Abstract
Background: Various trivalent radiometals are well suited for labeling of DOTA-conjugated variants of Glu-ureido-based prostate-specific membrane antigen (PSMA) inhibitors. The DOTA-conjugate PSMA-617 has proven high potential in PSMA radioligand therapy (PSMA-RLT) of prostate cancer as well as PET imaging when labeled with lutetium-177 and gallium-68 respectively. Considering the relatively short physical half-life of gallium-68 this positron emitter precludes prolonged acquisition periods, as required for pre-therapeutic dosimetry or intraoperative applications. In this context, the positron emitter scandium-44 is an attractive alternative for PET imaging. We report the synthesis of [44Sc]Sc-PSMA-617 as radiopharmaceutical with generator produced scandium-44, its in vitro characterization and clinical translation as part of a first in-human study.
Methods: Scandium-44 was obtained from a 44Ti/44Sc radionuclide generator. PSMA-617 was labeled with 142.4±12.7 MBq of scandium-44 in analogy to [68Ga]Ga-PSMA-617 and evaluated in vitro and in cell studies using PSMA+ LNCaP cells. A first-in-human investigation was subsequently carried out in a cohort of 4 patients (mean age 70±1.8 a) registered for [177Lu]Lu-PSMA-617 therapy. 50.5±9.3 MBq (40 µg, 38.4 nmol) [44Sc]Sc-PSMA-617 were applied via intravenous injection (i.v.), respectively. A Siemens Biograph 2 PET/CT system was used to acquire initial dynamic PET data (30 min) of abdomen in list mode followed by static PET/CT data (skull to mid-thigh) at 45 min, 2 and 18 h post-injection (p.i.). For quantitative analysis, dynamic images were reconstructed as 6 data sets of 300 s each. The noise ratio was measured in liver, lung and an additional region outside the body. SUV values in different organs and lesions were measured and compared to [68Ga]Ga-PSMA-11 data of the same patients. Residence times and organ absorbed doses were calculated using OLINDA/EXM software.
Results: Quantitative radiochemical yields of ≥98 % were achieved using 18 nmol of PSMA-617 after 20 min at 95 °C with apparent molar activity of 6.69±0.78 MBq/nmol. Following purification, >99 % radiochemical purity was obtained. [44Sc]Sc-PSMA-617 showed high stability (>95 %) in serum for 24 h. The binding affinity and internalization fraction were determined in PSMA+ LNCaP cells (IC50 = 4.72±0.7 nM and internalization fraction: 15.78±2.14 % IA/106 LNCaP cells) and compared to [68Ga]Ga-PSMA-11 (12.0±2.8 nM and 9.47±2.56 % IA/106 LNCaP cells). Physiological tracer uptake was observed in kidneys, liver, spleen, small intestine, urinary bladder, and salivary glands and pathological uptake in both soft and skeletal metastases. SUV values were significantly lower in the kidneys (14.0) compared to [68Ga]Ga-PSMA-11 OET (30.5). All other measured SUV values did not show a statistically significant difference. Tumor to liver ratios were found to lie between 1.9 and 8.3 for [68Ga]Ga-PSMA-11 and between 2.5 and 8.8 for [44Sc]Sc-PSMA-617 after 120 min. For [44Sc]Sc-PSMA-617 the ratios were higher and no statistically significant differences were observed. Total and % activity were highest in liver followed by kidneys, spleen, small intestine and salivary glands. Rapid wash out was seen in liver and spleen and gradually over time in kidneys. Kidneys received the highest radiation absorbed dose of 0.354 (0.180-0.488) mSv/MBq. No adverse pharmacological effects were observed.
Conclusion: In conclusion [44Sc]Sc-PSMA-617 PET is suitable for PET imaging of prostate cancer tissue. [44Sc]Sc-PSMA-617 shows promise to enable pre-therapeutic dosimetry in clinical settings. However, the clinical advantages for individual dosimetry or other applications like intraoperative applications have to be investigated in further studies.
Keywords: prostate cancer, PSMA-617, scandium-44, PET, theranostics.
Introduction
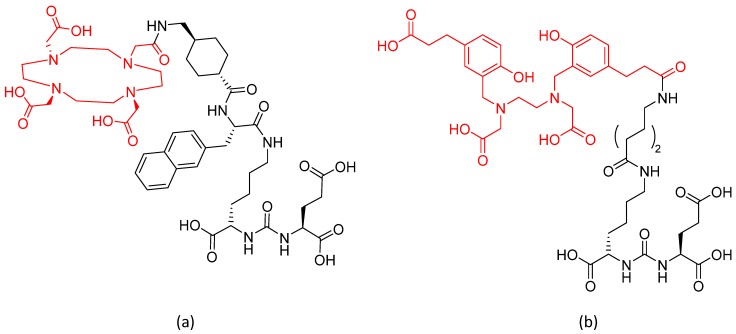
Prostate specific membrane antigen (PSMA) is expressed in prostate tissue and highly upregulated in prostate cancer 1, 2. As PSMA is a transmembrane molecule, its extracellular N-terminal part containing the catalytic domain is suitable for selective tumor targeting 3. A number of different radiopharmaceuticals binding to PSMA have been developed and correlated to prostate specific antigen (PSA) levels. Remarkably, radiolabeled low-molecular weight compounds presented as very promising prostate cancer imaging agents 4-7. Consequently, low-molecular-weight urea-based peptidomimetic inhibitors with high affinity to the catalytic domain of PSMA were developed 5, 8 of which the DOTA derivative PSMA-617 labeled with lutetium-177 (therapeutic radionuclide) or gallium-68 (diagnostic radionuclide) turned out to be a highly powerful theranostic tool for prostate cancer (Figure 1).
Figure 1.
Structures of (a) PSMA-617 and (b) PSMA-11.
First experiences showed that [177Lu]Lu-PSMA-617 is safe and effective in treatment of metastasized castration-resistant prostate cancer (mCRPC) and pre-therapeutic dosimetry may support to improve therapy planning as well as pre-selection of patients 9-14. Pre-therapeutic estimation of the dose of lutetium-177 delivered to the target site, organs at risk, and the whole body would help to predict therapy response and side effects of a certain therapeutic activity and facilitate individual dose adjustment 15. Currently pre-therapeutic dosimetry is performed using small amounts of [177Lu]Lu-PSMA-617 as suitable alternatives using positron emission tomography (PET) nuclides are still to be established 13. Due to the regulatory necessity of having to hospitalize the patient when using lutetium-177 for pre-therapeutic dosimetry it is often not performed at all. However, patients may benefit from higher therapeutic doses and therefore ways to implement individual dosimetry in the clinical workflow have to be investigated.
Currently gallium-68 is the most frequently used radionuclide for PET imaging utilized as [68Ga]Ga-PSMA-11, which is currently the most commonly used PSMA-targeted PET tracer worldwide 8,16. Even though its decay characteristics are ideal for PET imaging and its good availability via 68Ge/68Ga generators, gallium-68 has disadvantages. Due to its high positron energy (β+ = 89 %, Ē = 0.74 MeV) images tend to be more noisy compared to 18F-labeled tracers. Moreover, its short physical half-life of 68 min limits its application as it only covers imaging periods of a few hours. Late imaging as well as extended dosimetric calculations simply cannot be performed using gallium-68. Another application gallium-68 is not suitable for is the use for intraoperative applications several hours post-injection (p.i.). For this reason, the positron-emitter scandium-44 as an alternative to gallium-68 is in the focus of current research 17-20 resulting in initial human applications of [44Sc]Sc-DOTATOC for imaging neuroendocrine tumors 21, 22. First preclinical investigations with [44Sc]Sc-PSMA-617 utilizing cyclotron-produced scandium-44 have been performed as well 23.
Scandium-44 (β+ = 94 %, Ē = 0.597 MeV) has a physical half-life of 3.97 h and can be generated by a titanium-44 generator or produced by a cyclotron 22, 24. Just like gallium-68, scandium-44 is a trivalent cation but its physical half-life is almost 4 times longer. It provides a longer imaging period covering up to 24 h post injection. Moreover, its coordination chemistry is similar to lutetium‑177. Another potential advantage of Scandium(III) in nuclear medicine is the potential usage of its radioisotope scandium-47 (β-, primary γ-ray of 159 keV and t½ = 3.3 d) for therapy, constituting a matched pair of radioisotopes both for imaging and therapy thereby applicable in authentic Sc-labelled theranostic radiopharmaceuticals 20, 25-29.
The growing interest in scandium-44 motivated research for production routes providing activities in the GBq range, which are not accessible via the generator. Scandium-44 production using the 180 MBq titanium-44 generator requires adequate quantities of the parent radionuclide titanium-44 which can be produced by the 45Sc(p,2n)44Ti nuclear reaction. This production route limits the availability of titanium-44 because of the prolonged target irradiation times 30, 31. In comparison cyclotron-production using the 44Ca(p,n)44Sc nuclear reaction provides scandium-44 in sufficiently high activities with radionuclidic purities >99 % 32 and circumvents the problem of 44Ti-waste management.
The aim of this work was to determine optimal conditions for radiolabeling of PSMA-617 with generator produced scandium-44 used as prostate cancer imaging agent. Investigation of parameters influencing reaction kinetics like incubation time, temperature and amount of precursor were performed. Stability of the labeled compound was examined in vitro. Binding affinity and internalization of [44Sc]Sc-PSMA-617 were studied in LNCaP cells and compared to those of the hitherto known lutetium-177 and gallium-68 labelled analogues. Ultimately [44Sc]Sc‑PSMA-617 was successfully transferred to human application with four patients being examined, imaging features evaluated and compared to those obtained during [68Ga]Ga-PSMA-617 studies.
Materials and Methods
Reagents were purchased from Sigma-Aldrich (Germany) and used without further purification, unless otherwise stated. TraceSelect water was used for all generator and reaction solutions. No further special measures were taken regarding working under strict metal-free conditions. GMP-grade PSMA-617 was obtained from ABX advanced biochemical compounds (Radeberg, Germany).
Radioactivity was determined using an ionization chamber (ISOMED 2010; Nuklear-Medizintechnik Dresden GmbH, Germany). RadioTLC was performed using silica gel coated aluminum sheets (silica-gel 60 F254; Merck, Darmstadt, Germany) and glass microfiber chromatography paper impregnated with silica-gel (iTLC-SG, Agilent Technologies, Santa Clara, California). Analysis was performed with a single trace TLC scanner (PET-miniGita; Elysia-raytest, Straubenhardt, Germany). RadioHPLC was performed using an Agilent 1260 reverse phase HPLC system equipped with a Gabi γ-HPLC flow detector (Elysia-raytest, Straubenhardt, Germany) and a PC interface running Gina Star software (Elysia-raytest, Straubenhardt, Germany). Identification and quantification of long-living impurities was performed by a multi-channel analyzer for γ-spectroscopy (Mucha, Elysia-raytest, Straubenhardt, Germany). pH was measured using a pH-meter (SevenCompact 220, Mettler-Toledo, Columbus, USA).
Synthesis
Labelling of PSMA-617 with scandium-44
Scandium-44 was obtained from a prototype 185 MBq 44Ti/44Sc generator 26. Scandium-44 was eluted and post-processed according to literature 20, 26, 28. The resulting small volume (3 mL) of oxalate-free scandium-44 solution containing 142.4±12.7 MBq was used for further processing 20.
For labeling of PSMA-617 with scandium-44, different volumes of PSMA-617 stock solution (1 mg/mL in water) were mixed with 3 mL of post-processed 44Sc-eluate in 0.25 M ammonium acetate buffer (pH 4.0). The effect of PSMA-617 concentration on labeling yield was evaluated by use of different amounts of precursor varying from 14 to 26 nmol. Solutions were heated in a thermoblock (Thermomixer MHR 13; Hettich Benelux, Geldermalsen, Netherlands) for 20 min at 95 °C. The crude product was purified by solid-phase extraction utilizing C-18 cartridges (e.g. Sep-Pak C-18 Plus Short, Waters). The cartridges were preconditioned with 4 mL of ethanol and washed with 10 mL of water. The sample was subsequently loaded onto the cartridge providing retention of [44Sc]Sc‑PSMA-617. After washing the cartridge with 5 mL of water purified [44Sc]Sc‑PSMA-617 was eluted with 0.5 mL of pure ethanol.
Apparent molar activities were calculated on calibration time (end of synthesis).
natSc-PSMA-617
100 µg of PSMA-617 was dissolved in 250 µL of 0.25 M ammonium acetate buffer (pH 4). An aliquot of natScCl3 (1 mg/mL in 0.05 M HCl) was added to obtain a molar ratio of PSMA-617 to metal of 1:5. The reaction mixture was incubated at 95 °C for 25 min. The crude cold complex was purified by solid-phase extraction analogue to the purification of [44Sc]Sc-PSMA-617. natSc-PSMA-617 was obtained in 500 µL of ethanol and was analyzed by HPLC.
Quality control
All radiochemical yields of [44Sc]Sc-PSMA-617 formation were monitored by thin-layer chromatography (TLC) using 0.1 M sodium citrate (pH 4.0) as eluent and instant thin layer chromatography (iTLC) with a mixture 1:1 v/v of 1 M ammonium acetate and methanol. In 0.1 M sodium citrate free scandium-44 moved with an Rf = 0.8 and [44Sc]Sc-PSMA-617 remained at the origin with Rf = 0. In the ammonium acetate/methanol mixture free scandium-44 remained at the origin with Rf = 0 while [44Sc]Sc-PSMA-617 moved with Rf = 0.8.
HPLC (Nucleodur 100-3 C18 ec 125/4; Macherey-Nagel GmbH & Co. KG, Germany) was also performed. The gradient elution system utilized mobile phase A (deionized H2O + 0.01 % TFA) and mobile phase B (100 % acetonitrile + 0.01 % TFA) at a flow rate of 0.7 mL/min, starting with 100 % A/0 % B for 20 min to 0 % A/100 % B, after which gradient parameters returned to 50 % A/50 % B during next 5 min. The retention time of free 44Sc was Rt = 1.37 min, Rt = 8.85 min for [44Sc]Sc-PSMA-617 and Rt = 9.00 min for PSMA-617.
Determination of 44Ti-content was determined at least 4 days, more than 20 half-lives of scandium-44, after the end of synthesis using a multi-channel analyzer for γ-spectroscopy. Titanium-44 breakthrough was calculated based on the 44Sc activity at the end of synthesis.
In vitro evaluation
In vitro stability
Stability was tested by addition of 20 µL (4.7±0.6 MBq) of purified [44Sc]Sc-PSMA-617 to 500 µL of different solutions which were incubated for 24 h at 37° C and aliquots retrieved after t = 30 min, 1, 2, 4, 8 and 24 h. The investigated solutions are 0.9 % NaCl, human serum, aqueous solutions of different metal cations (Fe3+, Ca2+ and Mg2+) at concentration levels of 10-2 M as well as chelators (DTPA, ETDA) with a final molar ratio of chelator to PSMA-617 equal to 100:1 as recommended in literature for 68Ga-tracers 20,33.
Binding affinity and internalization
All cell studies were performed similar to literature 11. Competitive binding and internalization was determined using the PSMA-positive (PSMA+) LNCaP cell line (European Collection of Cell Cultures, Salisbury, UK) derived from an androgen-sensitive human lymph node metastatic lesion of prostatic adenocarcinoma (ATCC CRL-1740). The cells were grown in RPMI 1640 medium (PAN-Biotech, Aidenbach, Germany) containing 10 % of fetal calf serum (FCS) and 1 % of L-glutamine. Cell cultures were kept in an atmosphere of 5 % CO2 at 37 °C in a humidified incubator.
A cell-based competitive assay with 68Ga-labeled Glu-urea-Lys(Ahx)-HBED-CC dimer ([68Ga]Ga-PSMA-10 16) was used for the determination of binding affinity and expressed as Ki values according to literature 11. Twelve concentrations of natSc-PSMA-617 (0-5000 nM) were incubated for 45 min at 37°C with 0.75 nM (90 kBq) of [68Ga]Ga-PSMA-10 together with PSMA+ LNCaP cells (105 LNCaP cells/well) followed by washing three times with ice-cold PBS. Radioactivity accumulated in the cells was measured using a gamma counter and the data fitted using a nonlinear regression algorithm (GraphPad Software) to calculate 50 % inhibitory concentrations (IC50 values).
Internalization was evaluated with PSMA+ LNCaP cells (105 LNCaP cells/well) seeded in poly-L-lysine coated plates 24 h before the experiment. The cells were incubated for 45 min at 37 °C with 32 nM of [44Sc]Sc-PSMA-617 in 250 μL Opti-MEM medium. Additionally, for confirmation of specific cellular uptake, treatment of a second set of compounds with 500 μM/well of PSMA inhibitor 2-PMPA (2‑(phosphonomethyl)pentane-1,5-dioic acid) was performed. Incubation was followed by washing with ice-cold PBS four times and twice with 50 mM glycine (pH 2.8) to remove surface-bound radioactivity. The internalized fraction was determined by lysis of the washed PSMA-positive LNCaP cells using 0.3 M NaOH. The radioactivity collected from the glycine and hydroxide fractions was measured in a gamma counter and calculated as %IA/106 LNCaP cells (%IA = percentage of added radioactivity).
First in-human study
[44Sc]Sc-PSMA-617 for clinical application
Labeling was performed with 40 µL (38 nmol) of PSMA-617 and 3 mL of post-processed scandium-44 in 0.25 M ammonium acetate and 160 µL of ethanol for 20 min at 95 °C. After C-18 purification with 1.5 mL 50 % of ethanol, the final product was formulated with 8.5 mL of 0.9 % NaCl and subsequent sterile filtration.
Quality control was performed according to the monograph of the European Pharmacopoeia for the preparation of [68Ga]Ga-DOTA-TOC 34.
[44Sc]Sc-PSMA-617 clinical application. The four first patients who underwent [44Sc]Sc-PSMA-617 PET/CT as part of individual treatment attempt were retrospectively analyzed. All four patients (mean age 70±1.8a) had histological proven prostate carcinoma and showed progress during hormonal treatment, more patient details are given in Table 1. All patients gave written and informed consent for the examination. Due to the retrospective character of the study the ethic vote was waived by the local ethics committee.
Table 1.
Patient characteristics.
| Patient | Age [years] | Weight [kg] | Previous therapy | Number of previous cycles [177Lu]Lu-PSMA-617 |
|---|---|---|---|---|
| 1 | 70 | 78 | HTx, RTx, CTx, | 3 |
| 2 | 72 | 80 | HTx | 1 |
| 3 | 67 | 70 | HXt, RTx, bisphosphonates | 2 |
| 4 | 70 | 80 | HTx, RTx | 0 |
HTx: anti-hormonal therapy; RTx: local radiation therapy; CTx: chemotherapy
[44Sc]Sc-PSMA-617 was administered via slow intravenous injection (30-60 s) using 50.45±9.25 MBq in a total volume of 10 mL. A Siemens Biograph 2 PET/CT (Siemens Medical Solutions, Erlangen, Germany) was used to acquire initial dynamic PET (30 min) of abdomen in list mode followed by static PET/CT (skull to mid-thigh) 45 min, 2 and 18 h post injection (p.i.). Reconstruction was performed using the iterative reconstruction algorithm implemented by the manufacturer including attenuation and scatter correction based on the low dose CT. For quantitative analysis, the dynamic list mode data were reconstructed as 6 images of 300 s.
Images at 120 min were compared visually to [68Ga]Ga-PSMA-11 data sets of the same patients acquired 32 to 97 days before acquisition of the [44Sc]Sc-PSMA-617 data. For evaluation of the signal-to-noise ratio in all [44Sc]Sc-PSMA-617 data sets the mean activity concentration and the standard deviation was measured in fixed size regions in the liver (60 mm diameter), the lung (60 mm diameter) and a VOI outside the body (60 mm diameter). The ratio between standard deviation and mean uptake was used as measure for the noise ratio.
Mean standardized uptake values (SUV) were measured in fixed size volumes of interest (VOIs) in the kidneys (20 mm diameter), the liver (60 mm diameter), the parotic glands (15 mm diameter), and the spleen (30 mm diameter) in both, [44Sc]Sc-PSMA-617 and [68Ga]Ga-PSMA-11 data sets and compared to each other (students t-test, paired). One patient was excluded from the comparison as in the time interval between [68Ga]Ga-PSMA-11 and [44Sc]Sc-PSMA-617 PET examination another treatment cycle was performed which may limit comparability. In addition, the SUVs of two reference lesions per patient were measured and compared to the liver uptake. These values again were compared between [44Sc]Sc-PSMA-617 and [68Ga]Ga-PSMA-11 datasets (students t-test, paired).
Consequently for initial dosimetric evaluation VOIs were drawn around the source organs in the CT and transferred to all [44Sc]Sc-PSMA-617 data sets for calculating average counts/mL over time. Total and % activity in source organs were determined and residence times were calculated accordingly using OLINDA/EXM software (Hermes, Germany). Organ absorbed doses and effective doses were calculated using OLINDA/EXM applying correction factor for patient weight. Results presented as mean organ absorbed dose and effective dose.
All data analysis was performed on MEDISO interview fusion software (MEDISO Medical Imaging Systems, Budapest, Hungary).
Results & Discussion
Synthesis
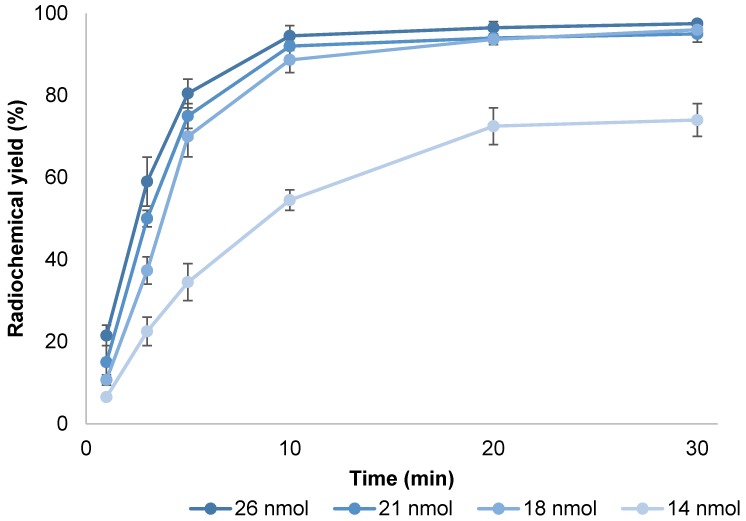
Figure 2. Complex formation of scandium-44 with the macrocyclic ligand DOTA and its derivatives requires elevated reaction temperatures. 95 °C were chosen as reaction temperature as degradation of the compound needs to be taken into account using elevated temperatures. Influence of precursor amount and reaction time on radiochemical yield was evaluated at constant temperature (Figure 3). Incubation at this temperature resulted in an average radiochemical yield of 74 % within 20 min when adding 14 nmol of PSMA-617 to an average of 142.36±12.65 MBq of post-processed scandium-44 eluate (pH 4.0). Increasing the amount of compound increased radiochemical yield, e.g. yield approached 98 % utilizing 18 nmol of PSMA-617 after 20 min with an apparent molar activity of 6.50±0.76 MBq/nmol. Higher amounts of precursor amount did not influence radiochemical yield after 20 min of reaction time but facilitated quantitative yields after 10 min. Utilizing 21 nmol resulted in a yield of 92 % within 10 min with apparent molar activity of 6.69±0.78 MBq/nmol. Values of 5 MBq/nmol up to 10 MBq/nmol using cyclotron produced scandium-44 are described in literature 23.
Figure 2.
Putative structure of [44Sc]Sc-PSMA-617.
Figure 3.
Kinetics of [44Sc]Sc-PSMA-617 formation using different amounts of precursor at pH 4.0 (T = 95 °C, V = 3.3 mL, n = 3).
Solid phase extraction with C-18 cartridges (Sep-Pak C-18 Plus Short, Waters) showed to be a useful method to remove uncomplexed scandium-44 and acetate ions from the crude product solution. In a first step the cartridge was equilibrated with 5 mL of ethanol followed by 10 mL of water. Passing the reaction mixture through the cartridge allowed almost quantitative retention of [44Sc]Sc-PSMA-617 with non-chelated scandium-44 being collected in the waste fraction. After a washing step with 10 mL of water the purified product was recovered in 0.5 mL of pure ethanol with > 90 % efficacy and used for further in vitro studies containing <0.9 % of uncomplexed scandium-44. Utilizing 21 nmol of the tracer resulted in an activity concentration of 233.93±27.33 MBq/mL in the purified fraction. In all quality control samples of the final product, no titanium-44 was detected.
For patient application, modifications of the radiolabeling procedure were performed in terms of precursor amount to ensure quantitative yields and additional ethanol content to prevent radiolysis in the initial reaction mixture. C-18 purification was included by default to remove potentially remaining uncomplexed scandium-44 as well as ammonium acetate buffer prior to reformulation in 0.9 % of NaCl. The apparent molar activity was 3.05±0.36 MBq/nmol (volume activity 11.70±1.37 MBq/mL) at time of calibration (end of synthesis) which is considerably lower compared to [68Ga]Ga-PSMA-11 (14-355 MBq/nmol) or [68Ga]Ga-DOTA-TOC (4-72 MBq/nmol) due to the limited activity derived from the generator system. As there is no monograph for the preparation of scandium-44 or 44Sc-radiopharmaceuticals available, quality control was performed according to the monograph for [68Ga]Ga-DOTA-TOC 34. With respect to the use of generator-derived scandium-44, titanium-44 content in the final formulation was of major interest. Titanium-44 was not traceable in any of the quality control samples.
In vitro evaluation
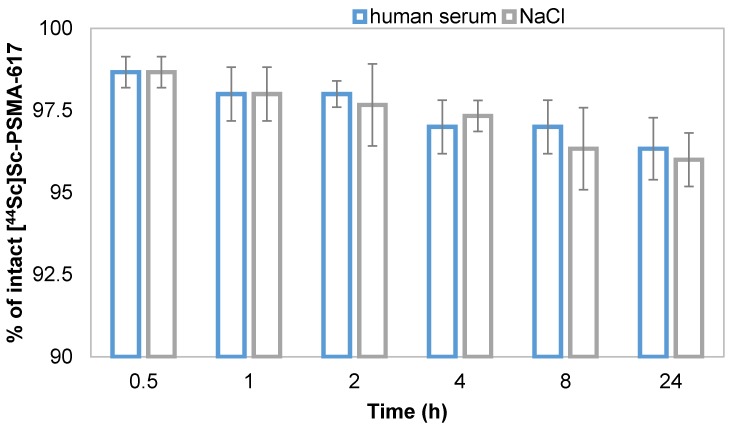
Stability of [44Sc]Sc-PSMA-617 against transmetallation, transchelation, its stability in human serum and in 0.9 % NaCl, representing physiological conditions and final formulation media were analyzed. The presence of different metal cations may cause transmetallation of the radionuclide-conjugate inducing a release of scandium-44 into solution 20. It is Therefore important to determine whether [44Sc]Sc-PSMA-617 is stable in the presence of relevant metal cations typically being present in vivo. The addition of Ca2+, Mg2+ and Fe3+ induced minimal transmetallation of scandium-44 even at metal concentration levels of 10-2 M, which is significantly higher compared to normal in vivo levels, within 4 h. The phenomenon of transchelation may occur in addition leading to release of scandium-44 into solution. Stability of [44Sc]Sc-PSMA-617 in terms of transchelation was determined against DTPA and EDTA (10-2 M) as these chelators have shown to form complexes with scandium-44 at room temperature without the need of elevated temperatures. [44Sc]Sc-PSMA-617 presented high stability with >95 % of intact tracer throughout the investigated period of 24 h against transmetallation as well as transchelation (Table 2). As PSMA-617 contains DOTA as chelating moiety these results were expected and comparable to literature 17, 20.
Table 2.
Stability of [44Sc]Sc-PSMA-617 at 37 °C in the presence of different metal cations and in the presence of DTPA and EDTA, at 10-2 M concentration respectively (n = 3).
| Time (h) | % intact [44Sc]Sc-PSMA-617±SD | ||||
|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Fe3+ | EDTA | DTPA | |
| 0.5 | 98.0±0.0 | 98.7±0.5 | 97.3±0.9 | 96.7±0.1 | 96.7±1.2 |
| 1 | 98.3±0.5 | 98.3±0.5 | 98.0±0.8 | 97.3±0.1 | 97.7±0.5 |
| 2 | 97.0±0.1 | 98.0±0.8 | 98.0±0.8 | 97.3±0.1 | 97.0±0.8 |
| 4 | 97.0±1.4 | 97.7±0.5 | 96.7±0.5 | 97.3±0.1 | 96.7±0.5 |
| 24 | 96.7±0.8 | 97.2±0.8 | 95.0±1.4 | 95.9±1.2 | 95.1±0.8 |
Results for stability in physiological saline and human serum are similar to the ones presented above. After 24 h of incubation, more than 95 % of the labeled compound remained intact (Figure 4).
Figure 4.
Stability of [44Sc]Sc-PSMA-617 in 0.9 % NaCl and human serum at 37 °C (n = 3).
The binding affinity of natSc-PSMA-617 to the target was determined indirectly by measuring its ability to compete with the dimeric PSMA inhibitor PSMA-10 labeled with gallium-68. The experimental setup was equivalent to those published for [177Lu]Lu-PSMA-617 in order to allow direct comparison of data 11. Various concentrations of natSc-PSMA-617 were allowed to compete with a fixed concentration of 0.75 nM of the 68Ga-dimer for PSMA binding. Whilst the concentration of unlabeled ligand increased, the amount of radioligand bound to PSMA decreased. Nanomolar binding affinity of 4.7±0.8 nM was obtained for natSc-PSMA-617 with LNCaP cells. These results are in the same range as the values for binding affinities of natGa- and natLu-labelled versions of PSMA-617 (Table 3).
Table 3.
Inhibition constants and internalization values of natSc-, natGa- and natLu-PSMA-617 determined in equal experimental set up for all three compounds.
| Ki±SD / nM | Internalization±SD / %IA/106 cells | |
|---|---|---|
| natSc-PSMA-617 | 4.72±0.78 | 15.78±2.14 |
| natGa-PSMA-617 | 6.40±1.02(11) | 17.67±4.35(11) |
| natLu-PSMA-617 | 6.91±1.32(11) | 17.51±3.10(11) |
| natGa-PSMA-11 | 12.0±2.80(8) | 9.47±2.56(11) |
[44Sc]Sc-PSMA-617 was specifically internalized into human prostate carcinoma (LNCaP) cells up to 15.8±2.1 % IA/106 LNCaP cells (n = 4). The internalization value is within the limits of the standard deviation and directly comparable with the values for gallium-68 and lutetium-177 analogs (Table 3, Figure 5). The results of all three radiotracers did not show any significant differences.
Figure 5.
Internalization of 44Sc-, 68Ga- and 177Lu-labeled PSMA-617 in LNCaP cells 11. Surface refers to the surface exposed fraction of the radioligand.
First in-human study
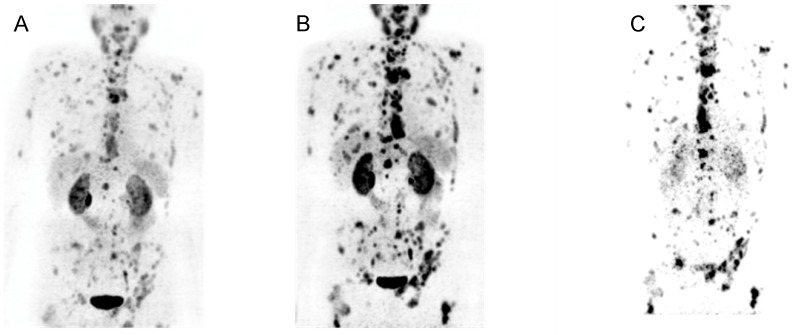
Following the promising labeling efficiency, in vitro stability, binding affinity and internalization of [44Sc]Sc-PSMA-617 a first-in-human application was performed in four patients. No adverse side effects were observed. Multiple PSMA-positive metastases were detected by [44Sc]Sc-PSMA-617 PET/CT in all four patients. Using a single dose of 50.45±9.25 MBq of [44Sc]Sc-PSMA-617 the acquired images were at least equal to the previously obtained images using [68Ga]Ga-PSMA-11-PET/CT in visual comparison. A direct comparison of [68Ga]Ga-PSMA-11 and [44Sc]Sc-PSMA-617 PET/CT images in one patient is depicted in Figure 6.
Figure 6.
Maximal intensity projection (top) and representative slice (bottom) of PET/CT examination of a 77-year-old patient suffering of mCRPC with high tumor load using (A) [44Sc]Sc-PSMA-617 (50 MBq, 60 min p.i.), and (B) [68Ga]Ga-PSMA-11 (120 MBq, 60 min p.i.). (C) On the right hand side the planar scintigraphy is shown (top) and a representative slice of the post therapy SPECT/CT scan, about 24 h after application of 6700 MBq of [177Lu]Lu-PSMA-617.
Quantitative comparison yielded noise ratios (standard deviation divided by mean uptake in the volume of interest) in mean 0.44 for liver, 0.28 for lung and 1.2 for a volume outside the patient in the four [44Sc]Sc-PSMA-617 patient images. These results are comparable to the ones obtained with [68Ga]Ga-PSMA-11 PET/CT giving 0.34 for liver, 0.31 for lung and 0.7 for the area outside the patient.
SUV values were statistically significantly lower in kidneys for the scandium-44 tracer (30.5 for [68Ga]Ga-PSMA-11 averaged over right and left side and all patients versus 14.0 for [44Sc]Sc-PSMA-617). All other measured SUV values did not show a significantly statistical difference. Detailed SUV values are presented in Figure 7. The differences found are in line with previous clinical studies 11.
Figure 7.
Mean SUV values for kidneys, liver, parotic glands and spleen in three patients for [44Sc]Sc-PSMA-617 (boxes) and [68Ga]Ga-PSMA-11 (crosses). P-values for students t-test revealed significant differences only in kidneys.
The tumor to liver ratios were in a range of 1.9 to 8.3 for [68Ga]Ga-PSMA-11 and 2.5 to 8.8 for [44Sc]Sc-PSMA-617. Although ratios using [44Sc]Sc-PSMA-617 tend to be slightly higher, no significant statistical difference was found.
Using doses with little activity and scans performed at late time points of 19 h p.i., lesions were still detected using [44Sc]Sc-PSMA-617 (Figure 8). Accumulated activity in the urinary tract or kidneys were no longer observed at this time point. Almost all remaining activity was accumulated in the metastatic tissue. Qualitative detection of PSMA-positive lesions was feasible as background activity was very low in the urinary tract and kidneys. Estimated residence times of the tracer in various organs are listed in Table 4. For all organs the estimated residence times appear larger compared to the ones published in literature for [68Ga]Ga-PSMA-617 35.
Figure 8.
PET/CT whole body images at different time points post-injection using [44Sc]Sc-PSMA-617: (A) 30 min (B) 120 min (C) 19 h.
Table 4.
Residence times 36 [h] of the source organs.
| Organ | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Mean | [68Ga]Ga-PSMA-617 |
|---|---|---|---|---|---|---|
| Salivary glands | 0.021 | 0.0007 | 0.010 | 0.026 | 0.016 | - |
| Kidneys | 0.215 | 0.361 | 0.121 | 0.341 | 0.260 | 0.132 (35) |
| Liver | 0.253 | 0.779 | 0.067 | 0.292 | 0.348 | 0.093 (35) |
| Spleen | 0.083 | 0.086 | 0.018 | 0.053 | 0.060 | 0.009 (35) |
Based on this calculation an organ absorbed dose of 0.354 mSv/MBq of injected activity was obtained for the kidneys (range 0.180 - 0.488 mSv/MBq) which is about twice the value of [68Ga]Ga-PSMA-617 (0.206 mSv/MBq) as reported in literature 35. The values for other organs are given in Table 5.
Table 5.
Organ absorbed doses [mSv/MBq].
| Organ | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Mean | [68Ga]Ga-PSMA-617 |
|---|---|---|---|---|---|---|
| Salivary glands | 0.097 | 0.035 | 0.052 | 0.012 | 0.075 | - |
| Kidneys | 0.293 | 0.488 | 0.180 | 0.456 | 0.354 | 0.206 (35) |
| Liver | 0.085 | 0.229 | 0.030 | 0.097 | 0.110 | 0.029 (35) |
| Spleen | 0.236 | 0.248 | 0.064 | 0.163 | 0.177 | 0.029 (35) |
Conclusion
Radiolabeling of PSMA-617 with the generator-derived PET radionuclide scandium-44 and its eligibility as PET imaging agent for prostate cancer was investigated in detail. A manual synthesis procedure, guaranteeing safe preparation and reproducible quality of the final radiopharmaceutical as well as an adequate quality control protocol with respect to the guidelines of the European Pharmacopoeia was developed. During in vitro studies, the radiopharmaceutical presented high stability and showed similar binding and specific internalization in LNCaP cells compared to the 68Ga- and 177Lu-labeled derivatives.
Based on the obtained in vitro data, [44Sc]Sc-PSMA-617 was used as imaging agent for pre-therapeutic dosimetry in four patients scheduled for 177Lu-PSMA-treatment for the first time. Data obtained with [44Sc]Sc-PSMA-617 was comparable with [68Ga]Ga-PSMA-11 in terms of image quality and noise level. More importantly, it allowed acquisition of excellent images and quantitative inspection at late time points of 19 h p. i.. [44Sc]Sc-PSMA-617 shows improved pre-therapeutic dosimetry for therapy with [177Lu]Lu-PSMA-617. Scans with [44Sc]Sc-PSMA-617 can be easily implemented as part of a clinical setting without the need of hospitalization of the patient, which is needed when performing dosimetry using [177Lu]Lu-PSMA-617 in small amounts directly. Further clinical trials now need to show the influence of individual dosimetry for the patient outcome in a more general approach.
Additional potential application of scandium-44 radiotracers, is image-guided radiosurgery using positron probes intraoperatively e.g. for detection of lymph node metastases. This procedure is feasible due to the longer half-life of scandium-44 compared to gallium-68. This concept has been well established 37 and it may benefit from higher resolution imaging using PET instead of SPECT tracers.
Potential drawbacks of scandium-44 as PET imaging agent may be the presence of additional high-energy gamma lines. However, in the visual comparison of gallium-68 and scandium-44 as well as the calculation of the signal to noise-ratio no significant differences were found. This observation may be caused by the reduced noise-level of scandium-44 due to the lower positron energy compared to gallium-68. In addition, optimization of PET acquisition parameters and noise reduction algorithms may be part of future work. Another general limitation of scandium-44 is the currently limited availability. As soon as clinical advantages of scandium-44 are shown and confirmed as part of future studies, availability of this promising nuclide will hopefully be granted.
The study presented as part of this publication confirms suitability of [44Sc]Sc-PSMA-617 for PET imaging and pre-therapeutic dosimetry of prostate cancer.
Acknowledgments
This work was financially supported by BONFOR (O-140.0008).
Ethics Guidelines
All patients gave written and informed consent for the examination. Due to the retrospective character of the study the ethic vote was waived by the local ethics committee.
Abbreviations
- 2-(phosphonomethyl)pentane-1,5-dioic acid
2-PMPA
- computed tomography
CT
- 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
DOTA
- diethylenetriaminepentaacetic acid
DTPA
- ethylenediaminetetraacetic acid
EDTA
- fetal calf serum
FCS
- high pressure liquid chromatography
HPLC
- half maximal inhibitory concentration
IC50
- intravenous injection
i.v.
- percentage of overall radioactivity added to the cells
% IA
- instant thin layer chromatography
iTLC
- binding affinity expressed as inhibition potency
Ki
- thermodynamic stability constant
log K
- metastatic castration-resistant prostate cancer
mCRPC
- reduced serum media
Opti-MEM
- post injection
p.i.
- phosphate buffered saline
PBS
- personal computer
PC
- positron emission tomography
PET
- prostate specific antigen
PSA
- prostate specific membrane antigen
PSMA
- prostate specific membrane antigen positive
PSMA+
- radioligand therapy
RLT
- Rosswell Park Memorial Institute medium
RPMI
- region of interest
ROI
- standard deviation
SD
- standardized uptake values
SUV
- thin layer chromatography
TLC
- volume of interest
VOI.
References
- 1.Israeli RS, Powell CT, Corr JG, Fair WG, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Research; 1994. pp. 541807–11. [PubMed] [Google Scholar]
- 2.Israeli RS, Powell CT, Fair WG, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Research; 1993. pp. 53227–30. [PubMed] [Google Scholar]
- 3.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc Natl Acad Sci U S A. 2005;102(17):5981–6. doi: 10.1073/pnas.0502101102. doi:10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, Holland-Letz T, Hadaschik BA, Giesel FL, Debus J, Haberkorn U. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41(1):11–20. doi: 10.1007/s00259-013-2525-5. doi:10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SAMW, Rosenbaum-Krumme S, Bockisch A, Gotthardt M, Rijpkema M, Boerman OC. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics. 2015;5(12):1388–401. doi: 10.7150/thno.13348. doi:10.7150/thno.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinnab L, Simon J, Hautmann RE, Cronauer MV, Hohl K, Buck AK, Reske SN, Mottaghy FM. (11)Ccholine PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J Urol. 2009;27(5):619–25. doi: 10.1007/s00345-009-0371-7. doi:10.1007/s00345-009-0371-7. [DOI] [PubMed] [Google Scholar]
- 7.Small EJ. Monoclonal antibody therapy for prostate cancer: finally a reality? J Clin Oncol. 2004;22(13):2515–6. doi: 10.1200/JCO.2004.04.901. doi:10.1200/JCO.2004.04.901. [DOI] [PubMed] [Google Scholar]
- 8.Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, Haberkorn U, Eisenhut M. 68 Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjugate Chem. 2012;23(4):688–97. doi: 10.1021/bc200279b. doi:10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E, Gartner F, Rogenhofer S, Schafers M, Essler M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015;5(1):114.. doi: 10.1186/s13550-015-0114-2. doi:10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, Schottelius M, Mueller D, Klette I, Wester H-J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016;57(7):1006–13. doi: 10.2967/jnumed.115.168443. doi:10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 11.Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, Haberkorn U, Kopka K, Eder M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015;56(6):914–20. doi: 10.2967/jnumed.114.147413. doi:10.2967/jnumed.114.147413. [DOI] [PubMed] [Google Scholar]
- 12.Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, Tritschler S, Stief CG, Kopka K, Haberkorn U, Bartenstein P, Boning G. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2016;43(1):42–51. doi: 10.1007/s00259-015-3174-7. doi:10.1007/s00259-015-3174-7. [DOI] [PubMed] [Google Scholar]
- 13.Kabasakal L, AbuQbeitah M, Aygun A, Yeyin N, Ocak M, Demirci E, Toklu T. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2015;42(13):1976–83. doi: 10.1007/s00259-015-3125-3. doi:10.1007/s00259-015-3125-3. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, Claesener M, Ahmadzadehfar H. Response and Tolerability of a Single Dose of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: A Multicenter Retrospective Analysis. J. Nucl. Med. 2016;57(9):1334–8. doi: 10.2967/jnumed.116.173757. doi:10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 15.Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, Joyal J, Kopka K, Debus J, Babich JW, Haberkorn U. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41(7):1280–92. doi: 10.1007/s00259-014-2713-y. doi:10.1007/s00259-014-2713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schäfer M, Bauder-Wüst U, Leotta K, Zoller F, Mier W, Haberkorn U, Eisenhut M, Eder M. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging Research; 2012. p. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domnanich KA, Müller C, Farkas R, Schmid RM, Ponsard B, Schibli R, Türler A, van der Meulen, Nicholas P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI radiopharm. chem. 2017;1(1):2.. doi: 10.1186/s41181-016-0013-5. doi:10.1186/s41181-016-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, Barnhart TE, Cai W. (44)Sc: an attractive isotope for peptide-based PET imaging. Mol Pharm. 2014;11(8):2954–61. doi: 10.1021/mp500343j. doi:10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller C, Bunka M, Reber J, Fischer C, Zhernosekov K, Turler A, Schibli R. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent beta-emitters: in vitro and in vivo study of a 44Sc-DOTA-folate conjugate. J. Nucl. Med. 2013;54(12):2168–74. doi: 10.2967/jnumed.113.123810. doi:10.2967/jnumed.113.123810. [DOI] [PubMed] [Google Scholar]
- 20.Pruszyński M, Majkowska-Pilip A, Loktionova NS, Eppard E, Roesch F. Radiolabeling of DOTATOC with the long-lived positron emitter 44Sc. Applied Radiation and Isotopes. 2012;70(6):974–9. doi: 10.1016/j.apradiso.2012.03.005. doi:10.1016/j.apradiso.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Singh A, Baum RP, Klette I, van der Meulen, Nicholas P, Muller C, Tuerler A, Schibli R. Scandium-44 DOTATOC PET/CT: First in-human molecular imaging of neuroendocrine tumors and possible perspectives for Theranostics. Journal of Nuclear Medicine. 2015;56(Supplement 3):267. [Google Scholar]
- 22.Rösch F. Scandium-44: Benefits of a Long-Lived PET Radionuclides Available from the 44Ti/44Sc Generator System. Curr. Radiopharm; 2012. pp. 5187–201. [DOI] [PubMed] [Google Scholar]
- 23.Umbricht CA, Benesova M, Schmid RM, Turler A, Schibli R, van der Meulen, Nicholas P, Muller C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017;7(1):9.. doi: 10.1186/s13550-017-0257-4. doi:10.1186/s13550-017-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alliot C, Kerdjoudj R, Michel N, Haddad F, Huclier-Markai S. Cyclotron production of high purity (44m,44)Sc with deuterons from (44)CaCO3 targets. Nuclear Medicine and Biology. 2015;42(6):524–9. doi: 10.1016/j.nucmedbio.2015.03.002. doi:10.1016/j.nucmedbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Trans. 2011;40(23):6104–11. doi: 10.1039/c0dt01504k. doi:10.1039/c0dt01398f. [DOI] [PubMed] [Google Scholar]
- 26.Filosofov DV, Loktionova NS, Rösch F. A 44Ti/44Sc radionuclide generator for potential application of 44Sc-based PET-radiopharmaceuticals. Radiochimica Acta. 2010;98(3):149–56. [Google Scholar]
- 27.Alenitzky YG, Novgorodov AF, Filosofov DV, Skripnik AV, Kaplun VG, Suzikov AG, Eliseev IA, Rösch F. 44Ti: Investigation of target preparation, irradiation and yields in the 45Sc(p,2n) process. Mainz: Institute of Nuclear Chemistry, University Mainz; 2005. Annual Report. [Google Scholar]
- 28.Pruszynski M, Loktionova NS, Filosofov DV, Rösch F. Post-elution processing of 44Ti/44Sc generator-derived 44Sc for clinical application. Appl. Rad. Isot. 2010;68(9):1636–41. doi: 10.1016/j.apradiso.2010.04.003. doi:10.1016/j.apradiso.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Müller C, Bunka M, Haller S, Köster U, Groehn V, Bernhardt P, van der Meulen N, Türler A, Schibli R. Promising prospects for 44Sc-/47Sc-based theragnostics: application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014;55(10):1658–64. doi: 10.2967/jnumed.114.141614. doi:10.2967/jnumed.114.141614. [DOI] [PubMed] [Google Scholar]
- 30.Qaim SM. The present and future of medical radionuclide production. Radiochimica Acta. 2012;100(8-9):635–51. doi:10.1524/ract.2012.1966. [Google Scholar]
- 31.Zhernosekov K, Bunka M, Hohn A, Schibli R, Türler A. Development and evaluation of (44)Ti production on high energy protons. J. Label Compd. Radiopharm. 2011;54(S1):S239.. doi:10.1002/jlcr.1926. [Google Scholar]
- 32.van der Meulen, Nicholas P, Bunka M, Domnanich KA, Müller C, Haller S, Vermeulen C, Türler A, Schibli R. Cyclotron production of (44)Sc: From bench to bedside. Nuclear Medicine and Biology. 2015;42(9):745–51. doi: 10.1016/j.nucmedbio.2015.05.005. doi:10.1016/j.nucmedbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Riss PJ, Burchardt C, Roesch F. A methodical 68Ga-labelling study of DO2A-(butyl-L-tyrosine)2 with cation-exchanger post-processed 68Ga: practical aspects of radiolabelling. Contrast Media Mol Imaging. 2011;6(6):492–8. doi: 10.1002/cmmi.451. doi:10.1002/cmmi.451. [DOI] [PubMed] [Google Scholar]
- 34.European pharmacopoeia. 8.6 to 8.8. Strasbourg: Council Of Europe; 2015. [Google Scholar]
- 35.Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, Eisenhut M, Kübler W, Holland-Letz T, Giesel FL, Mier W, Kopka K, Haberkorn U. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015;56(11):1697–705. doi: 10.2967/jnumed.115.161299. doi:10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 36.Stabin MG. Fundamentals of Nuclear Medicine Dosimetry; 1st ed. s.l.: Springer-Verlag; 2008: 241.
- 37.Schottelius M, Wirtz M, Eiber M, Maurer T, Wester H-J. (111)InPSMA-I&T: Expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015;5(1):68.. doi: 10.1186/s13550-015-0147-6. doi:10.1186/s13550-015-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]