium that preferentially infects mononuclear phagocytes (1, 2) and causes the emerging life-threatening tick-borne zoonosis human monocytotropic ehrlichiosis (HME). HME manifests as a disease with moderate to high severity, with meningitis, acute respiratory distress syndrome, and multisystem organ failure common in many fatal cases (3, 4). In order to survive intracellularly and evade innate defense mechanisms of the mononuclear phagocyte, Ehrlichia spp. have evolved sophisticated molecular strategies to reprogram the host cell defenses. Mononuclear phagocytes are abundant in lysosomes, and a key survival strategy of E. chaffeensis involves inhibition of phagolysosomal fusion associated with the autophagic process. The mechanisms whereby E. chaffeensis inhibits autophagy are not well understood; however, ehrlichial type 1 secreted tandem repeat protein (TRP) effectors activate Wnt and Notch signaling pathways (5, 6) that are associated with autophagy regulation (7, 8).
In eukaryotes, autophagy is a conserved, highly regulated cellular degradation pathway that sequesters and digests intracellular components, including invading pathogens (9, 10). Autophagy is a primary innate host defense mechanism against intracellular pathogens such as Rickettsia conorii, Salmonella enterica servovar Typhimurium, Listeria monocytogenes, Shigella, and Mycobacterium tuberculosis (11–15). In contrast, induction of autophagy benefits some intracellular bacteria such as Francisella, Brucella, and Coxiella through nutrient acquisition or by allowing cell-to-cell spread (16–18). The autophagy process involves membrane nucleation, elongation, and formation of a unique double-membrane vacuole called the autophagosome. Autophagosomes can fuse directly with lysosomes to form an autolysosome or can fuse with endosomes, creating a single-membrane amphisome that eventually fuses with lysosomes to form an autolysosome (19). Autophagy is normally induced as an innate immune response to pathogens, but this process is inhibited during Ehrlichia infection. A role for the functional two-component system in inhibition of autophagosome/lysosome fusion during ehrlichial infection has been reported (20), but the mechanistic details are unknown.
Recently, E. chaffeensis type IV secretion effector protein Etf-1 has been shown to interact with Rab5, phosphatidylinositol 3-kinase C3 (PI3KC3), and Beclin-1 and to induce autophagosome formation proximally to ehrlichial inclusions for nutrient acquisition. However, LC3 was not detected on E. chaffeensis inclusions, suggesting that fusion with the autophagophore does not occur; and yet, ehrlichial vacuoles resemble amphisomes in other ways (21). Moreover, the related rickettsial pathogen Anaplasma phagocytophilum also uses its type IV secretion effector, Ats-1, to initiate autophagy by binding Beclin-1 to activate class III PI3K, which, in contrast to the results seen with Ehrlichia, leads to membrane nucleation and autophagosome formation and fusion with the Anaplasma vacuole. The effector-driven autophagosome formation seen with both Ehrlichia and Anaplasma appears to bypass normal starvation signal-induced mTOR (mechanistic target of rapamycin) inhibition (21–23). Nevertheless, the mechanisms whereby lysosomal fusion is inhibited by Ehrlichia are unknown.
The central inhibitor of autophagy is the serine/threonine protein kinase mTOR. Under nutrient-deprived conditions, mTOR phosphorylates ULK1 (mammalian homolog of Atg1) and Atg13, inhibiting ULK1 complex coupling, which is the first step in autophagophore formation. Various host components such as LC3, Beclin-1, and p62 are involved in the autophagic pathway. Mammalian protein p62 (SQSTM1) directly binds to both ubiquitinated targets via its ubiquitin-associated domain (UBA) and to LC3 via the LIR domain, which links it to the autophagy machinery for degradation (24, 25). Beclin-1 is a component of the class III phosphatidylinositol 3-kinase (PtdIns3K) complex (PtdIns3K Vps34, Beclin-1/Vps30, and Atg14), which is required for nucleation and assembly of the initial phagophore membrane (26–29). Two ubiquitin-like systems acting at the Atg5-Atg12 conjugation step where conversion of cytosolic LC3I to membrane-bound phosphatidylethanolamine (PE)-conjugated LC3II occurs are critical in the autophagy process (19, 30). Maturation of the autophagosome involves fusion with early and late endosomes, which requires small G protein Rab5, Rab7 (GTP bound state), and presenilin protein, followed by lysosomal fusion (31, 32).
A major player in regulation of lysosomal biogenesis is transcription factor EB (TFEB), which regulates expression of multiple genes encoding lysosomal enzymes, including the vacuolar H+-ATPase (v-ATPase) complex that participates in lysosomal acidification and membrane proteins that mediate the interaction of the lysosome with other cellular compartments (33–35). When nutrients are present, nuclear translocation and the activity of TFEB are inhibited by mTOR-mediated phosphorylation (36). mTOR is tightly regulated by several signal transduction pathways such as the Wnt and phosphoinositide 3-kinase (PI3K)/ATP dependent tyrosine kinase (Akt) signaling pathways. mTOR is activated downstream of Akt and PI3K kinases and growth factor receptor signaling and acts to inhibit autophagy under these growth-promoting conditions (37). The underlying signaling involves inactivation of glycogen synthase kinase-3 (GSK3) by phosphorylation and regulation of tuberous sclerosis complex 2 (TSC2), two important negative regulators of mTOR (37). TSC2 is a GTPase-activating protein (GAP) for Rheb, a Ras family GTPase and an mTOR activator (38, 39). The conditional ability to perform Akt-mediated GSK3 phosphorylation depends on activation of Wnt signaling (40). Therefore, the Wnt pathway plays an essential role in inhibition of autophagy by regulating activation of the mTOR pathway (7, 41–43). Recently, we showed that host canonical and noncanonical Wnt pathways are activated by E. chaffeensis TRP effectors to facilitate ehrlichial entry and promote survival (6). However, the role of the Wnt pathway in inhibition of autophagy as a mechanism of ehrlichial survival has not been examined.
Here, we report that lysosomal fusion and autophagic degradation are inhibited by Ehrlichia-induced Wnt pathway activation. This report provides insight into this mechanism and demonstrates that the Wnt and PI3K/Akt pathway and mTOR activation, which inhibits TFEB nuclear localization and autolysosome generation, are central to this reprogramming strategy. We further show a primary role of E. chaffeensis TRP effectors in reprogramming these signal transduction pathways. Exploitation of Wnt-PI3K-mTOR signaling is an important strategy that enables Ehrlichia to evade host innate immune defenses.
RESULTS
E. chaffeensis induces autophagosome formation but inhibits lysosomal fusion and autophagic degradation.
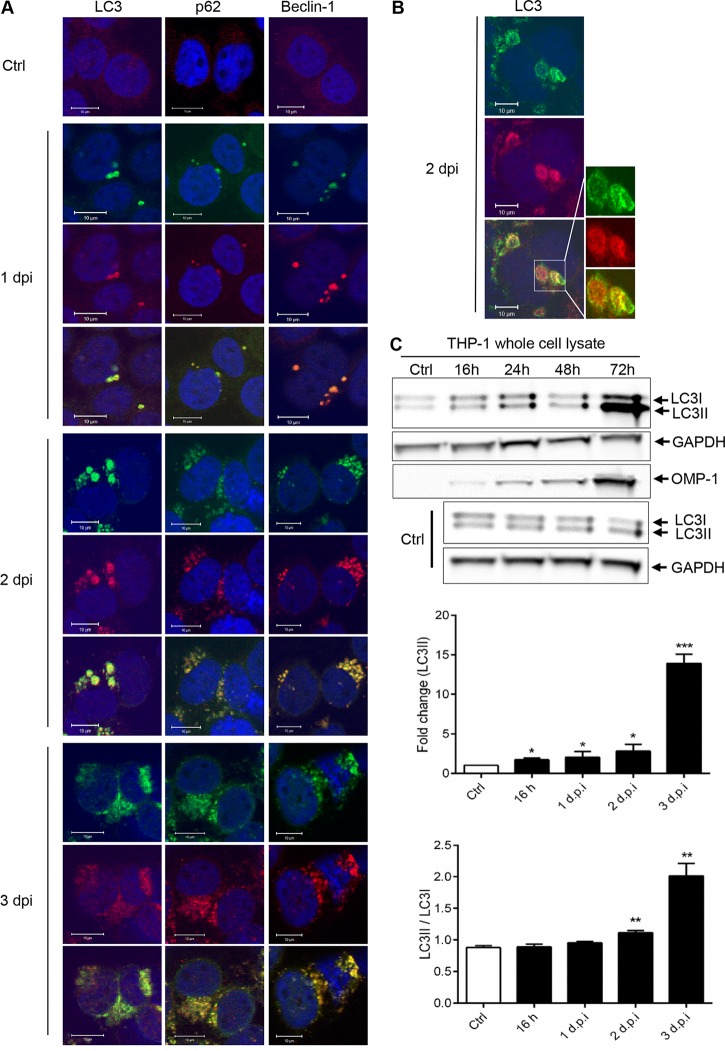
To understand the process of autophagy, the distribution and localization of the autophagosome in E. chaffeensis-infected THP-1 cells (at 1 to 3 days postinfection [dpi]) were analyzed by confocal immunofluorescence microscopy using anti-LC3, anti-p62, anti-Beclin-1, and anti-E. chaffeensis antibodies. Unlike the diffuse distribution of LC3 (cytosolic LC3) in control THP-1 cells, colocalization of ehrlichial vacuole with the autophagosome markers LC3, p62/SQSTM1, and Beclin-1 was observed (Fig. 1A), suggesting that the autophagosomes fused with the ehrlichial inclusions to form amphisomes. By confocal microscopy, LC3 was observed inside the morulae and associated with the morula membrane (Fig. 1B). LC3 was also examined in E. chaffeensis-infected RF/6A cells used in a previous study (21), and colocalization was again observed (see Fig. S1 in the supplemental material). During induction of autophagy, cytosolic microtubule-associated protein light chain 3 (LC3I), encoded by the mammalian homologue of Atg8, is conjugated to the carboxyl glycine of PE to generate the membrane-associated lipidated form, LC3II (44, 45), a key indicator of autophagophore formation (44). Therefore, conversion of LC3I to LC3II was examined by immunoblot analysis of E. chaffeensis-infected and uninfected THP-1 cells. Increasing amounts of low-level LC3II conversion were detected at 16, 24, and 48 h before they reached a maximal level at 72 h postinfection (hpi; Fig. 1C). The significantly increased LC3II levels observed at 72 hpi compared to 24 and 48 hpi suggest the possibility of additional induction of canonical autophagy due to nutrient depletion. These findings demonstrate that E. chaffeensis vacuoles colocalize with LC3 and that LC3II conversion occurs at some level throughout the infection.
FIG 1.
E. chaffeensis vacuole colocalization with the autophagosome components. (A) Uninfected and E. chaffeensis-infected THP-1 cells (1, 2, and 3 dpi) were probed with polyclonal anti-E. chaffeensis antibody (green), anti-LC3 antibody (red; left panels), anti-p62 antibody (red; center panels), and anti-Beclin-1 antibody (red; right panels). Colocalization of ehrlichial vacuole with LC3, p62, and Beclin-1 was observed. Cells were visualized by confocal microscopy (×40; bars, 10 μm). Cell nuclei were stained with DAPI (blue). Ctrl, control. (B) High (×100) magnification of E. chaffeensis morula showing localization of LC3 inside and associated with morula membrane. Cell nuclei were stained with DAPI (blue). (C) Immunoblot performed using anti-LC3I, LC3II, and OMP-1 antibodies from uninfected and E. chaffeensis-infected THP-1 cells (16, 24, 48, and 72 hpi). Data corresponding to fold change in LC3II levels and ratio of LC3I/LC3II and OMP-1 are normalized to GAPDH. Data are represented as means ± SD (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [Student's t test]; n = 3).
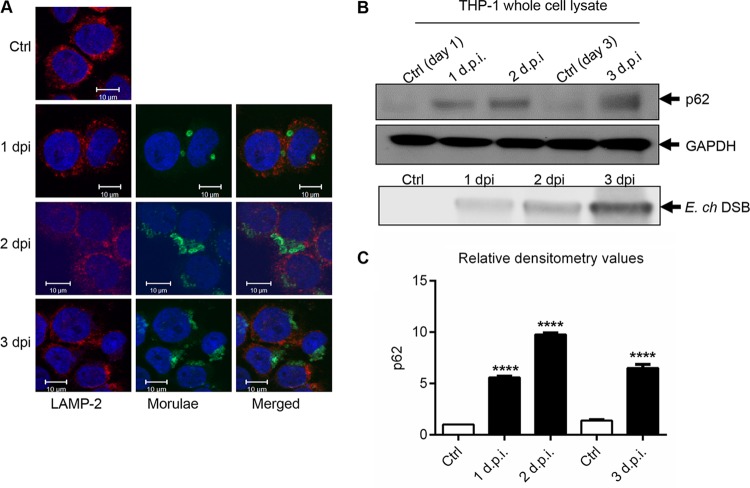
Since autophagy formation leads to destruction of invading bacterial pathogens in autolysosomes, we examined whether E. chaffeensis vacuoles colocalizing with LC3 were undergoing lysosomal fusion by examining the distribution and colocalization of lysosomal marker LAMP-2 with the ehrlichial vacuoles. Two lysosomal membrane-associated proteins, LAMP-1 and LAMP-2, are essential for autolysosomal fusion during the autophagic process (35, 46). Similarly to a previous study (47), colocalization of the ehrlichial vacuole with the lysosomal marker LAMP-2 was not detected (Fig. 2A). Ubiquitin-binding scaffold protein p62 binds directly to both LC3 and ubiquitinated proteins and serves as a marker of degradation. To understand the autophagic process during infection, autophagic degradation was further analyzed by measuring the level of p62 protein in uninfected and E. chaffeensis-infected cells. An increased level of p62 was detected in E. chaffeensis-infected cells compared to control cells (Fig. 2B). Densitometry data generated by the use of ImageJ software and normalized with the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) showed significant differences in the levels of p62 in E. chaffeensis-infected cells compared to uninfected cells (Fig. 2C). Collectively, these data not only support the conclusion that LC3, p62, and Beclin-1 participate in autophagy pathway and associate with E. chaffeensis vacuoles but also suggest that E. chaffeensis infection induces autophagosome formation while inhibiting lysosomal fusion and autophagic degradation.
FIG 2.
E. chaffeensis inhibits phagolysosomal fusion and autophagic degradation. (A) E. chaffeensis-infected or uninfected THP-1 cells (24 h) were fixed, permeabilized, and probed with polyclonal anti-E. chaffeensis antibody (green) and anti-LAMP2 antibody (red). Cells were visualized by confocal microscopy (×40; bars, 10 μm). Cell nuclei were stained with DAPI (blue). (B) The level of p62 was analyzed by Western immunoblotting in uninfected and E. chaffeensis (E. ch)-infected THP-1 cells (1, 2, and 3 dpi). Cell lysates were used to quantify E. chaffeensis infection based on levels of Dsb protein. (C) Relative band intensities of p62 normalized to the loading control (GAPDH) were determined using ImageJ software (****, P < 0.0001 [Student's t test]; n = 3).
E. chaffeensis-mediated inhibition of autolysosome formation and autophagic degradation depends on the Wnt signaling pathway.
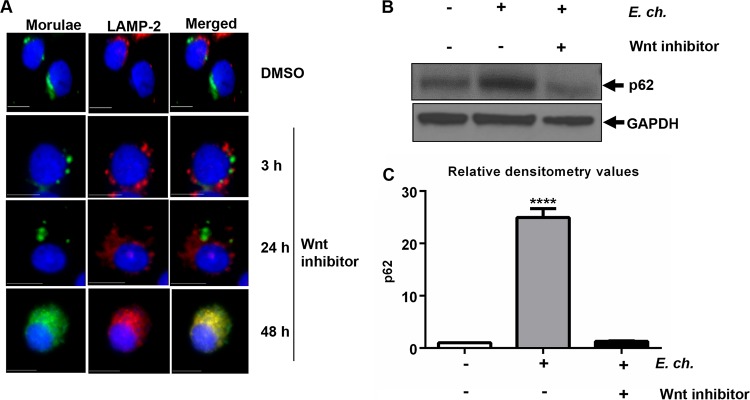
The Wnt pathway regulates autophagy and exhibits cross talk with other pathways, such as the Notch pathway, which also regulate autophagy (7, 8). Manipulation and reprogramming of host signaling are prerequisites for ehrlichial survival (5, 6, 48), and we recently demonstrated that E. chaffeensis-activated host Wnt cell signaling is involved (6). Since recent studies have shown that Wnt signaling plays a critical role in regulation of autophagy (7, 41, 49), we sought to determine the role of E. chaffeensis-mediated activation of Wnt signaling in inhibition of autophagy. Localization of lysosomes and the E. chaffeensis vacuole was examined in infected THP-1 cells treated with Wnt inhibitor. Immunofluorescence microscopy demonstrated that lysosomes are considerably reduced in level and are redistributed in E. chaffeensis-infected cells and that the ehrlichial vacuoles do not fuse with lysosomes in the presence of dimethyl sulfoxide (DMSO; vehicle) (Fig. 3A). In contrast, lysosomal fusion with ehrlichial vacuoles was observed when cells were treated with a Wnt inhibitor (Dvl-PDZ domain inhibitor; catalog no. 3289-8625) for 48 h (50) (Fig. 3A). Degradation of p62 during E. chaffeensis infection was further analyzed in the presence of the Wnt inhibitor. In comparison to the E. chaffeensis-infected control cells, the Wnt inhibitor-treated cells showed decreased level of p62 (Fig. 3B), suggesting induction of autophagic degradation in the absence of Wnt signaling. Densitometry data showed significant differences between the levels of p62 in E. chaffeensis-infected cells and in uninfected and inhibitor-treated cells (Fig. 3C). Collectively, these results demonstrate that activation of Wnt signaling by E. chaffeensis facilitates inhibition of lysosome fusion during infection.
FIG 3.
E. chaffeensis-mediated inhibition of phagolysosome fusion and p62 degradation depends on the Wnt signaling pathway. E. chaffeensis-infected THP-1 cells (24 h) were treated with either DMSO or Wnt inhibitor (3289-8625; 30 μM). (A) Cells were harvested at 3, 24, or 48 h posttreatment, fixed, permeabilized, and probed with polyclonal anti-E. chaffeensis (green) and anti-LAMP2 (red) antibodies. Colocalization of ehrlichial vacuole with lysosome (LAMP-2) was observed at 48 h posttreatment with Wnt inhibitor. Cells were visualized by immunofluorescence microscopy (×40; bars, 10 μm). Cell nuclei were stained with DAPI (blue). (B) Immunoblotting of cell lysates from infected or uninfected THP-1 cells (24 h) after treatment with Wnt inhibitor for 48 h. Membranes were probed with antibodies against p62 and GAPDH to measure lysosomal degradation. Data are representative of results from n = 4 experiments. (C) Relative band intensities of p62 normalized to the loading control (GAPDH) were determined using ImageJ software (****, P < 0.0001 [Student's t test]; n = 3).
E. chaffeensis-mediated Wnt activation regulates mTOR signaling and TFEB nuclear localization during infection.
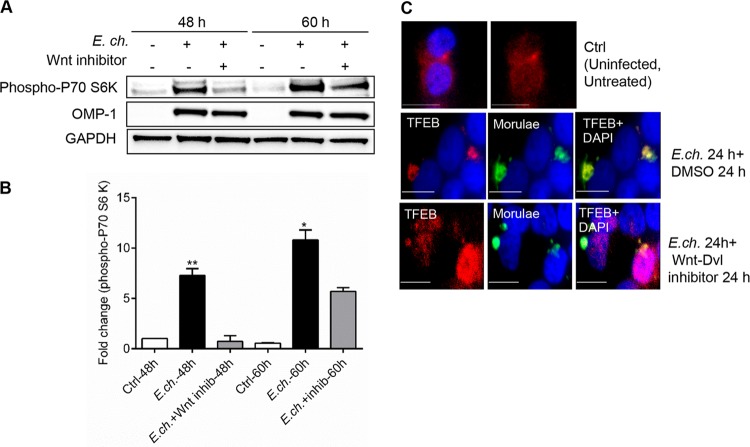
Autophagy can be activated by a wide range of signals, but the central regulator of autophagy is mTOR kinase (51–53). Since Wnt signaling has been shown to regulate mTOR activation and autophagy, the role of Wnt signaling in the activation of mTOR during E. chaffeensis infection was investigated by analyzing the level of phospho-p70 S6 kinase, a key downstream target of mTORC1 (54). THP-1 cells were infected with E. chaffeensis and treated with Wnt inhibitor, and the level of phospho-p70 S6 kinase was analyzed at different times posttreatment. A substantial induction of the phospho-p70 S6 kinase level was observed during E. chaffeensis infection, beginning at 2 dpi and continuing thereafter, compared to the control; however, induction was not observed in infected cells when Wnt signaling was inhibited (Fig. 4A). Thus, E. chaffeensis was unable to activate mTOR in the absence of Wnt signaling. Activation of mTOR was not observed at 1 dpi (data not shown). Western immunoblot densitometry results show a quantitative comparison of the levels of phopho-p70 S6 kinase (Fig. 4B).
FIG 4.
E. chaffeensis regulates mTOR activation and TFEB subcellular localization through the Wnt signaling pathway. THP-1 cells were infected with E. chaffeensis (24 h) and treated with either vehicle (DMSO) or Wnt inhibitor (3289-8625; 30 μM). (A) Immunoblot analysis was done using anti-phospho-p70 S6 kinase, anti-OMP-1, and anti-GAPDH antibody (48 and 60 hpi). (B) Relative band intensities of phospho-p70 S6 kinase and OMP-1 have been normalized to the loading control GAPDH and were determined using ImageJ software (*, P < 0.05; **, P < 0.01 [Student's t test]; n = 3). inhib, inhibitor. (C) Cells probed with polyclonal anti-E. chaffeensis antibody (green) and anti-TFEB (red) antibody. Cells were visualized by immunofluorescence microscopy (×40; bars, 10 μm). Cell nuclei were stained with DAPI (blue).
mTOR regulates the phosphorylation and retention of the transcription factor TFEB in the cytoplasm (36). TFEB plays an important role in autophagy since lysosomal biogenesis and function are regulated by this transcription factor (34). To demonstrate that Wnt-induced activation of mTOR results in inhibition of TFEB nuclear recruitment, cells were infected with E. chaffeensis and treated with Wnt inhibitor. As shown in Fig. 4C, during infection, TFEB was recruited to the E. chaffeensis inclusion. However, in the absence of Wnt signaling, nuclear translocation of TFEB was observed in more than 80% of cells. Collectively, these data support the idea of a critical role for Wnt signaling in mTOR activation and regulation of TFEB nuclear localization during Ehrlichia infection.
E. chaffeensis activates the PI3K/Akt pathway and modulates critical autophagic regulators GSK3, PTEN, and TSC2.
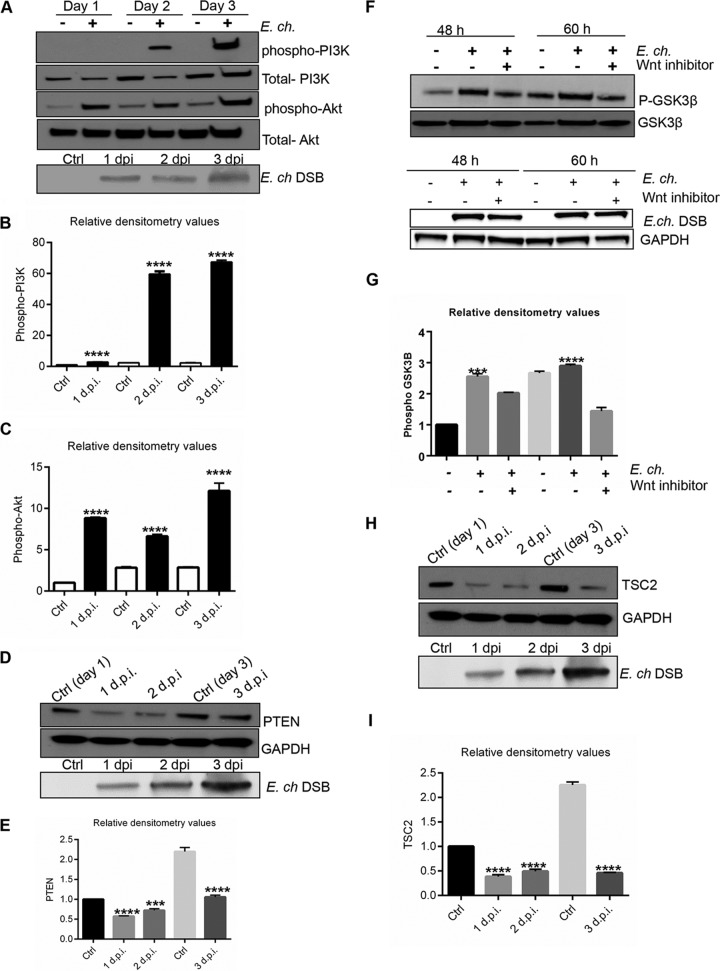
Since mTOR is regulated by the canonical PI3K/Akt pathway, we further analyzed the activation of this pathway by measuring the phosphorylated and total levels of PI3K and Akt in response to E. chaffeensis infection. The level of phosphorylated PI3K increased beginning at 2 dpi (Fig. 5A), indicating activation of PI3K signaling during infection. We analyzed phosphorylation of serine/threonine kinase Akt, the signaling molecule downstream of PI3K, usng an Akt (Ser473) phosphorylation-specific antibody to evaluate Akt activity. Compared to uninfected control cells, the level of phosphorylated Akt was substantially increased in E. chaffeensis-infected cells (Fig. 5A). Densitometry analysis confirmed significant activation of PI3K and Akt signaling during E. chaffeensis infection (Fig. 5B and C). These data demonstrated that E. chaffeensis is able to induce the PI3K/Akt phosphorylation and that this pathway is activated during infection. The tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a potent antagonist of PI3K/Akt signaling (55). Thus, PTEN protein levels were determined in E. chaffeensis-infected cells at different time points (1, 2, and 3 dpi). Immunoblotting analysis revealed that E. chaffeensis infection caused decreased PTEN expression in THP-1 cells (Fig. 5D and E).
FIG 5.
Activation of PI3K-Akt pathway and Wnt-dependent inhibition of GSK3 during E. chaffeensis infection. (A to C) Western blot analysis of control and E. chaffeensis-infected cells to determine phospho-PI3K (Tyr 458), total PI3K, phospho-Akt (Ser 473), and total Akt levels. E. chaffeensis infection was confirmed by detecting ehrlichial DSB protein. (D and E) PTEN levels were determined and quantified relative to GAPDH (1, 2, and 3 days pi). (F and G) THP-1 cells were infected with E. chaffeensis (24 h) and treated with either vehicle (DMSO) or Wnt inhibitor (3289-8625; 30 μM). Immunoblot analysis was performed to determine phospho-GSK3β and total GSK3 levels (48 and 60 h pi), and the results were quantified relative to GAPDH. (H and I) TSC2 levels were determined in E. chaffeensis-infected and uninfected controls by Western immunoblotting, and the results were quantified relative to GAPDH. Data are representative of results from n = 4 experiments. Quantitative analysis of the Western blot data was performed using Image J software (***, P < 0.001; ****, P < 0.0001 [Student's t test]; n = 3).
GSK3, the regulator of β-catenin levels in the cells, is inhibited by activation of canonical Wnt signaling (56). To investigate the role of Wnt signaling in regulation of GSK3 during ehrlichial infection, cells were treated with Wnt inhibitor and phosphorylated and total levels of GSK3-β were analyzed. Increased levels of phosphorylated GSK3-β were detected in E. chaffeensis-infected cells compared to controls. However, a decreased level of phospho-GSK3-β was detected in the cells that were treated with Wnt inhibitor (Fig. 5F and G). GSK3 is a critical downstream element of the PI3K/Akt pathway, and it regulates mTOR by inducing the negative regulator TSC2 (tuberous sclerosis complex). Therefore, the level of TSC2 was analyzed in E. chaffeensis-infected cells at 1, 2, and 3 dpi. Immunoblot analysis performed with anti-TSC2 demonstrated decreased levels of TSC2 in E. chaffeensis-infected cells compared to uninfected cells (Fig. 5H and I). These studies demonstrate that E. chaffeensis infection activates the PI3K/Akt pathway, phosphorylates and inactivates GSK3, and inhibits TSC2. Moreover, our results suggest that GSK3 serves to integrate PI3K/Akt and Wnt signals in the induction of the mTOR pathway during E. chaffeensis infection.
Wnt-GSK3-mTOR signaling is required for E. chaffeensis survival.
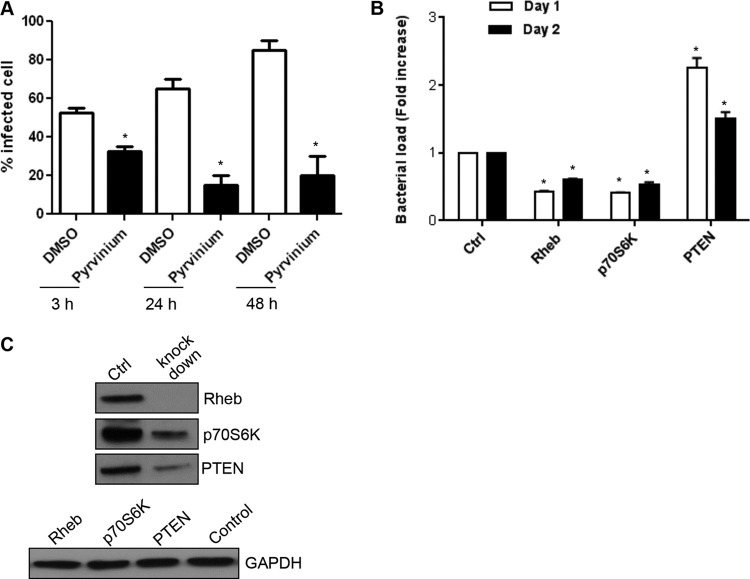
Using Wnt and PI3K/Akt inhibitors and small interfering RNAs (siRNAs), we previously demonstrated that these pathways are important for ehrlichial survival (6). In order to understand the role of the downstream molecule GSK3 in ehrlichial survival, we treated the E. chaffeensis-infected cells with an Akt inhibitor and GSK3 inducer pyrvinium and determined the percentage of infected cells by Diff-Quik staining and visualization of morulae by light microscopy. A significant decrease in infected cells was observed at 3, 24, and 48 h posttreatment (Fig. 6A). At 24 h posttreatment, the proportion of E. chaffeensis-infected cells was >70% in the presence of DMSO, and only 15% were infected after pyrvinium treatment. At 48 h, >80% of cells were infected, but only 20% of the pyrvinium-treated cells were infected (Fig. 6A). We further confirmed the role of host mTOR signaling in ehrlichial infection by RNA interference analysis performed by examining the role of Rheb because it is an important component of mTOR signaling. Rheb is an mTOR activator, and the presence of p70 S6 kinase is a hallmark of mTOR activation (38). siRNA knockdown of both Rheb and p70 S6 kinase decreased E. chaffeensis infection significantly (Fig. 6B). A significant increase in ehrlichial infection was observed in cells where the negative regulator, PTEN, was knocked down (Fig. 6B). Protein expression of Rheb, p70 S6 kinase, and PTEN was reduced in siRNA-transfected cells (Fig. 6C). These findings demonstrated that E. chaffeensis exploits conserved Wnt signaling to regulate mTOR activation and inhibits canonical autophagosome generation to promote intracellular survival.
FIG 6.
The GSK3/PI3K/mTOR pathway plays an important role in E. chaffeensis survival. (A) THP-1 cells were infected with E. chaffeensis (MOI of 50). Bacterial loads were determined at 3, 24, and 48 h posttreatment with pyrvinium by measuring the percentage of infected cells by counting 100 Diff-Quik-stained cells. Data represent means ± SD (n = 3) (P < 0.05). (B) THP-1 cells were transfected with specific or control siRNA to knock down Rheb/p70S6K/PTEN and were then infected with E. chaffeensis (1 day posttransfection). Ehrlichial infection was determined using qPCR measurement of the dsb copy numbers at 24 and 48 h pi. Data are represented as means ± SD (*, P < 0.05, n = 4). (C) Western immunoblot to confirm reduction of Rheb, p70 S6K, and PTEN protein levels after siRNA transfection.
TRP effectors modulate autophagic signal transduction pathways.
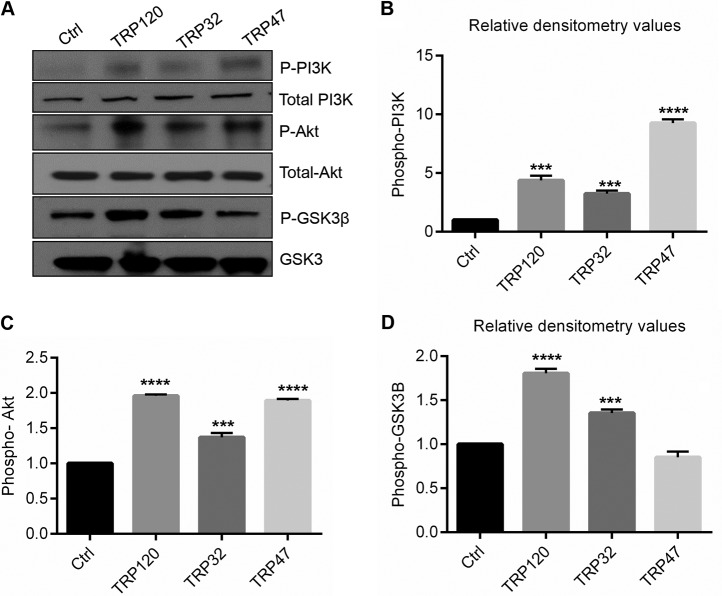
We determined that E. chaffeensis activates the Wnt signaling pathway and reprograms PI3K/Akt and the downstream mTOR pathway as a mechanism for survival. We have demonstrated that Wnt signaling is activated by TRPs (6); thus, we investigated the regulation of signaling molecules downstream of the Wnt pathway and the effect on mTOR activation in response to TRP120, TRP32, and TRP47. THP-1 cells were stimulated with TRPs (1 μg/ml), and activation of cell signaling molecules such as PI3K, Akt, and GSK3 was analyzed 24 h poststimulation. The PI3K/Akt pathway was activated by TRP stimulation as demonstrated by increased phosphorylation levels of PI3K and Akt compared to the results seen with thioredoxin-stimulated cells (Fig. 7A). Total PI3K and Akt protein levels remained unchanged in both groups. TRP120 and TRP32 stimulated increases in the levels of phospho-GSK3β; however, TRP47 did not induce an increase (Fig. 7A). Densitometry data showed a significant increase in phospho-PI3K (Fig. 7B), phospho-Akt (Fig. 7C), and phospho-GSK3β (Fig. 7D) levels during TRP stimulation. These results indicate that E. chaffeensis TRP effectors activate PI3K/Akt pathways and inhibit GSK3 activity by phosphorylation.
FIG 7.
Activation of PI3K/Akt pathway and inactivation of GSK3 by E. chaffeensis TRPs. (A) THP-1 cells were treated with thioredoxin (control) or TRP120, TRP32, or TRP47 in suspension (1 μg/ml) for 24 h. Data represent results of immunoblot analysis of levels of phospho-PI3K, total PI3K, phospho-Akt, total Akt, phospho-GSK3β, and GSK3. Data are representative of results from n = 4 experiments. (B to D) Quantitative analysis of the Western blot data was performed using Image J software (****, P < 0.0001; ***, P < 0.001 [Student's t test]; n = 3).
DISCUSSION
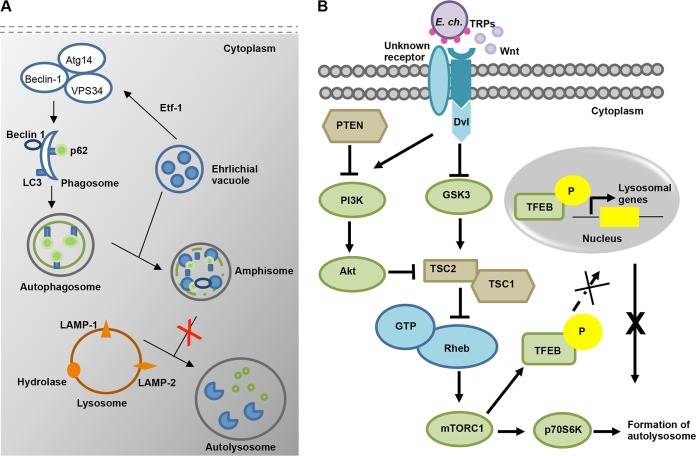
Autophagy has emerged as an innate immune response pathway targeting intracellular bacteria in the cytosol. However, increasing evidence suggests that these pathogens have evolved strategies to inhibit autolysosome generation. While some bacteria such as S. Typhimurium and M. tuberculosis inhibit autophagy induction by inhibiting signaling pathways (57–59), others block downstream events involved in autophagosome-lysosome fusion (60). Shigella and Listeria mask themselves with host proteins to inhibit autophagic recognition (14, 61, 62). An A. phagocytophilum effector protein, Ats-1, hijacks the Beclin-1-class III PI3K autophagy initiation pathway to induce autophagosome formation that fuses with the Anaplasma vacuole to deliver nutrients for growth (22, 23). Notably, a recent study also reported that an E. chaffeensis-secreted effector, Etf-1, induces autophagosome formation through interaction with Beclin-1 and PIK3C3 complexes, which favors ehrlichial growth through nutrient acquisition (21). However, in contrast to the results seen with A. phagocytophilum, the autophagosome marker LC3 was not detected on the E. chaffeensis vacuole. The present study demonstrated that E. chaffeensis vacuoles have characteristics of autophagosomes, including LC3; however, lysosomal fusion and autolysosome generation are inhibited, as illustrated in Fig. 8A. We further demonstrated that E. chaffeensis uses TRP effector proteins to exploit evolutionarily conserved Wnt and PI3K/Akt signaling pathways to modulate downstream signal transduction events to activate mTOR signaling and to regulate TFEB nuclear localization as a mechanism to inhibit autolysosome generation and autophagic destruction (Fig. 8B). This is the first study to provide a mechanistic explanation of E. chaffeensis inhibition of lysosomal fusion and, ultimately, destruction in the autolysosome, which has been a long-standing enigma in the field.
FIG 8.
(A) Proposed model for the autophagy process during E. chaffeensis infection. E. chaffeensis initiates induction of autophagosome formation through Etf-1 effector-driven interaction with Beclin-1 and activation of the class III phosphatidylinositol 3-kinase complex. Fusion of the ehrlichial vacuole with an autophagosome leads to formation of amphisome. However, it inhibits autolysosome formation through reprogramming of host cell signaling pathways. (B) Proposed model for E. chaffeensis TRP-mediated activation of the canonical Wnt-PI3K-mTOR signaling pathway and inhibition of autolysosome formation and autophagic degradation. E. chaffeensis TRP effectors activate canonical and noncanonical Wnt and PI3K/Akt pathways, which results in phosphorylation and inactivation of GSK3 and in inhibition of the negative regulator TSC2. Thus, mTORC1 is activated and then phosphorylates and inhibits nuclear translocation of TFEB. Exploitation of this signal cascade by E. chaffeensis plays a critical role in inhibition of autolysosome formation. The components which were not tested in this study are colored blue.
Increased levels of LC3II relative to LC3I are considered a hallmark of autophagy (19, 63). Ehrlichial vacuoles were LC3 positive during infection (days 1 to 3), suggesting that autophagosome nucleation is induced by the ehrichial effector Etf-1 as previously described and that the resulting autophagosome fuses with the ehrlichial vacuole to form an amphisome (fusion of endosome with autophagosome). A recent study did not detect LC3 punctate structures associated with the ehrlichial vacuole in RF/6A cells but did detect GFP-LC3 and endogenous LC3B puncta adjacent to the vacuole (21). Moreover, conversion of LC3I to LC3II was detected only on day 3 postinfection by immunoblotting, and this conversion was attributed to canonical autophagy starvation pathway induction (21). To further reconcile the differences in LC3 colocalization observed in our study, we performed LC3 staining on both THP-1 and RF/6A E. chaffeensis-infected cells and observed strong colocalization of LC3 with the ehrlichial vacuole in both cell lines. Moreover, we confirmed these results with two different anti-LC3 antibodies. In addition, several phenotypic hallmarks of autophagosomes, including colocalization of Beclin-1 and p62 with E. chaffeensis vacuoles, were also observed. Although the reasons for the differences in LC3 colocalization with ehrlichial inclusions in our study from the results seen in the previous report are unclear, the LC3 staining and the level of LC3II conversion that we report here are consistent with results previously reported to occur during A. phagocytophilum infection (22, 23). These findings suggest that, like Anaplasma inclusions, Ehrlichia inclusions fuse with proximally located effector nucleated autophagosomes, resulting in inclusions displaying major autophagy proteins.
To further understand the autophagic process as it relates to E. chaffeensis infection, localization of lysosomes in the infected cells was determined. Consistent with previous reports (21, 47), colocalization of E. chaffeensis vacuoles with the lysosomal marker LAMP-2 was not observed, suggesting that autolysosome generation is inhibited. Similarly to reports from studies performed with A. phagocytophilum, E. chaffeensis appears to induce autophagosome formation through noncanonical mechanisms involving effector protein Etf-1 with the Beclin-1 complex as a mechanism of nutrient acquisition (21).
Different signal transduction pathways and cross talk among them are important for regulation of any host cellular processes such as apoptosis, necrosis, or autophagy. Though autophagy can regulate Wnt signaling by degrading β-catenin (64), Wnt signaling has also been associated with inhibition of autophagy in different cancers (41, 49, 65, 66). Recently, we reported that E. chaffeensis activates both canonical and noncanonical Wnt signaling pathways and that inhibition of these pathways is detrimental for ehrlichial survival (6). Here, using a Wnt inhibitor, we further demonstrated that Wnt activation leads to inhibition of autolysosomal fusion and p62 degradation. These findings are significant because the involvement of Wnt signaling in regulation of autophagy during bacterial infection has never been reported. Thus, these findings offer new insights into pathogen exploitation of a conserved cellular pathway in order to regulate autophagy and promote survival.
Autophagy can negatively regulate the Wnt pathway through degradation of the Wnt pathway component Dishevelled (67). However, previous studies reported that Wnt-dependent activation of mTOR also regulates autophagy (7, 41). Thus, in order to understand the mechanism of ehrlichial inhibition of autophagy, and the role of the Wnt pathway in such inhibition, mTOR activity in E. chaffeensis-infected cells was analyzed by measuring the level of phospho-p70 S6 kinase. Substantial activation of mTOR after 2 dpi and dependence of this activation on Wnt pathway were observed. These data further support the conclusion that the induction of autophagosome formation is independent of mTOR and mediated by Etf-1. Activation of Wnt signaling by E. chaffeensis prevents canonical autophagy in response to the presence of a pathogen through mTOR activation to inhibit lysosome biosynthesis and fusion with the ehrlichial inclusion.
In eukaryotes, lysosomal gene transcription is coordinated to respond to cellular needs and regulated by TFEB, a basic helix-loop-helix (bHLH) leucine zipper transcription factor of the Myc family (68). TFEB binds and targets the coordinated lysosomal expression and regulation (CLEAR) motif in the promoter region which regulates lysosomal biogenesis and function (34). A recent study showed that mTOR-dependent phosphorylation of TFEB on Ser 211 results in retention of the transcription factor in the cytoplasm (36). To investigate the relationship between Wnt-mediated mTOR activation and autophagy, the localization of TFEB in E. chaffeensis-infected cells was determined. Our data demonstrated that TFEB retention in the cytoplasm during E. chaffeensis infection is regulated by Wnt activation. A key concept that emerged from this study is that E. chaffeensis activates Wnt signaling and regulates mTOR activation and TFEB nuclear localization to inhibit autophagy. Similarly to E. chaffeensis, S. Typhimurium has been shown to escape autophagy by promoting mTORC1 activation, but the bacterial factor responsible for this virulence strategy is unknown (58).
Phosphorylation and activation of PI3K increases phosphatidylinositol-3,4,5-triphosphate (PIP3) levels, resulting in recruitment and phosphorylation of Akt. The canonical PI3K/Akt pathway known to activate mTOR was also activated in this study. Active Akt phosphorylates an array of proteins involved in regulation of cellular processes such as proliferation, survival, growth, apoptosis, autophagy, and metabolism (37, 69–71). Thus, this finding is very significant as these Akt targets can be used to study the mechanisms of some of the cellular processes which have been shown to be regulated by E. chaffeensis. PI3K/Akt signaling is regulated by both the Wnt pathway and the tumor suppressor protein PTEN, which serve as a positive regulator and a negative regulator, respectively (71–74). PTEN blocks the action of PI3K by dephosphorylating signal lipid PIP3 and also serves as an inducer of autophagy. Activation of Wnt signaling during E. chaffeensis infection and inhibition of PTEN expression were shown here, demonstrating how these cell survival pathways are tightly regulated during E. chaffeensis infection (6). The mechanism underlying the decreased level of PTEN can be explained by the activation of the Notch pathway and by increased expression of hes1 during E. chaffeensis infection (5, 42, 71).
To explore the signaling mechanisms that couple the Wnt and PI3K/Akt pathways to downstream components of the mTOR pathway, known regulators of mTOR such as GSK3 and TSC2 were investigated. Wnt signaling and PI3K/Akt signaling are required for phosphorylation and inhibition of GSK3 activity (37, 40, 75). E. chaffeensis was shown to induce phospho-GSK3β levels in a Wnt-dependent manner, and a decreased level of TSC2 was also observed during the infection. The importance of TSC1/TSC2 in regulation of cell growth has been well established (76). The major cellular function of TSC1/TSC2 is to inhibit the phosphorylation of p70 S6 kinase, through Rheb and mTOR (38, 77). Inhibition of Rheb by TSC1/TSC2 is critical for the function of these tumor suppressor proteins because Rheb potently regulates the phosphorylation of p70 S6 kinase. These findings shed some light on the mechanism of mTOR activation during E. chaffeensis infection. We previously used RNA interference and pharmacological inhibitors to demonstrate that the Wnt and PI3K pathways are required for E. chaffeensis survival (6). Here, using GSK3 inducer pyrvinium and siRNA specific for PTEN, Rheb, and p70 S6 kinase, we further demonstrated that activation of the mTOR pathway is required for E. chaffeensis survival. However, we did not use 3-methyladenine (MA) or rapamycin in our study since both of these inhibitors/inducers have off-target effects. 3-MA has been shown to have differential temporal effects on class I and class III PI3K (78).
TRP effectors play major roles in ehrlichial immune evasion through regulation of host gene expression and direct interactions with host proteins. Additionally, they have been shown to activate conserved cell signaling pathways such as Wnt and Notch (5, 6). Thus, we hypothesized that the downstream signaling molecules of these pathways are activated by TRPs. Our data demonstrated that TRP120 and TRP47 activated the PI3K/Akt pathway more efficiently than TRP32 and that both TRP32 and TRP120 were able to induce phosphorylation of GSK3β. These findings suggest that different TRPs play distinct roles in activation and inhibition of different cell signaling components. This conclusion is consistent with our previous findings demonstrating that TRP120 is an agonist of the Wnt pathway (6).
The present study revealed that E. chaffeensis activates the Wnt pathway to suppress canonical autophagy in order to prevent destruction in autolysosomes. The findings reported here provide a significant advancement in understanding of the molecular mechanisms involved in E. chaffeensis-mediated inhibition of autolysosome formation through regulation of Wnt-PI3K-mTOR signaling. The identification of these signal transduction pathways provides a new therapeutic target for HME. In addition, we have gained considerable knowledge of ehrlichial modulation of signal transduction pathways, such as that of PI3K/Akt, which regulate not only autophagy but also other cellular processes. This knowledge may be applicable to other intracellular bacterial infections where these pathways are modulated by the pathogen as a survival mechanism.
MATERIALS AND METHODS
Cell culture and cultivation of E. chaffeensis.
Human monocytic leukemia cells (THP-1; ATCC TIB-202) were propagated in RPMI medium 1640 with l-glutamine, 1 mM sodium pyruvate (Sigma, St. Louis, MO), 25 mM HEPES buffer (Invitrogen), 2.5 g/liter d-(+)-glucose (Sigma), and 10% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere. Rhesus monkey retina endothelial cells (RF/6A; ATCC CRL-1780RF/6A) were propagated in minimal essential medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). E. chaffeensis (Arkansas strain) was cultivated in THP-1 as previously described (79).
Antibodies and inhibitors.
A convalescent-phase anti-E. chaffeensis dog serum which was derived from an experimentally infected dog was previously described (80). The polyclonal mouse anti-TRP120 antibody used in this study was previously described (81). Other antibodies that were used for the indirect fluorescent-antibody assay (IFA) study include anti-mitogen-activated protein (anti-MAP) LC3α/β (H-47) antibody, anti-p62/SQSTM1 (H-290) antibody, anti-Beclin-1 (E8) (Santa Cruz Biotechnology) antibody, anti-LAMP-2 (H4B4), Developmental Studies Hybridoma Bank (University of Iowa) antibody, and anti-TFEB (Cell Signaling Technology) antibody. Anti-LC3A/B (D3U4C) antibody, anti-SQSTM1p62 (D5E2) antibody, anti-phospho-p70 S6 kinase (Thr389) (108D2) antibody, anti-phospho-Akt (Ser473; D9E) antibody, anti-Akt (pan, C67E7) antibody, anti-phospho-PI3K p85 (Tyr458)/p55 (Tyr 199) antibody, anti-PI3Kinase p85 (19H8) antibody, anti-Tuberin/TSC2 antibody, anti-PTEN antibody, anti-TFEB antibody, anti-Rheb antibody, anti-phospho-GSK3β antibody, anti-GSK3 (Cell Signaling Technology) antibody, anti-MAP1LC3B (catalog no. NB100-2220; NovusBio) antibody, and anti-GAPDH (clone 6C5; EMD Millipore) antibody were used for Western blotting. For inhibition of the Wnt pathway, Dvl inhibitor (catalog no. 3289-8625; Calbiochem) (30 μM) was used. Pyrvinium (Sigma) (20 nM) was used to inhibit Akt and induce GSK3.
siRNAs and transfection.
To knock down cell signaling components, THP-1 cells (1 × 105/well on a 96-well plate) were transfected with informational RNA (iRNA) for PTEN, Rheb, and S6K1 using Lipofectamine 2000 reagent (Life Technologies, CA) according to the manufacturer's instructions with a cocktail of 5 pM iRNA or a negative-control iRNA (Sigma-Aldrich, Germany).
Confocal and immunofluorescence microscopy.
Uninfected and E. chaffeensis-infected (1 dpi) THP-1 cells in the presence or absence of Wnt inhibitor (Dvl inhibitor 3289-8625; 30 μM) were cytospun onto glass slides, fixed for 15 min using 3% paraformaldehyde–phosphate-buffered saline (PBS), and blocked and permeabilized for 30 min using 0.3% Triton X-100–2% bovine serum albumin (BSA)–PBS at room temperature (RT). Cells were then incubated with primary antibodies dog anti-E. chaffeensis serum antibody (1:100), anti-LC3 antibody (1:100), mouse anti-p62 antibody (1:100), mouse anti-Beclin-1 antibody (1:100), mouse anti-LAMP2 antibody (1:50), and rabbit anti-TFEB antibody (1:50) for 1 h, washed, and incubated with Alexa Fluor 488 IgG (H+L) and Alexa Fluor 568 IgG (H+L) secondary antibodies (Molecular Probes) (1:100) for 30 min. Slides were mounted with ProLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen) after washing. Confocal laser micrographs were obtained with a model 510 Meta confocal laser scanning microscope (LSM) and analyzed with LSM Meta software (version 4.0), and immunofluorescence images were obtained using an Olympus BX61 epifluorescence microscope and analyzed using Slidebook software (ver. 5.0; Intelligent Imaging Innovations, Denver, CO).
Pharmacological inhibitor treatment and determination of bacterial load.
THP-1 cells were infected with E. chaffeensis at a multiplicity of infection (MOI) of 50 and treated with pyrvinium (20 nM) or DMSO at 1 dpi. At 3, 24, and 48 h posttreatment, cells were collected and infection was determined by calculating the percentage of infected cells present after Diff-Quick staining. Cells from an infected culture grown without the inhibitors and uninfected cells were used as positive and negative controls, respectively. To confirm that host cell death did not represent decreased levels of ehrlichial inclusions, differences in cell viability were assessed at 1, 2, and 3 dpi using trypan blue staining.
Stimulation of THP-1 cells with TRPs.
Recombinant E. chaffeensis (thioredoxin-fused) TRP120, TRP32, and TRP47 were expressed and purified as described previously (81–83). THP-1 cells were seeded in 6-well plates and then treated with either TRPs or thioredoxin (control) suspended in RPMI media to obtain a final concentration of 1 μg/ml. Cells were collected after 24 h of stimulation.
Western immunoblotting.
Approximately 20 μg (10 μg for LC3) of total cell lysate was prepared as previously described (84), separated by dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membrane using a semidry transfer apparatus. Immunoblotting was performed with primary antibodies, including rabbit anti-LC3, rabbit anti-p62, rabbit anti-p70 S6 kinase, rabbit anti-TSC2, rabbit anti-phospho-PI3K, rabbit anti-PI3K, rabbit anti-phospho-Akt, rabbit anti-Akt (pan), rabbit anti-TFEB, rabbit anti-Rheb, rabbit anti-PTEN, rabbit anti-phospho-GSK3β, rabbit anti-GSK3, and mouse anti-GAPDH antibodies. Horseradish peroxidase-labeled goat anti-rabbit or mouse IgG (heavy-chain and light-chain) secondary conjugate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) and ECL or SuperSignal West Dura chemiluminescent substrate were used for detection (Thermo Scientific). Quantitative analysis of the Western blot data was done using Image J software. The levels of target proteins were normalized with the housekeeping protein GAPDH.
Quantification of E. chaffeensis by qPCR.
THP-1 cells were washed with PBS, lysed in SideStep lysis and stabilization buffer (Agilent, Santa Clara, CA) for 30 min at room temperature, and analyzed for bacterial load using real-time quantitative PCR (qPCR) amplification of the integral ehrlichial gene dsb. Amplification was performed using Brilliant II SYBR green Mastermix (Agilent), 200 nM forward primer (5′-GCTGCTCCACCAATAAATGTATCCCT-3′), and 200 nM reverse primer (5′-GTTTCATTAGCCAAGAATTCCGACACT-3′). The absolute E. chaffeensis dsb copy number in the cells was determined against the standard curve, or the fold change of dsb copy number relative to the control was normalized to qPCR-detected levels of the host genomic glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene as previously described (85). The qPCR thermal cycling protocol (denaturation at 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 58°C for 1 min, and 72°C for 1 min) was performed on an EP Realplex2 S Mastercycler (Eppendorf).
Statistics.
The results were expressed as means ± standard deviations (SD) of data obtained from at least three independent experiments performed in triplicate, unless otherwise indicated. Differences between means were evaluated by using the two-tailed Student t test. A P value of 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI105536 and AI106859, by a University of Texas Medical Branch Jeane B. Kempner postdoctoral fellowship to Taslima T. Lina, and by funding from the Clayton Foundation for Research.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00690-17.
REFERENCES
- 1.Paddock CD, Childs JE. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev 16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paddock CD, Sumner JW, Shore GM, Bartley DC, Elie RC, McQuade JG, Martin CR, Goldsmith CS, Childs JE. 1997. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol 35:2496–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paparone PW, Ljubich P, Rosman GA, Nazha NT. 1995. Ehrlichiosis with pancytopenia and ARDS. N J Med 92:381–385. [PubMed] [Google Scholar]
- 4.Patel RG, Byrd MA. 1999. Near fatal acute respiratory distress syndrome in a patient with human ehrlichiosis. South Med J 92:333–335. doi: 10.1097/00007611-199903000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Lina TT, Dunphy PS, Luo T, McBride JW. 2016. Ehrlichia chaffeensis TRP120 activates canonical Notch signaling to downregulate TLR2/4 expression and promote intracellular survival. mBio 7:e00672-16. doi: 10.1128/mBio.00672-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo T, Dunphy PS, Lina TT, McBride JW. 2015. Ehrlichia chaffeensis exploits canonical and noncanonical host Wnt signaling pathways to stimulate phagocytosis and promote intracellular survival. Infect Immun 84:686–700. doi: 10.1128/IAI.01289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petherick KJ, Williams AC, Lane JD, Ordonez-Moran P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik K, Paraskeva C, Greenhough A. 2013. Autolysosomal beta-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J 32:1903–1916. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian JJ, Li JJ, Chen F, Wu HH, Han LX, Lu SH, Zheng YZ, Han ZC. 2015. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem 36:1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 9.Cuervo AM. 2004. Autophagy: in sickness and in health. Trends Cell Biol 14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Levine B, Klionsky DJ. 2004. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 11.Walker DH, Popov VL, Crocquet-Valdes PA, Welsh CJ, Feng HM. 1997. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab Invest 76:129–138. [PubMed] [Google Scholar]
- 12.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Kageyama S, Omori H, Saitoh T, Sone T, Guan JL, Akira S, Imamoto F, Noda T, Yoshimori T. 2011. The LC3 recruitment mechanism is separate from Atg9L1-dependent membrane formation in the autophagic response against Salmonella. Mol Biol Cell 22:2290–2300. doi: 10.1091/mbc.E10-11-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, Mimuro H, Nakagawa I, Yanagawa T, Ishii T, Kakizuka A, Sztul E, Chakraborty T, Sasakawa C. 2009. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol 11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 15.Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT, van der Goot FG, Sansonetti PJ, Lafont F. 2009. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe 6:137–149. doi: 10.1016/j.chom.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. 2005. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol 7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 17.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele S, Brunton J, Ziehr B, Taft-Benz S, Moorman N, Kawula T. 2013. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog 9:e1003562. doi: 10.1371/journal.ppat.1003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N. 2007. Autophagy: process and function. Genes Dev 21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z, Kumagai Y, Lin M, Zhang C, Rikihisa Y. 2006. Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cell Microbiol 8:1241–1252. doi: 10.1111/j.1462-5822.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin M, Liu H, Xiong Q, Niu H, Cheng Z, Yamamoto A, Rikihisa Y. 2016. Ehrlichia secretes Etf-1 to induce autophagy and capture nutrients for its growth through RAB5 and class III phosphatidylinositol 3-kinase. Autophagy 12:2145–2166. doi: 10.1080/15548627.2016.1217369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu H, Yamaguchi M, Rikihisa Y. 2008. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell Microbiol 10:593–605. doi: 10.1111/j.1462-5822.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 23.Niu H, Xiong Q, Yamamoto A, Hayashi-Nishino M, Rikihisa Y. 2012. Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc Natl Acad Sci U S A 109:20800–20807. doi: 10.1073/pnas.1218674109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. 2008. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A 105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. 2007. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 26.Itakura E, Kishi C, Inoue K, Mizushima N. 2008. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kihara A, Noda T, Ishihara N, Ohsumi Y. 2001. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. 2008. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A 105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkin V, McEwan DG, Novak I, Dikic I. 2009. A role for ubiquitin in selective autophagy. Mol Cell 34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez MG, Munafo DB, Beron W, Colombo MI. 2004. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 32.Jäger S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. 2004. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 33.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. 2011. TFEB links autophagy to lysosomal biogenesis. Science 332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. 2009. A gene network regulating lysosomal biogenesis and function. Science 325:473–477. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri M, Impey S, Kang H, di Ronza A, Pelz C, Sardiello M, Ballabio A. 2011. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet 20:3852–3866. doi: 10.1093/hmg/ddr306. [DOI] [PubMed] [Google Scholar]
- 36.Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. 2012. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manning BD, Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoki K, Li Y, Xu T, Guan KL. 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF. 1994. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem 269:16333–16339. [PubMed] [Google Scholar]
- 40.Ma T, Tzavaras N, Tsokas P, Landau EM, Blitzer RD. 2011. Synaptic stimulation of mTOR is mediated by Wnt signaling and regulation of glycogen synthetase kinase-3. J Neurosci 31:17537–17546. doi: 10.1523/JNEUROSCI.4761-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, Zhu J, Mi M. 2014. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One 9:e102535. doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailis W, Pear WS. 2012. Notch and PI3K: how is the road traveled? Blood 120:1349–1350. doi: 10.1182/blood-2012-06-435099. [DOI] [PubMed] [Google Scholar]
- 43.Lapierre LR, Gelino S, Melendez A, Hansen M. 2011. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol 21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. 2000. A ubiquitin-like system mediates protein lipidation. Nature 408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 46.Eskelinen EL. 2006. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502. doi: 10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Y, Liu Y, Wu B, Zhang JZ, Gu J, Liao YL, Wang FK, Mao XH, Yu XJ. 2014. Proteomic analysis of the Ehrlichia chaffeensis phagosome in cultured DH82 cells. PLoS One 9:e88461. doi: 10.1371/journal.pone.0088461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin M, Rikihisa Y. 2004. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU1 and inhibits lipopolysaccharide activation of NF-kappa B, ERK 1/2 and p38 MAPK in host monocytes. Cell Microbiol 6:175–186. doi: 10.1046/j.1462-5822.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Cui W, Liu B, Hu H, Liu J, Xie R, Yang X, Gu G, Zhang J, Zheng H. 2014. Atorvastatin protects vascular smooth muscle cells from TGF-beta1-stimulated calcification by inducing autophagy via suppression of the beta-catenin pathway. Cell Physiol Biochem 33:129–141. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- 50.Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ. 2009. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem 284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J. 2011. Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noda T, Ohsumi Y. 1998. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 54.Dann SG, Selvaraj A, Thomas G. 2007. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29–39. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 56.Logan CY, Nusse R. 2004. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 57.Shahnazari S, Namolovan A, Mogridge J, Kim PK, Brumell JH. 2011. Bacterial toxins can inhibit host cell autophagy through cAMP generation. Autophagy 7:957–965. doi: 10.4161/auto.7.9.16435. [DOI] [PubMed] [Google Scholar]
- 58.Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE. 2012. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11:563–575. doi: 10.1016/j.chom.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, Lee SH, Lee ZW, Cho SN, Kim JM, Friedman RL, Jo EK. 2010. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog 6:e1001230. doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chargui A, Cesaro A, Mimouna S, Fareh M, Brest P, Naquet P, Darfeuille-Michaud A, Hebuterne X, Mograbi B, Vouret-Craviari V, Hofman P. 2012. Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death. PLoS One 7:e51727. doi: 10.1371/journal.pone.0051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. 2005. Escape of intracellular Shigella from autophagy. Science 307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 62.Dortet L, Mostowy S, Samba-Louaka A, Gouin E, Nahori MA, Wiemer EA, Dussurget O, Cossart P. 2011. Recruitment of the major vault protein by InlK: a Listeria monocytogenes strategy to avoid autophagy. PLoS Pathog 7:e1002168. doi: 10.1371/journal.ppat.1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirkegaard K, Taylor MP, Jackson WT. 2004. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2:301–314. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jia Z, Wang J, Wang W, Tian Y, XiangWei W, Chen P, Ma K, Zhou C. 2014. Autophagy eliminates cytoplasmic beta-catenin and NICD to promote the cardiac differentiation of P19CL6 cells. Cell Signal 26:2299–2305. doi: 10.1016/j.cellsig.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 65.Lin R, Feng J, Dong S, Pan R, Zhuang H, Ding Z. 2015. Regulation of autophagy of prostate cancer cells by beta-catenin signaling. Cell Physiol Biochem 35:926–932. doi: 10.1159/000369749. [DOI] [PubMed] [Google Scholar]
- 66.Chang HW, Lee YS, Nam HY, Han MW, Kim HJ, Moon SY, Jeon H, Park JJ, Carey TE, Chang SE, Kim SW, Kim SY. 2013. Knockdown of beta-catenin controls both apoptotic and autophagic cell death through LKB1/AMPK signaling in head and neck squamous cell carcinoma cell lines. Cell Signal 25:839–847. doi: 10.1016/j.cellsig.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 67.Gao C, Chen YG. 2010. Dishevelled: the hub of Wnt signaling. Cell Signal 22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 68.Steingrímsson E, Copeland NG, Jenkins NA. 2004. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 38:365–411. doi: 10.1146/annurev.genet.38.072902.092717.Steingrimsson. [DOI] [PubMed] [Google Scholar]
- 69.Fantauzzo KA, Soriano P. 2014. PI3K-mediated PDGFRalpha signaling regulates survival and proliferation in skeletal development through p53-dependent intracellular pathways. Genes Dev 28:1005–1017. doi: 10.1101/gad.238709.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malemud CJ. 2015. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem 7:1137–1147. doi: 10.4155/fmc.15.55. [DOI] [PubMed] [Google Scholar]
- 71.Zhai C, Cheng J, Mujahid H, Wang H, Kong J, Yin Y, Li J, Zhang Y, Ji X, Chen W. 2014. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS One 9:e90563. doi: 10.1371/journal.pone.0090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. 2006. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernis ME, Oksdath M, Dupraz S, Nieto Guil A, Fernandez MM, Malchiodi EL, Rosso SB, Quiroga S. 2013. Wingless-type family member 3A triggers neuronal polarization via cross-activation of the insulin-like growth factor-1 receptor pathway. Front Cell Neurosci 7:194. doi: 10.3389/fncel.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. 2001. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem 276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 75.Vigneron F, Dos Santos P, Lemoine S, Bonnet M, Tariosse L, Couffinhal T, Duplaa C, Jaspard-Vinassa B. 2011. GSK-3beta at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res 90:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- 76.Potter CJ, Huang H, Xu T. 2001. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105:357–368. doi: 10.1016/S0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 77.Inoki K, Li Y, Zhu T, Wu J, Guan KL. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 78.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. 2010. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuriakose JA, Miyashiro S, Luo T, Zhu B, McBride JW. 2011. Ehrlichia chaffeensis transcriptome in mammalian and arthropod hosts reveals differential gene expression and post transcriptional regulation. PLoS One 6:e24136. doi: 10.1371/journal.pone.0024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuriakose JA, Zhang X, Luo T, McBride JW. 2012. Molecular basis of antibody mediated immunity against Ehrlichia chaffeensis involves species-specific linear epitopes in tandem repeat proteins. Microbes Infect 14:1054–1063. doi: 10.1016/j.micinf.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo T, Zhang X, McBride JW. 2009. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol 16:982–990. doi: 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doyle CK, Nethery KA, Popov VL, McBride JW. 2006. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun 74:711–720. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo T, Zhang X, Wakeel A, Popov VL, McBride JW. 2008. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun 76:1572–1580. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakeel A, den Dulk-Ras A, Hooykaas PJ, McBride JW. 2011. Ehrlichia chaffeensis tandem repeat proteins and Ank200 are type 1 secretion system substrates related to the repeats-in-toxin exoprotein family. Front Cell Infect Microbiol 1:22. doi: 10.3389/fcimb.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunphy PS, Luo T, McBride JW. 21 July 2014. Ehrlichia chaffeensis exploits host SUMOylation pathways to mediate effector-host interactions and promote intracellular survival. Infect Immun doi: 10.1128/IAI.01984-14. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.