ABSTRACT
Heterogeneity among Aspergillus fumigatus isolates results in unique virulence potential and inflammatory responses. How these isolates drive specific immune responses and how this affects fungally induced lung damage and disease outcome are unresolved. We demonstrate that the highly virulent CEA10 strain is able to rapidly germinate within the immunocompetent lung environment, inducing greater lung damage, vascular leakage, and interleukin 1α (IL-1α) release than the low-virulence Af293 strain, which germinates with a lower frequency in this environment. Importantly, the clearance of CEA10 was consequently dependent on IL-1α, in contrast to Af293. The release of IL-1α occurred by a caspase 1/11- and P2XR7-independent mechanism but was dependent on calpain activity. Our finding that early fungal conidium germination drives greater lung damage and IL-1α-dependent inflammation is supported by three independent experimental lines. First, pregermination of Af293 prior to in vivo challenge drives greater lung damage and an IL-1α-dependent neutrophil response. Second, the more virulent EVOL20 strain, derived from Af293, is able to germinate in the airways, leading to enhanced lung damage and IL-1α-dependent inflammation and fungal clearance. Third, primary environmental A. fumigatus isolates that rapidly germinate under airway conditions follow the same trend toward IL-1α dependency. Our data support the hypothesis that A. fumigatus phenotypic variation significantly contributes to disease outcomes.
KEYWORDS: Aspergillus fumigatus, IL-1, aspergillosis, innate immunity, invasive fungal infection, lung defense, lung infection, mucosal immunity, neutrophils, respiratory pathogens
INTRODUCTION
Aspergillus fumigatus is a ubiquitous mold whose conidia humans inhale on a daily basis. An individual with a sufficient immune response clears the conidia from the body without the conidia causing disease. In immunocompromised populations, the anti-Aspergillus immune response is altered, leading to a significant risk of developing invasive aspergillosis (IA). Patients at an increased risk of developing IA include those receiving chemotherapy treatments for cancer as well as patients receiving immunosuppressive regimens for hematopoietic stem cell transplants or solid-organ transplants (1). Additionally, individuals with primary immunodeficiencies, such as chronic granulomatous disease, which alter antifungal effector pathways are highly susceptible to IA (2–4). In all these contexts, prolonged leukopenia or altered leukocyte function is a major risk factor for IA. For multiple reasons, which include difficulty in diagnosis and a limited repertoire of antifungal drugs for clinical use (1, 5), the mortality rate from IA remains at between 30 and 50% (6–10). Thus, novel stand-alone or adjunctive therapeutic options are sought after for the treatment of IA. One line of research that holds great promise for improving IA outcomes is modulation of the host immune response (11–15).
Typically, in an immunocompetent individual, conidia are removed through mucociliary action, and the physical barriers within the respiratory tract prevent conidia from entering the lung environment. If this primary barrier is bypassed, airway epithelial cells and lung-resident macrophages comprise the first line of defense against inhaled conidia, while neutrophils and inflammatory monocytes are then sequentially recruited to the site to prevent fungal growth (16, 17). Defining the immunological events that are necessary for A. fumigatus conidia to be cleared from the lungs without excessive and immune-mediated lung damage is a critical step toward understanding how the response is altered in different immunocompromised populations. Ultimately, this understanding will be required for the development of immunomodulatory therapies for the treatment of IA.
Interleukin 1 (IL-1) has been shown to be important for host defense against numerous fungal infections, including histoplasmosis, candidiasis, and aspergillosis (18–27). Additionally, single-nucleotide polymorphisms in the IL-1 gene cluster have been shown to be associated with greater risks of patients developing IA (28, 29), while polymorphisms in the NLRP3 gene were associated with recurrent vulvovaginal candidiasis in a subset of women (30). The IL-1 gene cluster codes for IL-1α, IL-1β, as well as the IL-1 receptor antagonist (IL-1ra), all of which can bind to IL-1 receptor type I (IL-1RI) (31). In several different disease models, IL-1α and IL-1β have been shown to have different inflammatory activities (19, 22, 32–34).
Following A. fumigatus challenge, IL-1RI- and MyD88-dependent signals are necessary for optimal leukocyte recruitment and antifungal effector functions to prevent the development of fungal growth and disease (22, 35, 36). However, there is controversy in the literature concerning the specific roles of IL-1α and IL-1β in the prevention of fungal growth and disease (22, 26, 27). The differing conclusions drawn from those studies could be due to a number of factors, including different host immune statuses in the murine models used, different strains of fungi used, and/or different morphologies of the fungi used. Most notably, in those studies, different Aspergillus fumigatus strains were used, CEA10 (22) and Af293 (26, 27). It was recently shown that Af293 is significantly less virulent than CEA10 in the triamcinolone model of IA (37). Moreover, both the inflammatory response elicited by these strains and the mortality associated with each strain significantly differed in immunocompetent mice (38). However, the mechanism(s) behind how different inflammatory responses are induced by different A. fumigatus strains is unknown.
Here, we explore the role of IL-1α in maintaining host resistance to A. fumigatus using an array of strains and isolates. We demonstrate that A. fumigatus strains and isolates that can rapidly germinate within the lung environment induced higher levels of pulmonary damage, which corresponded with a requirement for IL-1α for the prevention of fungal growth and mortality. In contrast, A. fumigatus strains and isolates that undergo limited germination within the lungs of immunocompetent mice drive lower levels of pulmonary damage and can be controlled through an IL-1α-independent mechanism. Intriguingly, germlings of a less pathological and less virulent isolate are sufficient to induce greater lung damage and an IL-1α-dependent inflammatory response, which is likely due to increased cytotoxicity to macrophages. Finally, calpain activity, rather than caspase 1/11 activity or P2XR7 signaling, was necessary for IL-1α release. Thus, our data highlight a role for early pathogen adaptation and growth in the pulmonary environment in establishing the host inflammatory pathways that are necessary to maintain resistance against this ubiquitous and increasingly important human pathogen.
RESULTS
The CEA10 and Af293 strains of A. fumigatus induce different levels of lung damage and inflammation.
Previous reports indicated variation in virulence among the commonly studied “wild-type” Aspergillus fumigatus strains CEA10 and Af293 (37, 38). To examine the overall lung damage induced by each of these strains in an immunocompetent pulmonary inhalation model of Aspergillus fumigatus infection, C57BL/6 mice were challenged with 4 × 107 conidia of CEA10 or Af293. We noted that by 24 h postinoculation (hpi), lungs from mice challenged with CEA10 appeared to have more edema and, overall, appeared more damaged than did lungs from mice challenged with Af293 (Fig. 1A). Histological analysis of lungs 40 h after challenge with CEA10 displayed markedly greater inflammatory cell recruitment than did lungs from Af293-challenged animals (Fig. 1A). Moreover, blood vessels in the lungs of Af293-challenged mice appeared to be intact (Fig. 1A). To quantitatively assess lung damage and vascular leakage, we measured lactate dehydrogenase (LDH) and albumin levels, respectively, in bronchoalveolar lavage fluid (BALF) (39, 40). Mice inoculated with CEA10 had significantly higher levels of LDH and albumin in their BALF than did mice inoculated with Af293 by 12 hpi (Fig. 1B), indicating greater lung damage and vascular leakage, respectively. IL-1α is a cytokine that is constitutively expressed in cells and is typically released in highly pathological situations, such as during necrotic or necroptotic cell death (31, 41). Given the role of IL-1α in driving inflammation during highly pathological inflammatory responses, we wanted to know whether IL-1α protein levels were also differentially abundant after exposure to CEA10 or Af293. IL-1α protein levels were significantly higher in the lungs of CEA10-inoculated mice than in Af293-inoculated lungs at 12 hpi (Fig. 1C). Furthermore, using Spearman's rank-order correlation analysis, we found a positive correlation between the amount of IL-1α protein in the lung and the amount of LDH in the BALF following A. fumigatus exposure (Spearman's rho [r] = 0.8846; P = 0.0001) (Fig. 1D). Values on the higher end of the correlation plot were obtained from mice inoculated with CEA10 (Fig. 1D, blue dots), whereas values on the lower end of the plot were obtained from mice inoculated with Af293 (red dots), suggesting that the CEA10 strain is more pathological than the Af293 strain, which corresponds to the release of the IL-1α protein.
FIG 1.
CEA10 and Af293 induce different levels of pulmonary damage. C57BL/6 mice were challenged i.t. with 4 × 107 CEA10 or Af293 conidia. (A) At 24 or 40 hpi, mice were euthanized, and lungs were removed to observe the gross pathology of infected lungs at 24 h or for histological analysis of inflammation by H&E staining at 40 h in C57BL/6 mice infected with CEA10 (left) or Af293 (right). (B) At 12 hpi, mice were euthanized, BALF was collected, and lung tissue was homogenized. Lung damage and leakage were quantified by measuring LDH and albumin levels, respectively, in the BALF. (C) IL-1α levels in the lung homogenate. (D) Spearman rank-order correlation for LDH and IL-1α levels in mice infected with CEA10 (blue dots) or Af293 (red dots) (Spearman r = 0.8846; P = 0.0001). Data are representative of results from at least 2 independent experiments with at least 5 mice per group. Bar graphs show group means ± 1 standard error of the mean. Statistically significant differences in panel B were determined by using Kruskal-Wallis one-way ANOVA with Dunn's posttest. Statistically significant differences in panel C were determined by using a Mann-Whitney U test (*, P < 0.05; **, P < 0.01).
IL-1α-dependent neutrophil recruitment and fungal clearance are fungal strain dependent.
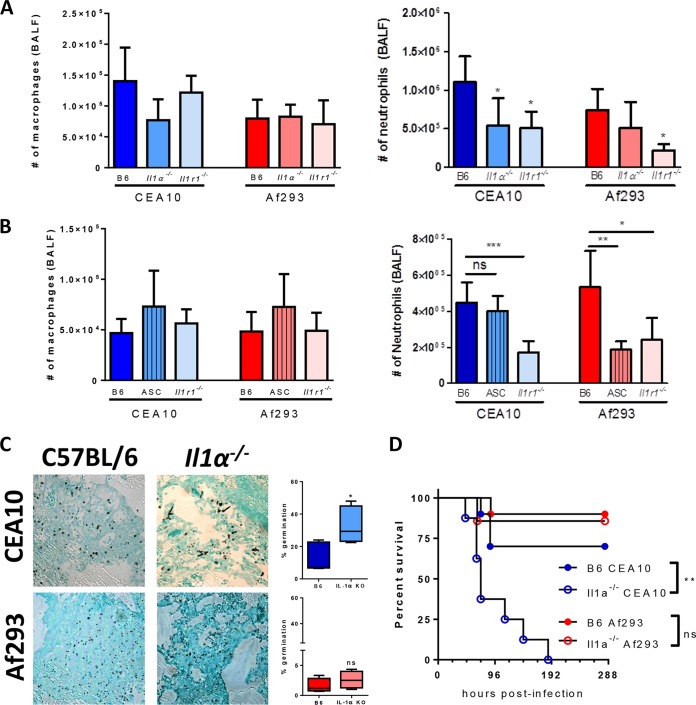
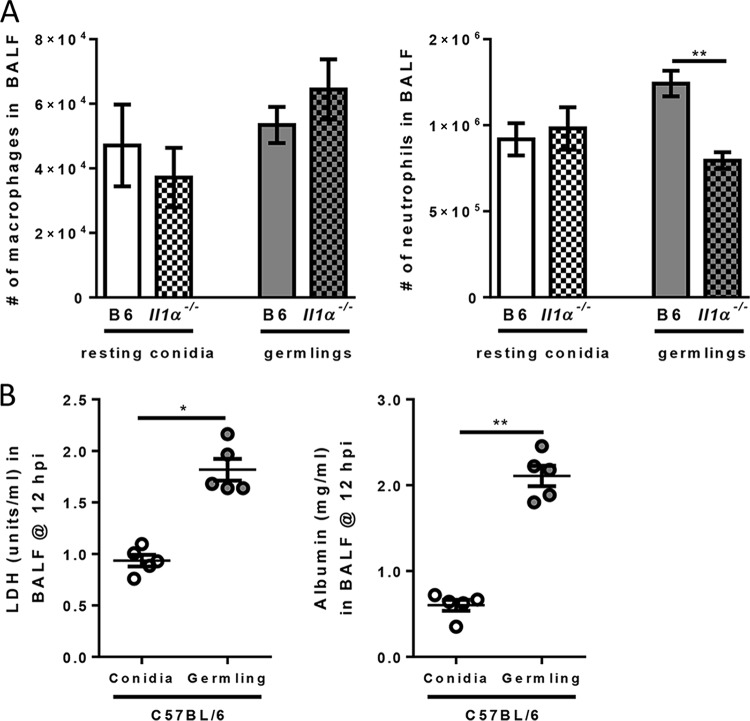
While the importance of IL-1RI/MyD88 signaling in host resistance against A. fumigatus infection is unequivocal (22, 35, 42), the specific roles of IL-1α and IL-1β for the prevention of fungal growth and disease are controversial in the literature (22, 26, 27). Previously, our laboratory showed that IL-1α was critical for host resistance against A. fumigatus infection, but Pycard (apoptosis-associated speck-like protein containing a CARD [ASC]) was largely dispensable (22). In contrast, Karki and colleagues found that Pycard (ASC) and Casp1/11 (caspase 1/11) were essential for host resistance against A. fumigatus infection downstream of the NLRP3 and AIM2 sensors (26). The inflammasomes are critical for the maturation and secretion of both IL-1β and IL-18. Karki and colleagues showed that IL-1β-deficient mice are more susceptible to A. fumigatus challenge, whereas IL-18-deficient mice survive infection, similarly to controls (26). In those studies, the major difference was the strain of A. fumigatus used, CEA10 in our studies and Af293 in the studies by Karki and colleagues. We have now shown that the CEA10 strain induces greater inflammation and lung damage than Af293 in immunocompetent mice (Fig. 1) (38). Thus, to address whether the increase in IL-1α protein levels observed following CEA10 challenge was immunologically relevant, we challenged C57BL/6, Il1a−/−, Pycard−/− (ASC-deficient), and Il1r1−/− mice with 4 × 107 conidia of either CEA10 or Af293. Twelve hours after fungal challenge, BALF was collected to assess macrophage and neutrophil numbers in the airways. Il1r1−/− mice had a defect in neutrophil recruitment when challenged with either CEA10 or Af293, whereas macrophage numbers were similar (Fig. 2A and B). Thus, IL-1 signaling is essential for driving the accumulation of neutrophils in the airways following A. fumigatus challenge in response to multiple A. fumigatus strains, similar to what was previously observed (22, 35). Interestingly, Il1a−/− mice had a defect in neutrophil recruitment only when inoculated with CEA10 (Fig. 2A). In contrast, Pycard−/− (ASC-deficient) mice were impaired in neutrophil recruitment to the airways only following inoculation with Af293 (Fig. 2B). Taken together, these data demonstrate that the CEA10 strain induces IL-1α-dependent neutrophil recruitment, while Af293 drives inflammasome-dependent neutrophil recruitment early after challenge, clarifying the findings of previously reported work (22, 26, 27).
FIG 2.
Il1a−/− mice are more susceptible to infection by CEA10 than by Af293. (A and B) C57BL/6, Il1a−/−, Pycard−/−, or Il1r1−/− mice were challenged with 4 × 107 conidia of either CEA10 or Af293. At 12 hpi, mice were euthanized, and BALF was collected for the quantification of macrophage and neutrophil recruitment to the airways. Data are representative of results from 1 to 2 experiments with 5 to 7 mice per group. Bar graphs show group means ± 1 standard error of the mean. (C) At 42 hpi, mice were euthanized, and lungs were saved for histological analysis. Formalin-fixed lungs were paraffin embedded, sectioned, and stained with GMS for analysis by microscopy. Representative lung sections from C57BL/6 mice (left) or Il1a−/− mice (right) infected with CEA10 (top) or Af293 (bottom) are shown with a 40× objective. A. fumigatus germination rates were determined by microscopically counting both the number of conidia and the number of germlings in GMS-stained sections. (D) Survival analysis of CEA10 and Af293 in immunocompetent C57BL/6 or Il1a−/− mice that had been inoculated with 5 × 107 conidia given intratracheally. **, P = 0.0016 by a log rank test. Statistical significance in panels A and B was determined by using one-way ANOVA with Bonferroni's posttest (*, P = 0.05; **, P = 0.01; ***, P = 0.001). Statistical significance in panel C was determined by using a Mann-Whitney U test (ns, not significant; *, P < 0.05).
Because neutrophils are widely acknowledged to be critical effector cells in mediating the clearance of A. fumigatus from the lungs, we hypothesized that following CEA10 challenge, the control of fungal growth and tissue invasion would be IL-1α dependent, while Af293 would be controlled in an IL-1α-independent manner. To test this, C57BL/6 mice and Il1a−/− mice were challenged with 4 × 107 conidia of CEA10 or Af293. At 42 hpi, lungs were collected for histological analysis to assess fungal growth by Grocott-Gomori's methenamine silver (GMS) staining. Following CEA10 challenge, there was significantly greater fungal germination and significantly greater tissue invasion in the absence of IL-1α than in C57BL/6 mice (Fig. 2C, top). In contrast, after Af293 challenge, both C57BL/6 and IL-1α-deficient mice were able to control fungal growth (Fig. 2C, bottom). Although both CEA10 and Af293 are typically cleared from C57BL/6 lungs, mice challenged with CEA10 consistently had greater fungal growth at 42 hpi: approximately 10 to 20% of the stained fungi were germinated (Fig. 2C, top), while almost no fungal germination (<2%) was observed following Af293 challenge at this time point (Fig. 2C, bottom). The enhanced fungal growth in the lungs of IL-1α-deficient mice challenged with the CEA10 strain corresponded to increased mortality, which was not seen with the Af293 strain (Fig. 2D). Taken together, these results demonstrate that the CEA10 strain of A. fumigatus drives neutrophil recruitment and the control of fungal germination through an IL-1α-dependent mechanism, whereas neutrophil recruitment and the control of fungal germination of the Af293 strain occurs in an IL-1α-independent manner.
IL-1α is produced by radiosensitive cells after A. fumigatus challenge.
To determine which cells contribute to the production of IL-1α after A. fumigatus challenge, we made a series of bone marrow chimeric mice. C57BL/6 and Il1a/b−/− mice were lethally irradiated and then reconstituted with either C57BL/6 or Il1a/b−/− bone marrow intravenously to develop the following groups: C57BL/6 mice possessing C57BL/6 bone marrow, Il1a/b−/− mice possessing Il1a/b−/− bone marrow, C57BL/6 mice possessing Il1a/b−/− bone marrow, and Il1a/b−/− mice possessing C57BL/6 bone marrow. Mice were then rested for 6 to 8 weeks prior to challenge with 4 × 107 conidia of CEA10. At 12 hpi, mice were euthanized, and lungs were collected to measure IL-1α levels. As expected, C57BL/6 mice possessing C57BL/6 bone marrow were able to produce IL-1α, while Il1a/b−/− mice possessing Il1a/b−/− bone marrow could not produce IL-1α in the lungs (Fig. 3). Interestingly, mice that were devoid of IL-1α in radiosensitive cells had significantly decreased levels of IL-1α protein in the lungs, whereas mice lacking IL-1α in radioresistant cells had levels of IL-1α protein comparable to those of C57BL/6 mice (Fig. 3). Similarly, levels of IL-1β protein in the airways were dependent on a radiosensitive cell (data not shown). These data show that radiosensitive cells are the major source of IL-1α after challenge with A. fumigatus.
FIG 3.

Cells of hematopoietic origin contribute to IL-1α production after challenge with A. fumigatus in vivo. Bone marrow chimeric mice were made by irradiating C57BL/6 and Il1a/b−/− mice and reconstituting the mice with bone marrow from either C57BL/6 or Il1a/b−/− mice to develop the following groups: C57BL/6→C57BL/6, Il1a/b−/−→Il1a/b−/−, C57BL/6→Il1a/b−/−, and Il1a/b−/−→C57BL/6. These mice were infected i.t. with 4 × 107 CEA10 conidia, and at 12 hpi, mice were euthanized, and lung tissue was homogenized to quantitate IL-1α levels by using an ELISA. Data are representative of results from at least 2 independent experiments consisting of 5 to 7 mice per group. Each graph shows data for individual mice as symbols, with the lines representing group means ± 1 standard error of the mean. Statistical significance was determined by using Kruskal-Wallis one-way ANOVA with Dunn's posttest (***, P = 0.001; ****, P = 0.0001).
Aspergillus fumigatus germlings are more cytotoxic to macrophages, resulting in greater IL-1α release.
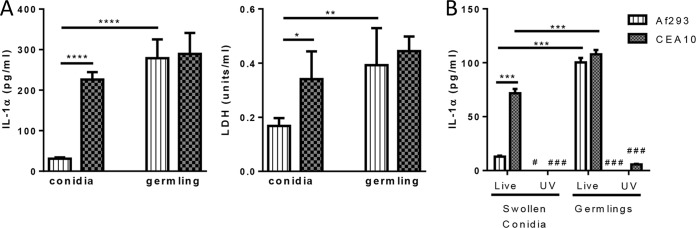
We have previously shown that following A. fumigatus inoculation, IL-1α secretion is dependent on the presence of CCR2+ monocytes (22). Thus, we next wanted to determine if Af293 and CEA10 were interacting differently with macrophages in an in vitro system. Swollen conidia or germlings of Af293 and CEA10 were incubated with bone marrow-derived macrophages (BMDM) from C57BL/6 mice at a 10:1 ratio for 24 h. Interestingly, swollen Af293 conidia induced low levels of IL-1α and LDH release from BMDM cells compared to those induced by swollen CEA10 conidia (Fig. 4A). In contrast, Af293 germlings induced levels of IL-1α and LDH release from BMDM that were similar to those induced by CEA10 germlings (Fig. 4A). The fungal factor(s) important for driving IL-1 cytokine release is poorly understood. Previous work suggested that Dectin-1 recognition of the β1,3-glucan molecules of the A. fumigatus cell wall is critical for inflammatory cytokine release, including both IL-1α and IL-1β (43). To test whether Aspergillus fumigatus had to be alive or whether cell wall alterations between the fungal growth stages were necessary to drive IL-1α secretion from BMDM, swollen conidia or germlings of Af293 and CEA10 were UV inactivated prior to their coculture with BMDM. Interestingly, we found that UV-inactivated swollen conidia and germlings were unable to induce IL-1α release from BMDM (Fig. 4B). These data demonstrate that different morphotypes of A. fumigatus strains can drive specific IL-1α and damage responses from macrophages; moreover, metabolically active fungi are required to drive the IL-1α response from macrophages, which suggests that alterations in the fungal cell wall and carbohydrate exposure are not essential for the differences observed between fungal strains and morphotypes.
FIG 4.
IL-1α production from macrophages is dependent on live A. fumigatus fungi. (A) BMDM were incubated with swollen conidia or germlings of Af293 or CEA10 for 24 h, and the supernatant was collected to quantify IL-1α and LDH levels. (B) BMDM were incubated with swollen conidia or germlings of Af293 or CEA10 that were either untreated or UV inactivated. In both cases, BMDM were cocultured with A. fumigatus for 24 h, at which point the supernatants were collected to quantify IL-1α secretion. Data are representative of results from 2 independent experiments consisting of 6 biological replicates per group. Bar graphs show group means ± 1 standard error of the mean. Statistical significance in panel A was determined by using two-way ANOVA with a Tukey posttest (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Statistical significance in panel B was determined by using two-way ANOVA with a Tukey posttest for comparisons of live A. fumigatus fungi (***, P < 0.001) or for comparisons between live and UV-inactivated A. fumigatus fungi (#, P < 0.05; ###, P < 0.001).
Aspergillus fumigatus induces macrophage IL-1α release through a calpain-dependent mechanism.
Both IL-1α and IL-1β are produced as precursor proteins, but their maturation process and secretion from cells occur by distinct pathways. It is well established that the NLRP3 (26, 27, 44) and AIM2 (26) inflammasomes are critical for IL-1β secretion, but the pathways critical for IL-1α secretion during fungal infections have not been well elucidated. Gross and colleagues previously showed that IL-1α can be released through both inflammasome-dependent and -independent pathways (45). Thus, we wanted to test whether or not IL-1α release following A. fumigatus challenge was caspase 1/11 dependent. Germlings of Af293 and CEA10 were incubated with BMDM derived from C57BL/6 or Casp1/11−/− mice at a 10:1 ratio for 24 h. We found that caspase 1/11 molecules were not necessary for IL-1α release but were essential for IL-1β secretion (Fig. 5A). This finding fits with our previous observation that Pycard-deficient mice do not have a decrease in IL-1α secretion into their airways (22).
FIG 5.
IL-1 cytokine production from macrophages is dependent on calpain activity and independent of caspase 1/11 activity and P2X7R signaling. (A) BMDM from either C57BL/6 or Casp1/11-deficient mice were incubated with germlings of Af293 or CEA10 for 24 h, at which point the supernatants were collected to quantify levels of IL-1α and IL-1β secretion. (B) BMDM from either C57BL/6 or P2x7r-deficient mice were incubated with germlings of Af293 or CEA10 for 24 h, at which point the supernatants were collected to quantify levels of IL-1α and IL-1β secretion. (C) BMDM from C57BL/6 mice were preincubated with the vehicle (DMSO), 30 μM calpeptin, or 40 μM MDL28170, followed by overlaying the BMDM cells with germlings of Af293 or CEA10 for 24 h, at which point the supernatants were collected to quantify levels of IL-1α and IL-1β secretion. Data are representative of results from 2 independent experiments consisting of 4 to 6 biological replicates per group. Bar graphs show group means ± 1 standard error of the mean. Statistical significance was determined by using two-way ANOVA with a Tukey posttest for comparisons between treatment groups and each A. fumigatus strain (***, P < 0.001; ****, P < 0.0001).
Alternatively, Dagvadorj and colleagues showed that lipopolysaccharide (LPS) drives alveolar macrophage cell death and IL-1α release through a P2X7R-dependent mechanism (46). Thus, we wanted to test whether or not P2X7R was necessary for IL-1α release following A. fumigatus challenge. Germlings of Af293 and CEA10 were incubated with BMDM derived from C57BL/6 or P2rx7−/− mice at a 10:1 ratio for 24 h. We found that P2rx7−/− BMDM secreted at least equivalent levels of both IL-1α and IL-1β (Fig. 5B). Moreover, in vivo challenge of P2rx7−/− mice with the CEA10 strain of A. fumigatus did not result in decreased IL-1 cytokine release into the BALF at either 12 h postinoculation (6.6 ± 1.1 pg/ml IL-1α in C57BL/6 mice versus 9.6 ± 0.9 pg/ml IL-1α in P2rx7−/− mice and 341.9 ± 23.8 pg/ml IL-1β in C57BL/6 mice versus 510.7 ± 39.9 pg/ml IL-1β in P2rx7−/− mice) or 40 h postinoculation (59.6 ± 20.8 pg/ml IL-1α in C57BL/6 mice versus 87.7 ± 8.1 pg/ml IL-1α in P2rx7−/− mice and 410.8 ± 123.8 pg/ml IL-1β in C57BL/6 mice versus 632.2 ± 86.2 pg/ml IL-1β in P2rx7−/− mice). Thus, it does not appear that P2X7R is necessary for the IL-1 cytokine response after A. fumigatus challenge.
Although both the full-length precursor form of IL-1α (p33) and the cleaved mature form of IL-1α (p17) can bind to IL-1RI to initiate downstream signaling, p17 has nearly a 50-fold-higher binding affinity for IL-1RI (47). Cleavage of p33 to the mature p17 form occurs through calpain, a calcium-dependent protease (48, 49). To determine if calpain cleavage contributes to IL-1α secretion from macrophages following A. fumigatus exposure, BMDM were incubated with germlings of CEA10 or Af293 in the presence or absence of the calpain inhibitor calpeptin (30 μM) or MDL28170 (40 μM). In the presence of calpain inhibition, IL-1α secretion from macrophages was significantly decreased in response to swollen conidia and germlings of both Af293 and CEA10 (Fig. 5C). Unexpectedly, IL-1β secretion from macrophages was also decreased following calpain inhibition (Fig. 5C). Taken together, these results suggest that calpain activity contributes to IL-1 secretion from macrophages following interaction with A. fumigatus.
Early germination of the CEA10 strain of A. fumigatus within the lung environment corresponds to its greater virulence and lung damage potential.
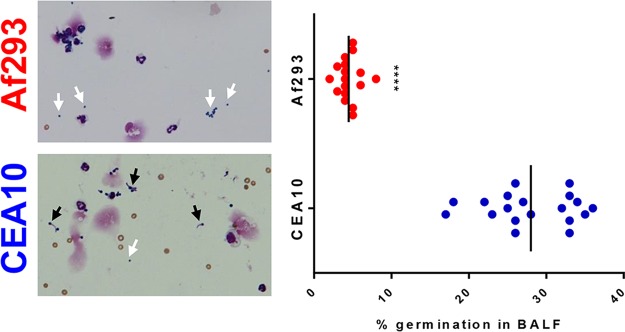
In A. fumigatus infection models, resting conidia are instilled into the airways, where they can begin to swell and subsequently begin polarized cell growth, forming germlings and eventually hyphae if not controlled. Aspergillus hyphae are highly invasive and express an array of secondary metabolites and hydrolytic enzymes that could be highly damaging to the host (50). Based on our observation that A. fumigatus germlings were highly cytotoxic and inflammatory when cocultured with BMDM, we wanted to examine whether the initial growth of CEA10 and Af293 was different within mouse lungs. In BALF cytospins, we noted significant fungal material that was recovered from the airways (Fig. 6) and included both conidia (white arrows) and germlings (black arrows). As early as 6 h after challenge, CEA10 showed an increased ability to germinate within the airway compared to Af293 (Fig. 6). CEA10 is able to germinate more extensively under these conditions than Af293, which corresponds to its abilities to induce high levels of lung damage and drive an IL-1α-dependent inflammatory response.
FIG 6.
CEA10 germinates more efficiently than does Af293 in C57BL/6 airways. C57BL/6 mice were challenged i.t. with 4 × 107 conidia of Af293 (top) or CEA10 (bottom). At 6 hpi, mice were euthanized, and BALF was collected. BALF was spun onto slides and stained with a Differential Quik staining kit. White arrows indicate conidia in the BALF, while black arrows indicate germlings. Fungal germination was quantified by counting conidia and germlings on the slides and is represented as a percentage of the fungal matter that was germinated. Each symbol represents data for an individual mouse, and the lines represent the group means. Data were pooled from 3 independent experiments consisting of 5 to 8 mice per group. Statistical significance was determined by using a Mann-Whitney U test (****, P < 0.0001).
Heterogeneous germination of A. fumigatus isolates in vitro in lung homogenate medium.
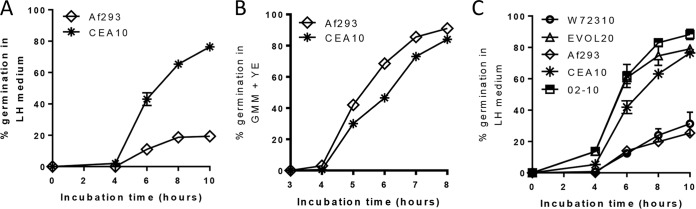
Based on our in vivo observation that CEA10 germlings were more prevalent in the airways at early times after instillation, we next sought to test whether the CEA10 strain was better able to utilize the nutrients found in the respiratory tract. It is well established that only certain carbon and nitrogen sources are able to trigger Aspergillus species growth (51, 52). Since it is unclear which nutrients are available to conidia upon instillation into the airways, we conducted our in vitro germination assay with mouse lung homogenate medium. In line with our in vivo data, CEA10 was able to germinate more extensively in lung homogenate medium than Af293 (Fig. 7A). This is not caused by an inherent growth defect in the Af293 strain because it germinates as extensively as the CEA10 strain in nutrient-rich medium (Fig. 7B). Thus, our data support a role for inherent differences in nutrient sensing and/or utilization by A. fumigatus strains in the airways of immunocompetent mice for their germination, which corresponds to their pathological and inflammatory potential.
FIG 7.
Heterogeneity in germination potentials of A. fumigatus isolates in lung homogenate medium. (A) Lung tissue from C57BL/6 mice was homogenized in PBS and used as the medium for in vitro germination assays. The lung homogenate (LH) was diluted 1:4 with PBS and then inoculated with 2 × 107 conidia of either CEA10 or Af293. Beginning at 4 h postinoculation, germination was quantified every 2 h by microscopically counting the numbers of conidia and germlings. Data are represented as the percentage of fungal matter that was germinated. (B) To show that Af293 does not have an inherent germination defect, 2 ml of nutrient-rich medium containing 0.05% yeast extract (YE) was inoculated with 2 × 107 conidia of Af293 or CEA10, and a germination assay was performed. In panels A and B, data are representative of results from at least 3 independent experiments consisting of 3 biological replicates per group. Each symbol represents the group mean ± 1 standard error of the mean. (C) Environmental isolates 02-10 and W72310, the microevolved Af293 strain EVOL20, and our wild-type reference strains CEA10 and Af293 were used for germination assays with lung homogenate medium as described above for panel A. Data in panel C were pooled from 3 independent experiments consisting of 3 biological replicates per group, except for EVOL20, for which the data were pooled from 2 independent experiments consisting of 3 biological replicates per group. Each symbol represents the group mean ± 1 standard error of the mean.
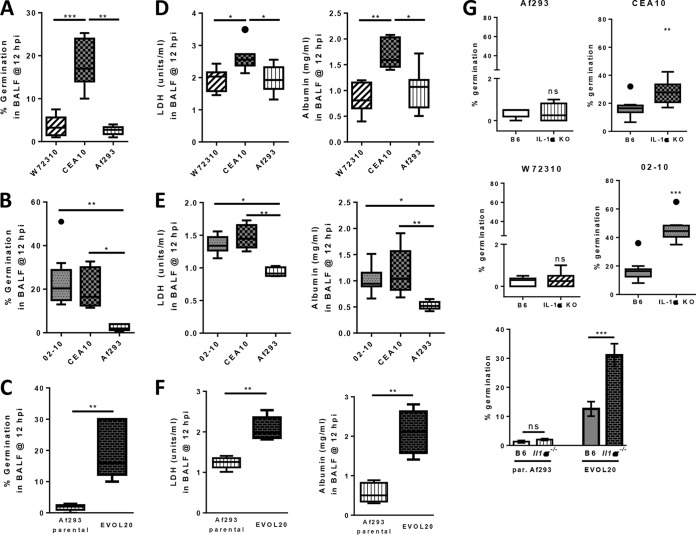
Our observations until now have been based on two commonly used reference strains of A. fumigatus. To extend these observations, we screened a number of strains and isolates of A. fumigatus that we previously studied (37) for their ability to germinate in lung homogenate medium. Interestingly, the EVOL20 strain of A. fumigatus, which is a more virulent strain recently generated by in vitro serial passaging of Af293 under low-oxygen conditions (37), was able to germinate more extensively than its parent strain Af293 in lung homogenate medium (Fig. 7C). Additionally, we found environmental isolates of A. fumigatus that had either Af293-like germination potential (W72310) or CEA10-like germination potential (02-10) (Fig. 7C). Thus, A. fumigatus isolates display significant heterogeneity in their abilities to germinate under airway-mimicking conditions in vitro, which may be an important determinant of fungal virulence in immunocompetent mice.
The ability of A. fumigatus strains and isolates to germinate in lung homogenate medium predicts in vivo airway germination and IL-1α-dependent fungal clearance.
To extend our work with the CEA10 and Af293 strains, we next wanted to determine if the ability of A. fumigatus isolates to germinate in vitro in lung homogenate medium could predict which isolates are more pathological and virulent and induce IL-1α-dependent inflammation in an in vivo murine infection model. To do this, C57BL/6 mice were inoculated with 4 × 107 conidia of either Af293, CEA10, W72310, 02-10, or EVOL20. At 12 hpi, BALF was collected to analyze fungal germination and levels of LDH and albumin in the airways. Strains and isolates that were unable to germinate in lung homogenate medium (Af293 and W72310) were similarly unable to germinate within the airways (Fig. 8A), whereas strains and isolates that germinated extensively in lung homogenate medium (CEA10, 02-10, and EVOL20) were also able to germinate within the lung airways (Fig. 8B and C). Moreover, significantly higher levels of LDH and albumin were present in the BALF of mice challenged with CEA10, 02-10, and EVOL20 (Fig. 8E and F) than in the BALF of mice challenged with Af293 and W72310 (Fig. 8D). To test whether the ability to germinate within the host is associated with an IL-1α-dependent immune response, C57BL/6 mice and Il1a−/− mice were challenged with 4 × 107 conidia of either Af293, CEA10, W72310, 02-10, or EVOL20. At 42 hpi, lungs were collected for histological analysis to determine fungal germination. GMS staining of lung sections demonstrated that mice lacking IL-1α lost the ability to control fungal growth and tissue invasion only when inoculated with the strains and isolates that were able to rapidly germinate within the airways (CEA10, 02-10, and EVOL20) (Fig. 8G; see also Fig. S1 in the supplemental material). Enhanced fungal germination in IL-1α-deficient mice challenged with 02-10 corresponded with 100% mortality (8/8 mice) within 2 days, while only ∼22% (2/9) of C57BL/6 mice succumbed to 02-10 infection in this time period (data not shown). In contrast, mice deficient in IL-1α that were inoculated with Af293 and W72310 showed normal control of fungal growth and germination (Fig. 8G and Fig. S1). Taken together, our data reveal a specific role of IL-1α for the control of fungal germination in hosts inoculated with strains and isolates of A. fumigatus that rapidly sense and grow in the nutrient environment of the lung.
FIG 8.
A. fumigatus isolates that are able to germinate within the airways induce greater lung damage and IL-1α-dependent control of fungal germination. (A to F) C57BL/6 mice were infected with 4 × 107 conidia of W72310 (A and D), 02-10 (B and E), or EVOL20 (C and F) along with our wild-type reference strains CEA10 and Af293. At 12 hpi, mice were euthanized, and BALF was collected to quantify germination in the airways (A to C) and to measure LDH and albumin levels to quantify lung damage and leakage, respectively (D to F). Data in panels A to F are representative of results from 2 independent experiments with 5 to 9 mice per group. Data are presented as box-and-whisker plots with Tukey whiskers and outliers displayed as dots. Statistical significance in panels A, B, D, and E was determined by Kruskal-Wallis one-way ANOVA with Dunn's posttest (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Statistical significance in panels C and F was determined by a Mann-Whitney U test (**, P < 0.01). (G) For histological analysis and quantification of germination at 42 hpi (36 hpi for 02-10, since IL-1α-deficient mice succumb to 02-10 infection by 40 hpi), C57BL/6 and Il1a−/− mice were infected with 4 × 107 conidia of Af293, CEA10, W72310, 02-10, or EVOL20 (from left to right). Formalin-fixed lungs were paraffin embedded, sectioned, and stained with GMS for analysis by microscopy. A. fumigatus germination rates were determined by microscopically counting both the number of conidia and the number of germlings in GMS-stained sections. Data for Af293, CEA10, W72310, and 02-10 are representative of results from at least 2 independent experiments consisting of 4 to 10 mice per group and are presented as box-and-whisker plots with Tukey whiskers and outliers displayed as dots. Data for EVOL20 and the parental Af293 strain were pooled from 2 independent experiments consisting of 5 to 9 mice per group and are presented as bar graphs showing group means ± 1 standard error of the mean. Statistical significance in panel G for Af293, CEA10, W72310, and 02-10 was determined by a Mann-Whitney U test (ns, not significant; **, P < 0.01; ***, P < 0.001). Statistical significance for EVOL20 and the parental Af293 strain was determined by two-way ANOVA with Tukey's posttest (***, P < 0.001).
Af293 germlings are sufficient to induce greater lung damage and initiate an IL-1α-dependent neutrophil response.
Our data strongly suggest that the presence of increased numbers of A. fumigatus germlings in the airways causes extensive lung damage and an IL-1α-dependent inflammatory response. Because our analysis until now has been correlative between the presence of fungal germlings and lung damage, we next sought to address whether Af293 germlings are sufficient to induce greater lung damage and IL-1α-dependent inflammation. To do this, Af293 conidia were pregerminated in liquid culture prior to instillation into mice. At the time of challenge, approximately 20 to 30% of all fungal matter in the culture initiated germ tube formation. This level of growth is very similar to the level of airway germination observed with hypervirulent strains and isolates of A. fumigatus (Fig. 8). C57BL/6 and Il1a−/− mice were challenged with 2.7 × 107 resting conidia or germlings of Af293. At 12 hpi, leukocyte recruitment to the airways and lung damage were quantified. Il1a−/− mice challenged with resting Af293 conidia had normal macrophage and neutrophil recruitment to the airways (Fig. 9A). In contrast, Il1a−/− mice challenged with Af293 germlings had a significant defect in neutrophil recruitment to the airways compared to C57BL/6 mice, while macrophage numbers were similar (Fig. 9A). Moreover, Af293 germlings induced significantly greater lung damage, as evidenced by elevated levels of both LDH and albumin in the BALF, than did Af293 conidia (Fig. 9B). Together, these data demonstrate that Af293 germlings are sufficient to drive enhanced pulmonary damage, which results in an IL-1α-dependent inflammatory neutrophil response.
FIG 9.
Af293 germlings are sufficient to enhance lung damage and drive IL-1α-dependent neutrophil recruitment in vivo. C57BL/6 and Il1a−/− mice were infected with 2.7 × 107 resting conidia or germlings of the Af293 strain of A. fumigatus. At 12 hpi, mice were euthanized, and BALF was collected to analyze macrophage and neutrophil recruitment to the airways (A) and LDH and albumin levels (B). Bar graphs in panel A show group means ± 1 standard error of the mean. Each symbol in panel B represents data for an individual mouse, and the black lines represent group means ± 1 standard error of the mean. Data in panels A and B are representative of results from 2 independent experiments with 5 to 8 mice per group. Statistical significance in panel A was determined by using two-way ANOVA with Tukey's posttest (**, P < 0.01). Statistical significance in panel B was determined by using a Mann-Whitney U test (*, P < 0.05; **, P < 0.01).
DISCUSSION
In this study, we reveal an essential function of IL-1α in the control of fungal growth and tissue invasion following challenge with isolates of A. fumigatus previously observed to be hypervirulent (relative to A293) in a clinically relevant disease model. We observe that rapid fungal germination in the airways corresponds to the induction of high levels of pulmonary damage, resulting in the IL-1α-dependent control of fungal growth of hypervirulent isolates that is necessary to prevent host mortality, while IL-1α was dispensable for the clearance of the less virulent isolates. Importantly, we demonstrated that A. fumigatus germlings are sufficient to induce excessive lung damage, which then necessitates IL-1α to recruit neutrophils and prevent fungal growth and mortality. Taken together, our data show that initial fungal germination following fungal deposition in the airways is a critical determinant of virulence and the subsequent immune response induced. This further validates the importance of using multiple isolates in studies of pathogenesis and immunity, in order to fully understand the virulence potential of specific pathogens (33, 37, 38, 53–59).
In order to cause disease, A. fumigatus must be able to sense, germinate, and grow within the lung microenvironment. Such growth, if left unchecked, leads to tissue invasion, destruction, and dissemination. Our data demonstrate that A. fumigatus isolates that can rapidly germinate within the airways cause greater tissue damage leading to inflammatory cell death, during which IL-1α is released. Following inoculation with these rapidly germinating A. fumigatus isolates, IL-1α is essential for fungal clearance. In contrast, A. fumigatus isolates that do not germinate extensively within the airways are not able to penetrate the epithelial barrier and are likely rapidly cleared by lung-resident macrophages through an IL-1α-independent mechanism. This concept is supported by previous work done in an oral candidiasis model (60–62). In oral candidiasis, the epithelial barrier is breached by Candida spp. in order to cause disease. In vitro, incubation of human epithelial cells with Candida spp. that were able to undergo hyphal growth induced higher levels of IL-1α expression, which were dampened in C. albicans mutants that were defective in hyphal growth (60–62). Moreover, IL-1α plays a critical role in regulating host resistance in murine models of oral candidiasis (63, 64). During oral candidiasis, IL-1α is secreted by keratinocytes (64). In contrast, we show that radiosensitive cells, including macrophages, are the major cellular source of IL-1α following A. fumigatus challenge (Fig. 3). This likely has to do with the epithelial structural differences between the tongue and lungs. Specifically, the tongue epithelium is highly stratified. Schonherr and colleagues found that the depth of hyphal growth corresponds to IL-1α release in the candidiasis model (64). In contrast, the respiratory epithelium can be as thin as a single cell to facilitate gas exchange. Thus, Aspergillus hyphae immediately enter the basement membrane, where innate phagocytes are located, driving IL-1α release from these phagocytes.
It is well established that following A. fumigatus challenge, IL-1β secretion is dependent on the NLRP3 and AIM2 inflammasomes (26, 27, 44), but how IL-1α is released from cells following fungal exposure is not completely understood. Previous work suggested the existence of two distinct pathways for IL-1α secretion that are either inflammasome dependent or independent (45). Viable Candida albicans and ATP led to inflammasome-dependent IL-1α release from cells (45). Interestingly, in the present study, the absence of P2X7R, which binds to extracellular ATP, did not result in diminished levels of IL-1α secreted from macrophages. Inflammasome-independent secretion of IL-1α was induced by particulate activators of the NLRP3 inflammasome, such as monosodium urate crystals and alum, as well as activators of calcium influx (45). Following interactions with A. fumigatus, macrophages secreted IL-1α in a caspase 1/11-independent manner. Further studies are necessary to determine the factors that induce inflammasome-independent IL-1α secretion, but it is possible that calcium influx is a contributing factor. Accordingly, following exposure to A. fumigatus, the inhibition of calpain, a calcium-dependent protease that cleaves full-length IL-1α into mature IL-1α (48, 49), resulted in lower levels of IL-1α secretion from macrophages. This study begins to elucidate the manner in which IL-1α secretion from macrophages occurs, but more work is needed to determine the fungal factors associated with calpain activation that were necessary for IL-1 release.
The observation that hypervirulent fungal strains and isolates drive excessive cell death, lung damage, and IL-1α-dependent inflammation appears to be conserved across numerous types of mucosal pathogens (33, 65–70). Highly virulent Pseudomonas aeruginosa isolates express a cytotoxin, ExoU, that has been found to induce necrosis and epithelial damage (65). Interestingly, ExoU-expressing isolates of Pseudomonas induced an immune response that was dependent on IL-1α, whereas isolates lacking ExoU induced an immune response that was dependent on IL-1β (33). For Staphylococcus-induced pneumonia, it was shown that a highly virulent Staphylococcus aureus isolate possessed toxins that caused necroptosis of host cells, which was shown to be a key factor in lung pathology and damage (66). During infection with highly virulent Mycobacterium tuberculosis isolates, rapid growth of the bacteria within macrophages resulted in cell damage and lung necrosis that were mediated by innate immune sensing of a damage signal from dying cells, extracellular ATP (71). Moreover, a specific genomic locus in M. tuberculosis, RD1, contributes to bacterial virulence, necrosis, and the secretion of mature IL-1α through the induction of calcium influx (54, 72). In an infection model of porcine reproductive and respiratory syndrome virus (PRRSV), animals that were infected with a highly pathogenic PRRSV isolate showed enhanced IL-1α expression in the lung, which correlated with high scores for lung pathology, compared to the low-virulence isolates (70). Together, data from those studies support our findings that the hypervirulent isolates of a given pathogen may induce an immune response that is highly dependent on IL-1α due to an increase in inflammatory cell death. Our results present an opportunity to identify fungal factors that promote host damage and highly pathological IL-1α responses, and approaches to identify these factors are ongoing in our laboratories.
Fungal germination is initiated immediately after the instillation of fungal conidia into the airways based on the nutrients present. Why certain isolates can germinate better than others remains elusive, but it could be because of genetic differences between isolates or due to the environmental origins of the Aspergillus conidia. In this regard, data from our in vitro germination assay using lung homogenate medium consistently corresponded with the results of our in vivo germination studies. These data suggest that the free nutrients in the lungs are sufficient to drive the in vivo growth differences. However, it is unclear which specific nutrients in the lungs/airways drive the growth of A. fumigatus isolates. Alternatively, a factor found within the lungs may directly inhibit the germination of the less-virulent isolates. More studies are necessary to determine the specific contributions of and mechanisms behind nutrient acquisition/utilization and other factors in the airways that lead to fungal isolate-specific growth and lung damage profiles.
Differences between how conidia and germlings interact with the respiratory epithelium and lung-resident macrophages are likely critical for establishing the early inflammatory response. Our data demonstrate that the germlings of A. fumigatus are much more pathological to macrophages and drive greater IL-1α secretion than do swollen conidia in general. However, it also appears that swollen conidia of the hypervirulent CEA10 strain are more damaging to macrophages. This suggests that even in the presence of macrophages, hypervirulent A. fumigatus isolates may continue to grow through either increased nutrient sensing in the phagosome or increased resistance to the antifungal effector functions of macrophages. Similarly, C. albicans drives pyroptosis-dependent and -independent cell death pathways in macrophages, which contributes to the IL-1β response from macrophages (63, 73–76), but its role in IL-1α secretion has not been explored. For C. albicans, it is well established that within the macrophage phagosome, the fungi encounter significant nutrient stress, which must be dealt with in order for the fungi to continue to grow and escape the macrophage (77, 78). Interestingly, Shah et al. showed that A. fumigatus conidia of the CEA10 strain have the ability to germinate within the late phagosomes of macrophages (79). This germination initiates necroptotic cell death of macrophages, resulting in the lateral transfer of hyphae to other macrophages in order to prevent hyphal escape and maintain control of fungal germination, which is associated with the transcriptional upregulation of Il1a (79). The metabolic response that enables this growth in A. fumigatus is unknown, but in C. albicans, amino acid catabolism is essential for the continued growth necessary for phagosome escape and the induction of pyroptosis (80, 81). Our data support that A. fumigatus must be metabolically active and alive to induce robust macrophage cell death and IL-1α release. Current studies are aimed at identifying the fungal effector molecule(s) responsible for inducing macrophage cell death and IL-1α release.
Given the drastic differences in the levels of virulence and the immune responses induced by numerous A. fumigatus isolates, it will be important to identify central inflammatory hubs that are necessary for host resistance against an array of isolates in order to maximize any potential immunotherapeutic interventions to treat IA. Further studies are needed to examine the factors that contribute to fungal germination in the host, which could lead to novel therapeutic targets for the treatment of IA. Understanding the response to several clinically relevant isolates will expand our understanding of host-pathogen interactions during aspergillosis and give insights into the fungal factors that contribute to adaptation to the lung environment and allow disease to manifest in different patient populations.
MATERIALS AND METHODS
Mice.
C57BL/6J (stock 000664; Jackson Laboratory), Il1r1−/− (stock 03245; Jackson Laboratory), Casp1/11−/− (stock 016621; Jackson Laboratory), P2rx7−/− (stock 005576; Jackson Laboratory), Il1a−/− (82), Pycard−/− (ASC-deficient) (83), and Il1a/b−/− (84) mice were bred in-house. All mice were 8 to 10 weeks of age at the time of challenge. All animal experiments were approved by either the Montana State University Institutional Animal Care and Use Committee or the Dartmouth College Institutional Animal Care and Use Committee.
Preparation of Aspergillus fumigatus conidia.
Aspergillus fumigatus strains and isolates CEA10, Af293, 02-10, W72310, and EVOL20 were used for this study, and the origins of these strains and isolates were reported previously (37). Each strain or isolate was grown on glucose minimal medium (GMM) agar plates for 3 days at 37°C. Conidia were harvested by adding 0.01% Tween 80 to plates and gently scraping conidia from the plates by using a cell scraper. Conidia were then filtered through sterile Miracloth, washed and resuspended in phosphate-buffered saline (PBS), and counted on a hemacytometer.
To make Af293 germlings for our sufficiency experiment, resting conidia of Af293 were incubated at 30°C in a shaking-platform incubator for 8 to 9 h in liquid glucose minimal medium containing 1% yeast extract. At 8 h, the conidia were checked microscopically for swelling/germ tube formation and subsequently returned to the shaker for incubation until germ tubes emerged. Once germ tubes formed, the sample was vortexed and transferred to a 10-ml tube containing 1.0-mm disruption beads (Research Products International Corp.). The sample and beads were vortexed on the highest setting for 2 min and subsequently transferred to a Dounce homogenizer to further break up any clumps of germlings. Conidia in the sample were then recounted on a hemacytometer, since during the shaking process, the fungi began to stick to the walls of the flask, decreasing the concentration. The concentration of resting conidia was then adjusted to the germling concentration, and the conidia were vortexed with beads and homogenized in a Dounce homogenizer for consistency. C57BL/6 mice or Il1a−/− mice were infected with ∼2.7 × 107 resting Af293 conidia or germlings, and at 12 hpi, BALF was collected for analysis of damage, vascular leakage, and inflammatory cell recruitment.
Aspergillus fumigatus pulmonary challenge model.
Mice were challenged with A. fumigatus conidia by the intratracheal (i.t.) route. Mice were anesthetized by the inhalation of isoflurane; subsequently, mice were challenged i.t. with ∼4 × 107 A. fumigatus conidia in a volume of 100 μl PBS. For the Af293 germling experiment, mice were challenged with ∼2.7 × 107 resting conidia or germlings in a volume of 100 μl of liquid GMM containing 1% yeast extract. At the indicated times after A. fumigatus challenge, mice were euthanized by using a lethal overdose of pentobarbital. BALF was collected by washing the lungs with 2 ml of PBS containing 0.05 M EDTA. BALF was clarified by centrifugation and stored at −20°C until analysis. After centrifugation, the cellular component of the BALF was resuspended in 200 μl of PBS, and total numbers of BALF cells were determined by counting with a hemacytometer. BALF cells were subsequently spun onto glass slides by using a Cytospin4 cytocentrifuge (Thermo Scientific) and stained with Diff-Quik stain set (Siemens) for differential counting. For histological analysis, lungs were filled with and stored in 10% buffered formalin phosphate for at least 24 h. Lungs were then embedded in paraffin and sectioned into 5-μm sections. Sections were stained with hematoxylin and eosin (H&E) and GMS by using standard histological techniques to assess lung inflammatory infiltrates and fungal germination, respectively. Representative pictures of lung sections were taken with an Olympus BX50WI microscope with a QImaging Retiga 2000R camera. For cytokine analysis, lungs were homogenized in 2 ml of PBS. After clarification, lung homogenates were stored at −20°C until analysis.
Determination of in vivo germination of A. fumigatus.
As described above, mice were challenged with A. fumigatus conidia by the i.t. route, and at 12 hpi, BALF was collected. BALF cells were spun onto glass slides by using a Cytospin4 cytocentrifuge (Thermo Scientific) and stained with Diff-Quik stain set (Siemens) for differential counting. Fungal conidia/germlings could be visualized in these cytospins. The percent germination of each A. fumigatus strain or isolate was quantified by manual counting of 100 to 400 fungal conidia and germlings at a ×100 magnification by using a standard upright microscope.
In vitro germination assays.
The germination potential of each A. fumigatus strain or isolate was tested with either GMM plus yeast extract or lung homogenate medium. GMM is a minimal medium that contains 1% glucose in a base of salts and trace elements (85). To make it a nutrient-rich medium, we added 0.5 g/liter of yeast extract (Amerco). Finally, to mimic the nutrient availability in the lungs at the time of challenge, we utilized lung homogenates. To make lung homogenate medium, lungs from 6- to 12-week-old C57BL/6J mice were homogenized through a 70-μm filter in 2 ml of PBS. The lung homogenate was then clarified and stored at −20°C until use. For experiments, the lung homogenate was diluted 1:4 in PBS prior to use. Experimental cultures were inoculated with 107 conidia/ml in 2 ml of medium in glass 20-ml disposable scintillation vials (VWR). Cultures were shaken at 300 rpm at 37°C in an Excella E24 shaking incubator (New Brunswick Scientific). Starting at 4 h, the germination of A. fumigatus conidia was determined every 2 h. To quantify A. fumigatus germination, a 200-μl sample was collected and added to a microtube containing 1.0-mm glass beads (Research Products International Corp.). The microtube was then vortexed to disrupt any clumps of A. fumigatus. The solution was then placed onto a glass slide, and a coverslip was placed onto the slide, at which point fungal germination was quantified manually by using the 40× objective lens of an upright VWR microscope. A minimum of 100 conidia and germlings was counted for each sample.
Preparation of bone marrow-derived macrophages.
Bone marrow cells were eluted from tibias and femurs of 8- to 12-week-old mice of the appropriate genotype (C57BL/6, Casp1/11−/−, or P2rx7−/−) and cultured for macrophages in RPMI containing 20% fetal calf serum (FCS), 5 mM HEPES buffer, 1.1 mM l-glutamine, 0.5 U/ml penicillin, and 50 mg/ml streptomycin supplemented with 30% (vol/vol) L929 cell supernatant (source of macrophage colony-stimulating factor [M-CSF]). Bone marrow cells for macrophages were plated in a volume of 10 ml in 100-mm by 15-mm sterile polystyrene petri dishes (catalog number FB0875712; Fisher Scientific). Bone marrow cells from each mouse were split evenly into 5 plates. The medium was supplemented on day 3 with an additional 10 ml of macrophage medium. Adherent BMDM were harvested on day 6 by using ice-cold PBS.
In vitro activation of bone marrow-derived macrophages with Aspergillus fumigatus.
BMDM cells were washed with clear RPMI containing 10% FCS, 5 mM HEPES buffer, 1.1 mM l-glutamine, 0.5 U/ml penicillin, and 50 mg/ml streptomycin (TC medium). Immediately prior to the addition of BMDM to A. fumigatus cultures, BMDM were stimulated with 1 μg/ml of LPS (InvivoGen). For the calpain inhibitor studies, BMDM were treated with either dimethyl sulfoxide (DMSO) (vehicle), 30 μM calpeptin (Enzo Life Sciences), or 40 μM MDL28170 (Cayman Chemical) immediately prior to the addition of A. fumigatus. Swollen conidia were generated by culturing resting conidia in TC medium for 16 h at room temperature followed by 2 h at 37°C in a flat-bottom 96-well plate. Germlings were generated by culturing resting conidia in TC medium overnight at 30°C and then shifting the culture to 37°C for 2 h in a flat-bottom 96-well plate. For the UV inactivation studies, swollen conidia and germlings were UV irradiated three times at 6,000 mJ/cm2 for 1 min each by using a CL-1000 ultraviolet cross-linker (UVP). When ready, the plates were centrifuged at 1,500 rpm to pellet the Aspergillus fungi. After centrifugation, the medium was removed, and BMDM cells were added on top of the swollen conidia or germlings of A. fumigatus in 0.2 ml TC medium at a density of 5 × 105 cells/ml in a 96-well plate. BMDM cells were stimulated for 24 h at 37°C, after which the supernatants were collected and stored at −20°C until use.
ELISA for detection of IL-1α and LDH secretion by bone marrow-derived macrophages.
Commercially available enzyme-linked immunosorbent assay (ELISA) kits for IL-1α (BioLegend) and IL-1β (R&D Systems) and an LDH CytoTox 96 cytotoxicity assay (Promega) were used according to the manufacturers' instructions. Plates were read by using a SpectraMax Paradigm plate reader (Molecular Devices).
Quantification of lung damage and leakage.
To assess lung damage (39), bronchoalveolar lavage fluid was analyzed by measuring lactate dehydrogenase levels using a CytoTox 96 cytotoxicity assay (Promega) according to the manufacturer's instructions. To assess vascular/pulmonary leakage (40), bronchoalveolar lavage fluid was analyzed by using an Albumin BCG Reagent set (Eagle Diagnostics). A standard curve was made by diluting the calibrator in PBS-EDTA. Next, 100 μl of the sample or standard was transferred to a 96-well flat-bottom plate, mixed with 100 μl of BCG reagent, allowed to sit at room temperature (RT) for 5 min, and then read on a plate reader at 630 nm.
Statistical analysis.
For correlation analyses, Spearman's correlation coefficient was calculated to assess correlation. For survival studies, a Mantel-Cox log-rank test was used to determine whether there was a significant difference in survival between C57BL/6 and Il1a−/− mice for each A. fumigatus isolate/strain. Statistical significance between experimental groups was determined by using a Mann-Whitney U test (comparison of two experimental groups that are not normally distributed), one-way analysis of variance (ANOVA) using a Bonferroni posttest (comparison of more than two experimental groups), Kruskal-Wallis one-way ANOVA with Dunn's posttest (comparison of more than two experimental groups), or two-way ANOVA with a Tukey posttest (comparison of experimental groups across two independent variables/factors). All graphs were generated and all statistical analysis were conducted by using GraphPad Prism 5 software.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brent Berwin (Dartmouth College), Russell Vance (UC Berkeley), Gabriel Nunez (University of Michigan), and Vishiva Dixit (Genetech) for providing the original breeding pairs of the genetic knockout mice. We thank Darius Armstrong-James for graciously providing the 02-10 isolate for these studies.
Research in this study was funded in part by NIH grant R01 AI081838 (R.A.C.). J.J.O. and R.A.C. were supported in part by institutional startup funds and in part through Dartmouth Lung Biology Center for Molecular, Cellular, and Translational Research grant P30 GM106394 (principal investigator, Bruce A. Stanton) and Center for Molecular, Cellular, and Translational Immunological Research grant P30 GM103415 (principal investigator, William R. Green). Research in this study was funded in part by a grant from the Munck-Pfefferkorn Novel and Interactive Research Fund at Dartmouth College (J.J.O.). This work was funded in part by NIH grants R01 AI093808 and R21 AI105617 to T.M.H. and in part through the NIH/NCI Cancer Center Support grant P30 CA008748. R.A.C. and T.M.H. are investigators in the pathogenesis of infectious diseases supported by the Burroughs Wellcome Fund. The funders had no role in the preparation or publication of the manuscript.
A.K.C.-C., R.A.C., and J.J.O. conceived of and designed the experiments. A.K.C.-C., C.H.K., S.R.B., N.A.B., A.T., and H.E.L. performed the experiments. A.K.C.-C., N.A.B., C.R.U., and H.E.L. analyzed the data. A.K.C.-C. and J.J.O. wrote the paper. T.H.M. and Y.-W.T. provided key reagents.
We have no conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00661-17.
REFERENCES
- 1.Segal BH. 2009. Aspergillosis. N Engl J Med 360:1870–1884. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari J, Wolach O, Gavrieli R, Wolach B. 2012. Infections associated with chronic granulomatous disease: linking genetics to phenotypic expression. Expert Rev Anti Infect Ther 10:881–894. doi: 10.1586/eri.12.77. [DOI] [PubMed] [Google Scholar]
- 3.Morgenstern DE, Gifford MAC, Li LL, Doerschuk CM, Dinauer MC. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med 185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollock JD, Williams DA, Gifford MAC, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 6.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis 50:1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan S-P, Meier-Kriesche H-U, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 65:453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. 2008. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 10.Thompson GR, Patterson TF. 2008. Pulmonary aspergillosis. Semin Respir Crit Care Med 29:103–110. doi: 10.1055/s-2008-1063849. [DOI] [PubMed] [Google Scholar]
- 11.Gresnigt MS, van de Veerdonk FL. 2014. The role of interleukin-1 family members in the host defence against Aspergillus fumigatus. Mycopathologia 178:395–401. doi: 10.1007/s11046-014-9776-y. [DOI] [PubMed] [Google Scholar]
- 12.Caffrey AK, Obar JJ. 2016. Alarmin(g) the innate immune system to invasive fungal infections. Curr Opin Microbiol 32:135–143. doi: 10.1016/j.mib.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liles WC, Huang JE, van Burik JA, Bowden RA, Dale DC. 1997. Granulocyte colony-stimulating factor administered in vivo augments neutrophil-mediated activity against opportunistic fungal pathogens. J Infect Dis 175:1012–1015. doi: 10.1086/513961. [DOI] [PubMed] [Google Scholar]
- 14.Bandera A, Trabattoni D, Ferrario G, Cesari M, Franzetti F, Clerici M, Gori A. 2008. Interferon-gamma and granulocyte-macrophage colony stimulating factor therapy in three patients with pulmonary aspergillosis. Infection 36:368–373. doi: 10.1007/s15010-008-7378-7. [DOI] [PubMed] [Google Scholar]
- 15.Peters BG, Adkins DR, Harrison BR, Velasquez WS, Dunphy FR, Petruska PJ, Bowers CE, Niemeyer R, McIntyre W, Vrahnos D, Auberry SE, Spitzer G. 1996. Antifungal effects of yeast-derived rhu-GM-CSF in patients receiving high-dose chemotherapy given with or without autologous stem cell transplantation: a retrospective analysis. Bone Marrow Transplant 18:93–102. [PubMed] [Google Scholar]
- 16.Obar JJ, Hohl TM, Cramer RA. 2016. New advances in invasive aspergillosis immunobiology leading the way towards personalized therapeutic approaches. Cytokine 84:63–73. doi: 10.1016/j.cyto.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa V, Rivera A. 2016. First line of defense: innate cell-mediated control of pulmonary aspergillosis. Front Microbiol 7:272. doi: 10.3389/fmicb.2016.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deepe GS, McGuinness M. 2006. Interleukin-1 and host control of pulmonary histoplasmosis. J Infect Dis 194:855–864. doi: 10.1086/506946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. 2006. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis 193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 20.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 21.Van't Wout JW, Van der Meer JWM, Barza M, Dinarello CA. 1988. Protection of neutropenic mice from lethal Candida albicans infection by recombinant interleukin 1. Eur J Immunol 18:1143–1146. doi: 10.1002/eji.1830180728. [DOI] [PubMed] [Google Scholar]
- 22.Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ. 2015. IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 24.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. 2009. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD. 2015. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 17:357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S, van de Veerdonk FL, Dinarello CA, Romani L. 2014. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog 10:e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sainz J, Pérez E, Gómez-Lopera S, Jurado M. 2008. IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol 28:473–485. doi: 10.1007/s10875-008-9197-0. [DOI] [PubMed] [Google Scholar]
- 29.Wójtowicz A, Gresnigt MS, Lecompte T, Bibert S, Manuel O, Joosten LAB, Rüeger S, Berger C, Boggian K, Cusini A, Garzoni C, Hirsch HH, Weisser M, Mueller NJ, Meylan PR, Steiger J, Kutalik Z, Pascual M, van Delden C, van de Veerdonk FL, Bochud P-Y, Binet I, De Geest S, van Delden C, Hofbauer GFK, Huynh-Do U, Koller MT, Lovis C, Manuel O, Meylan P, Mueller NJ, Pascual M, Schaub S, Steiger J. 2015. IL1B and DEFB1 polymorphisms increase susceptibility to invasive mold infection after solid-organ transplantation. J Infect Dis 211:1646–1657. doi: 10.1093/infdis/jiu636. [DOI] [PubMed] [Google Scholar]
- 30.Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. 2009. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol 200:303.e1–303.e6. doi: 10.1016/j.ajog.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Garlanda C, Dinarello CA, Mantovani A. 2013. The interleukin-1 family: back to the future. Immunity 39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. 2007. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 33.Al Moussawi K, Kazmierczak BI. 2014. Distinct contributions of interleukin-1alpha (IL-1alpha) and IL-1beta to innate immune recognition of Pseudomonas aeruginosa in the lung. Infect Immun 82:4204–4211. doi: 10.1128/IAI.02218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. 2011. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 35.Jhingran A, Kasahara S, Shepardson KM, Junecko BA, Heung LJ, Kumasaka DK, Knoblaugh SE, Lin X, Kazmierczak BI, Reinhart TA, Cramer RA, Hohl TM. 2015. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog 11:e1004589. doi: 10.1371/journal.ppat.1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bretz C, Gersuk G, Knoblaugh S, Chaudhary N, Randolph-Habecker J, Hackman RC, Staab J, Marr KA. 2008. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect Immun 76:952–958. doi: 10.1128/IAI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowalski CH, Beattie SR, Fuller KK, McGurk EA, Tang Y-W, Hohl TM, Obar JJ, Cramer RA. 2016. Heterogeneity among isolates reveals that fitness in low oxygen correlates with Aspergillus fumigatus virulence. mBio 7:e01515-16. doi: 10.1128/mBio.01515-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, Cavalieri D. 2013. Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PLoS One 8:e56651. doi: 10.1371/journal.pone.0056651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drent M, Cobben N, Henderson R, Wouters E, van Dieijen-Visser M. 1996. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 40.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M, Harmsen A. 2009. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One 4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallach D, Kang T-B, Dillon CP, Green DR. 2016. Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 42.Marr KA, Balajee SA, Hawn TR, Ozinsky A, Pham U, Akira S, Aderem A, Liles WC. 2003. Differential role of MyD88 in macrophage-mediated responses to opportunistic fungal pathogens. Infect Immun 71:5280–5286. doi: 10.1128/IAI.71.9.5280-5286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Said-Sadier N, Padilla E, Langsley G, Ojcius DM. 2010. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. 2012. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Dagvadorj J, Shimada K, Chen S, Jones HD, Tumurkhuu G, Zhang W, Wawrowsky KA, Crother TR, Arditi M. 2015. Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2x7 receptor leading to interleukin-1α release. Immunity 42:640–653. doi: 10.1016/j.immuni.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. 2013. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity 38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. 1990. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci U S A 87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carruth LM, Demczuk S, Mizel SB. 1991. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem 266:12162–12167. [PubMed] [Google Scholar]
- 50.Dagenais TRT, Keller NP. 2009. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayer K, Stratford M, Archer DB. 2014. Germination of Aspergillus niger conidia is triggered by nitrogen compounds related to l-amino acids. Appl Environ Microbiol 80:6046–6053. doi: 10.1128/AEM.01078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayer K, Stratford M, Archer DB. 2013. Structural features of sugars that trigger or support conidial germination in the filamentous fungus Aspergillus niger. Appl Environ Microbiol 79:6924–6931. doi: 10.1128/AEM.02061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller NP. 2017. Heterogeneity confounds establishment of “a” model microbial strain. mBio 8:e00135-17. doi: 10.1128/mBio.00135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR. 2003. The primary mechanism of attenuation of bacillus Calmette-Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Planet PJ, LaRussa SJ, Dana A, Smith H, Xu A, Ryan C, Uhlemann A-C, Boundy S, Goldberg J, Narechania A, Kulkarni R, Ratner AJ, Geoghegan JA, Kolokotronis S-O, Prince A. 2013. Emergence of the epidemic methicillin-resistant Staphylococcus aureus strain USA300 coincides with horizontal transfer of the arginine catabolic mobile element and speG-mediated adaptations for survival on skin. mBio 4:e00889-13. doi: 10.1128/mBio.00889-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker D, Planet PJ, Soong G, Narechania A, Prince A. 2014. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog 10:e1003951. doi: 10.1371/journal.ppat.1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Saïd-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol 175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, Whitney AR, Sturdevant DE, Dorward DW, Holland SM, Kreiswirth BN, Musser JM, DeLeo FR. 2010. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun 2:560–575. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 60.Jayatilake JA, Samaranayake LP, Lu Q, Jin LJ. 2007. IL-1alpha, IL-1ra and IL-8 are differentially induced by Candida in experimental oral candidiasis. Oral Dis 13:426–433. doi: 10.1111/j.1601-0825.2007.01318.x. [DOI] [PubMed] [Google Scholar]
- 61.Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. 2002. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol 118:652–657. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 62.Villar CC, Kashleva H, Dongari-Bagtzoglou A. 2004. Role of Candida albicans polymorphism in interactions with oral epithelial cells. Oral Microbiol Immunol 19:262–269. doi: 10.1111/j.1399-302X.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 63.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. 2016. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog 12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schonherr FA, Sparber F, Kirchner FR, Guiducci E, Trautwein-Weidner K, Gladiator A, Sertour N, Hetzel U, Le GTT, Pavelka N, d'Enfert C, Bougnoux ME, Corti CF, LeibundGut-Landmann S. 2017. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol 10:1335–1350. doi: 10.1038/mi.2017.2. [DOI] [PubMed] [Google Scholar]
- 65.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 66.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. 2015. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milora KA, Miller SL, Sanmiguel JC, Jensen LE. 2014. Interleukin-1α released from HSV-1 infected keratinocytes acts as a functional alarmin in the skin. Nat Commun 5:5230. doi: 10.1038/ncomms6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Paolo NC, Baldwin LK, Irons EE, Papayannopoulou T, Tomlinson S, Shayakhmetov DM. 2014. IL-1alpha and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog 10:e1004035. doi: 10.1371/journal.ppat.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Paolo NC, Miao EA, Iwakura Y, Kaja MK, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. 2009. Virus sensing at the plasma membrane triggers interleukin-1α-mediated pro-inflammatory macrophage response in vivo. Immunity 31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amarilla SP, Gómez-Laguna J, Carrasco L, Rodríguez-Gómez IM, Caridad y Ocerín JM, Morgan SB, Graham SP, Frossard J-P, Drew TW, Salguero FJ. 2015. A comparative study of the local cytokine response in the lungs of pigs experimentally infected with different PRRSV-1 strains: upregulation of IL-1α in highly pathogenic strain induced lesions. Vet Immunol Immunopathol 164:137–147. doi: 10.1016/j.vetimm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Amaral EP, Ribeiro SCM, Lanes VR, Almeida FM, de Andrade MRM, Bomfim CCB, Salles ÉM, Bortoluci KR, Coutinho-Silva R, Hirata MH, Alvarez JM, Lasunskaia EB, D'Império-Lima MR. 2014. Pulmonary infection with hypervirulent mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog 10:e1004188. doi: 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang R, Xi C, Sita DR, Sakai S, Tsuchiya K, Hara H, Shen Y, Qu H, Fang R, Mitsuyama M, Kawamura I. 2014. The RD1 locus in the Mycobacterium tuberculosis genome contributes to the maturation and secretion of IL-1α from infected macrophages through the elevation of cytoplasmic calcium levels and calpain activation. Pathog Dis 70:51–60. doi: 10.1111/2049-632X.12075. [DOI] [PubMed] [Google Scholar]
- 73.O'Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. 2015. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun 6:6741. doi: 10.1038/ncomms7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, Traven A. 2014. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio 5:e00003-14. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wellington M, Koselny K, Krysan DJ. 2012. Candida albicans morphogenesis is not required for macrophage interleukin 1β production. mBio 4:e00433-12. doi: 10.1128/mBio.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. 2014. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barelle CJ, Priest CL, MacCallum DM, Gow NAR, Odds FC, Brown AJP. 2006. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol 8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah A, Kannambath S, Herbst S, Rogers A, Soresi S, Carby M, Reed A, Mostowy S, Fisher MC, Shaunak S, Armstrong-James DP. 2016. Calcineurin orchestrates lateral transfer of Aspergillus fumigatus during macrophage cell death. Am J Respir Crit Care Med 194:1127–1139. doi: 10.1164/rccm.201601-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vylkova S, Lorenz MC. 2017. Phagosomal neutralization by the fungal pathogen Candida albicans induces macrophage pyroptosis. Infect Immun 85:e00832-16. doi: 10.1128/IAI.00832-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vylkova S, Lorenz MC. 2014. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog 10:e1003995. doi: 10.1371/journal.ppat.1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. 1998. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med 187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 84.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. 2007. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol 179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 85.Shimizu K, Keller NP. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.