Abstract
Aims
The detection of non‐ischaemic (mainly hypertension, diabetes, and obesity) stage B heart failure (SBHF) may facilitate the recognition of those at risk of progression to overt HF and HF prevention. We sought the relationship of specific electrocardiographic (ECG) markers of SBHF to echocardiographic features of SBHF and their prognostic value for development of HF. The ECG markers were Cornell product (Cornell‐P), P‐wave terminal force in lead V1 (PTFV1), ST depression in lead V5 V6 (minSTmV5V6), and increased heart rate. Echocardiographic assessment of SBHF included left ventricular hypertrophy (LVH), impaired global longitudinal strain (GLS), and diastolic dysfunction (DD).
Method and results
Asymptomatic subjects ≥65 years without prior cardiac history, but with HF risks, were recruited from the local community. At baseline, they underwent clinical assessment, 12‐lead ECG, and comprehensive echocardiography. New HF was assessed clinically at mean follow‐up of 14 ± 4 months, and echocardiography was repeated in subjects with HF. Of the 447 study subjects (age 71 ± 5, 47% men) with SBHF, 13% had LVH, 32% impaired GLS, and 65% ≥grade I DD (10% ≥grade II DD). Forty were lost to follow‐up. Clinical HF developed in 47 of 407, of whom 20% had echocardiographic LVH, 51% abnormal GLS, and 76% DD at baseline. Baseline LVH and abnormal GLS (not grade I DD) were independently associated with outcomes (clinical HF and cardiovascular death). Cornell‐P and heart rate (not minSTmV5V6 nor PTFV1) were independently associated with LVH, impaired GLS, and DD. Cornell‐P and minSTV5V6 (not heart rate nor PTFV1) were independently associated with outcomes. More ECG abnormalities improved sensitivity, but ECG‐markers were not independent of or incremental to echocardiographic markers to predict HF in SBHF.
Conclusions
In this elderly study population, ECG markers showed low diagnostic sensitivity for non‐ischaemic SBHF and low prognostic value for outcomes. Cornell‐P and minSTmV5V6 had predictive value for outcomes in non‐ischaemic SBHF independent of age, gender, and common comorbidities but were not incremental to echocardiography.
Keywords: Electrocardiography, Echocardiography, Stage B heart failure, Community screening
Introduction
Stage B heart failure (SBHF) is an early stage of heart failure (HF) with no symptoms despite evidence of cardiac structural or functional impairment.1, 2 Most often it is due to loss of functioning myocytes from myocardial infarction, valvular disease, or left ventricular hypertrophy (LVH) secondary to hypertension.1 Randomized trials have shown that early intervention can prevent or delay the onset of overt HF in patients with reduced left ventricular ejection fraction (LVEF) in the ischaemic population.3, 4 However, evidence is missing in the non‐ischaemic population with preserved LVEF about utility of early diagnosis and treatment. Using echocardiography, SBHF may be detected by LVH, diastolic dysfunction (DD), or impaired global longitudinal systolic strain (GLS).2 The assessment of left ventricular (LV) function has been strengthened by speckle‐tracking echocardiography. This semi‐automated method is highly sensitive for the detection of subtle myocardial impairment, provides incremental prognostic value over LVEF5 and can be considered to be a functional marker of SBHF.2, 6 However, the cost and feasibility of current echocardiographic techniques are a barrier to community‐based screening for SBHF. A selective screening strategy of identifying high‐risk individuals based on the use of simpler tools that are more feasible could improve the efficiency of a screening approach.
The association of abnormal electrocardiographic (ECG) markers and incident HF has been reported in the literature, including abnormal QRS duration,7 abnormal P‐wave terminal force in lead V1(PTFV1),8 ST changes,9 and various markers in combination.10, 11 ECG‐LVH has been associated with abnormal cardiac function and has predictive value for incident HF independent of echocardiographic LVH.12 ECG‐LVH by Cornell product (Cornell‐P) criteria is strongly associated with DD,13 and in a larger cohort of hypertensive patients, ECG‐LVH was associated with increased risk of LV systolic dysfunction,14 especially when combined with ST depression in the lateral precordial leads (V5–V6), even in the absence of coronary disease.15, 16 ECG markers [Cornell‐P, PTFV1, minimal ST deviation at m point of leads V5 and V6 (minSTmV5V6), and abnormally increased heart rate] may reflect underlying structural changes in the heart. Their associations with outcome have not been well studied. Accordingly, we aimed to evaluate the performance of commonly utilized ECG markers to predict echocardiographic features of SBHF2 and to compare the prognostic and incremental value of these ECG markers with echocardiographic indices for HF in this community population at risk of HF.
Materials and methods
Study population
Participants were enrolled through local media advertising. Data were prospectively collected from subjects ≥65 years old and living in the community. Inclusion was based on the presence of one or more of HF risk factors: (i) hypertension (based on systolic blood pressure (SBP) >140 mmHg and/or self‐report of anti‐hypertensive medication); (ii) type 2 diabetes mellitus (T2DM; based on self‐report of diagnosis including medication); (iii) obesity [body mass index (BMI) ≥30 kg/m2]; (iv) previous potentially cardio toxic chemotherapy; (v) family history of HF; and (vi) previous history of heart disease (but not existing HF). Excluded were subjects with (i) symptoms or a known history of HF; (ii) known coronary artery disease (CAD) including history of myocardial infarction and coronary artery bypass graft; (iii) more than moderate valvular heart disease; (iv) reduced LVEF (<40%) on baseline echocardiography; (v) atrial fibrillation; and (vi) inability to acquire interpretable images at baseline. This study was performed in accordance with a research protocol approved by the Tasmanian Human Research Ethics Committee and registered with the Australian and New Zealand Clinical Trials Registry (http://www.anzctr.org.au/; ACTRN12614000080628). Individual written informed consent was obtained from all participants
Data collection
Data were prospectively collected at facilities in the community from all participants enrolled in the study. All underwent a physical examination and symptom questionnaire. Anthropometric measurements were obtained, and BMI was calculated [body weight (kg)/height2 (m2)]. Blood pressure was measured twice after 10 min of rest. Data were also collected on socio‐economic indicators, complete medical history, and family history. Charlson comorbidity score index was used for comorbidity assessment.17
Electrocardiogram
A standard 12‐lead ECG was recorded at 25 mm/s and 1 mV/cm according to standard protocol. ECG measurements were performed using MUSE software (GE Healthcare, Milwaukee, WI, USA) including QRS duration and axis, PR, QT, and heart rate. Cornell voltage (Cornell‐V) was measured as SV3 + RaVL, and criteria for LVH were defined as ≥2.8 mV (28 mm) in men and ≥2.0 mV (20 mm) in women.14 Criteria for LVH using the Cornell‐P (the product of QRS duration times Cornell‐V [RaVL + SV3] plus 6 mm in women) was defined as ≥2440 mm · ms,14 and the 75th percentile of gender specific cut‐offs for Cornell‐P from the current study population were also used as categorical cut‐off. Sokolow‐Lyon voltage (SLV) was measured as SV1 + RV5 or RV6, and criteria for LVH were defined as ≥3.5 mV (35 mm).18 The cut‐off for absolute ST segment deviation (minSTmV5V6, the midpoint of the ST segment on median complexes in leads V5 and V6) was defined using upper 75th percentile of the cohort as −20 μV. Abnormal P‐wave terminal force in the right precordial lead V1 [PTFV1; the product of the negative P‐wave deflection from onset of the negative deflection to its nadir in lead V1 (μV) and the duration (ms)] was defined as ≤−4000 μVms.8 An abnormal ECG was defined as the combination of >1 of the following: (i) resting heart rate ≥80 beat per minute (upper 90th percentile); (ii) upper 75th percentile of Cornell‐P; (iii) upper 75th percentile of minSTmV5V6; and (iv) abnormal PTFV1.
Echocardiographic study
Standard transthoracic 2D and Doppler echocardiographic studies were performed using standard equipment (Siemens ACUSON SC2000, Siemens Healthcare, Mountain View, CA, USA) and transducer (4V1c, 1.25–4.5 MHz; 4Z1c, 1.5–3.5 MHz) in accordance with the American Society of Echocardiography guidelines.19 LV dimensions during diastole and systole and wall thicknesses were measured according to the recommended criteria, and LV mass index (LVMi) was calculated accordingly.19 Echocardiographic LVH (Echo‐LVH) was defined as LVMi >115 g/m2 in men and >95 g/m2 in women. LV and left atrial volumes were calculated by the Simpson biplane method19 indexed to body surface area. Left atrial enlargement defined as left atrium volume index ≥34 mL/m2. Mitral inflow peak early diastolic velocity (E), peak late diastolic velocity (A), E/A ratio, and E wave deceleration time were measured for diastolic function assessment.20, 21 Tissue Doppler mitral annular early diastolic velocity (e') was assessed at septal and lateral and averaged for calculation of E/e'; septal E/e' ≥15, lateral E/e' ≥13, and averaged ≥13 were defined as abnormal.21 DD grade was defined as previously described as21, 22 grade I DD: E/A <0.8, E/e' <10, pulmonary venous inflow S > D; grade II DD: 0.8 < E/A <1.5, E/e' >13 or left atrial enlargement, or presence of mid‐diastolic forward flow (L wave), or positive Valsalva (>50% decrease of E/A ratio); grade III DD: E/A >1.5, deceleration time <140 ms.
Left ventricular longitudinal strain measurements were obtained from grey scale‐recorded images in the apical four‐chamber, two‐chamber, and long‐axis views. Strain was analysed using velocity vector imaging (Syngo VVI, Siemens Medical Solutions). GLS was measured online by averaging all 18 segment strain from apical four‐chamber, two‐chamber, and long‐axis views. Impaired GLS was defined using cut‐off of <18%.23 Global circumferential strain was measured offline. Global diastolic strain was obtained by averaging all 18 segment strain values and measured according to method published by Ishii et al.24 Functional capacity was assessed using a 6‐min walk test distance following a standardized protocol.25
Follow‐up and primary endpoint
Potential HF symptoms were assessed through regular follow‐up phone calls, followed by symptom surveillance questionnaires and clinical visits. Possible HF signs and symptoms were reviewed by three independent cardiologists, and the HF diagnosis was confirmed using the Framingham criteria with the presence of two major or one major and two minor criteria.26 The primary composite endpoint was defined as new onset of HF or death from cardiovascular (CV) causes. Follow‐up echocardiographic assessment of LVEF was performed to classify the patients with HF with reduced (HFrEF, LVEF <40%), mid‐range (HFmrEF, LVEF 40–49%) or preserved EF.27
Statistical analysis
Data are presented as mean [±standard deviation (SD)] after testing for normal distribution (Shapiro–Wilk test). Data deviating from normality are expressed as median (interquartile range). Categorical variables are expressed as percentages. Correlation between variables was assessed with Pearson or Spearman correlation coefficients. For differences among groups, Mann–Whitney U test or t‐test were used for continuous variables. Pearson's χ2 tests or Fisher's exact test were used for categorical variables. Logistic regression analysis was used to examine the association of ECG markers and abnormal echocardiographic features of SBHF. The primary outcome of time to event was examined with univariable and multivariable Cox proportional hazards models. Receiver operator characteristic analysis was used to examine the discriminative ability of variables for outcome. Comparisons of areas under the curve were performed with the method suggested by Hanley and McNeil. Survival analysis was performed using the Kaplan–Meier method, and the differences in survival between groups were assessed by the log‐rank test. Net reclassification improvement (NRI) was based on quartile boundaries of probability calculated from the multivariable logistic regression for incremental value of ECG markers over clinical and echocardiographic measures for outcome. Statistical analyses were performed using a standard statistical software package (SPSS software 22.0, SPSS Inc., Chicago, IL). Statistical significance was defined by P < 0.05.
Results
Patient selection
Baseline ECG and echocardiography were obtained in 447 individuals from the community (age 71 ± 5 years, 47% men) who met the inclusion criteria. HF risk factors were present in all—most commonly hypertension (81%), T2DM (54%), and obesity (45%); 81% had more than one of the listed risk factors. The echocardiographic markers of SBHF were LVH (13%), DD (65% by ≥grade 1 DD and 10% by ≥grade 2 DD), and impaired GLS (32%). The median (interquartile range) for Cornell‐V was 9.8 (6.8–13.6) mm; SLV 18.0 (14.1–22.7) mm, Cornell‐P 1090 (786–1500) mm · ms. The mean (±SD) of minSTV5V6 was 3.1 ± 39 (μV) and PTFV1 −2918 ± 3532 (μVms). Using the conventional cut‐off values, ECG‐LVH was present in 1.6% by SLV, 2% by Cornell‐V, and 3.1% by Cornell‐P. Abnormal PTFV1 was present in 35%, abnormal minSTmV5V6 in 27%, and increased heart rate in 13%.
Association of electrocardiographic markers with echocardiographic feature of stage B heart failure
Baseline demographic, ECG, and echocardiographic characteristics are listed in Table 1, stratified according to the presence SBHF features. Subjects with LVH and DD were older, but impaired GLS was unrelated to age. However, more men had impaired GLS than women. Mean BMI was not different among groups. Hypertension and obesity were more prevalent in subjects with LVH; T2DM and obesity were more prevalent in subjects with impaired GLS. Functional capacity by 6‐min walk test was lower in those with DD (P = 0.02).
Table 1.
Baseline clinical and echocardiographic characteristics stratified by LVH, diastolic dysfunction, and impaired GLS
| LVH (−) (n = 389) | LVH (+) (n = 58) | P value | Normal diastolic (n = 158) | DD* (n = 289) | P value | Normal GLS (n = 305) | Impaired GLS** (n = 142) | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||||
| Age (years) | 70 (67–74) | 71 (68–77) | 0.019 | 69 ± 4 | 72 ± 5 | <0.001 | 71 ± 5 | 71 ± 5 | 0.787 |
| Male, n (%) | 188 (48) | 20 (35) | 0.049 | 83 (53) | 125 (43) | 0.060 | 119 (39) | 89 (63) | <0.001 |
| Systolic blood pressure (SBP) (mmHg) | 139 ± 16 | 146 ± 19 | 0.001 | 137 ± 14 | 141 ± 17 | 0.009 | 139 ± 15 | 141 ± 19 | 0.321 |
| Diastolic blood pressure (DBP) (mmHg) | 81 ± 10 | 84 (±10) | 0.078 | 80 ± 9 | 82 ± 11 | 0.057 | 80 ± 10 | 84 ± 11 | <0.001 |
| Body mass index (kg/m2) | 30 ± 5 | 30 (±5) | 0.458 | 29 ± 5 | 30 ± 6 | 0.332 | 29 ± 5 | 30 ± 6 | 0.078 |
| Charlson score | 1.0 (0–2.0) | 1.0 (0–2.0) | 0.744 | 1 (0–2) | 1 (0–2) | 0.138 | 1.0 (0–2.0) | 1.0 (0–2.0) | 0.177 |
| Type 2 diabetes, n (%) | 205 (53) | 36 (62) | 0.182 | 76 (48) | 165 (57) | 0.068 | 141 (46) | 100 (70) | <0.001 |
| Obese, n (%) | 167 (43) | 36 (62) | 0.006 | 66 (42) | 137 (47) | 0.253 | 125 (41) | 78 (55) | 0.006 |
| Hypertension, n (%) | 309 (79) | 53 (91) | 0.031 | 126 (80) | 236 (82) | 0.622 | 248 (81) | 114 (80) | 0.796 |
| Beta‐blocker, n (%) | 25 (6.4) | 6 (10) | 0.273 | 12 (7.6) | 19 (6.6) | 0.685 | 20 (6.6) | 11 (7.7) | 0.645 |
| ACEi/ARB, n (%) | 255 (66) | 46 (79) | 0.037 | 105 (67) | 196 (68) | 0.769 | 209 (69) | 92 (65) | 0.433 |
| Calcium channel blocker, n (%) | 74 (21) | 18 (33) | 0.052 | 32 (23) | 60 (23) | 0.960 | 59 (22) | 33 (26) | 0.365 |
| 6‐min walk (metre) | 469 ± 101 | 444 (±99) | 0.066 | 481 ± 98 | 457 ± 100 | 0.019 | 470 ± 96 | 456 ± 108 | 0.171 |
| ECG characteristics, continuous | |||||||||
| Heart rate (beat/min) | 68 ± 11 | 66 ± 10 | 0.172 | 65 ± 11 | 69 ± 11 | <0.001 | 67 ± 10 | 70 ± 12 | 0.002 |
| QRS duration (ms) | 82 (76–90) | 86 (78–95) | 0.047 | 82 (76–90) | 84 (76–92) | 0.489 | 82 (76–90) | 84 (76–94) | 0.147 |
| Cornell voltage (mm) | 9.5 (6.8–13.2) | 11.4 (7.5–16.3) | 0.006 | 9.1 (6.4–12.2) | 10.4 (7.3–14.3) | 0.015 | 9.1 (6.4–12.8) | 11.2 (7.4–15.2) | 0.001 |
| SL voltage (mm) | 18 (14–23) | 18 (13–24) | 0.851 | 18.7 (14.1–23.1) | 17.7 (14.0–22.6) | 0.515 | 17.9 (14.1–22.9) | 18.2 (14.0–22.6) | 0.920 |
| Cornell product (mm · ms) | 1062 (769–1440) | 1431 (1093–1821) | <0.001 | 1020 (724–1320) | 1123 (849–1558) | 0.001 | 1079 (776–1451) | 1132 (825–1637) | 0.096 |
| minSTmV5V6 (μV) | 4 (−20, 29) | −10 (−40, 19) | 0.021 | 9 (−20, 30) | 0 (−24, 24) | 0.166 | 4 (−20, 29) | −5 (−25, 19) | 0.035 |
| PTFV1 (μVms) | −3153 (−4864, −1328) | −2322 (−4193, −846) | 0.189 | −2844 (−4541, −1152) | −3185 (−4767, −1328) | 0.479 | −3139 (−4696, −1291) | −3042 (−5164, −1116) | 0.738 |
| ECG characteristics, categorical | |||||||||
| Cornell‐P 75th (n > 1442, f > 1518) | 85 (22) | 26 (45) | <0.001 | 26 (17) | 85 (29) | 0.002 | 69 (23) | 42 (30) | 0.113 |
| Abnormal PVTFV1 (≤4000 μV∙ms) | 139 (36) | 17 (29) | 0.338 | 51 (32) | 105 (36) | 0.390 | 108 (35) | 48 (34) | 0.740 |
| minSTmV5V6 75th (≤−20 μV) | 100 (26) | 19 (33) | 0.257 | 39 (25) | 80 (29) | 0.493 | 74 (24) | 45 (32) | 0.098 |
| Increased heart rate (≥80 bpm) | 52 (13) | 5 (9) | 0.312 | 12 (8) | 45 (16) | 0.016 | 31 (10) | 26 (18) | 0.016 |
| Echo characteristics continuous | |||||||||
| LV mass index (BSA) | 79 ± 14 | 112 ± 13 | <0.001 | 81 ± 17 | 45 ± 11 | 0.044 | 82 ± 16 | 87 ± 19 | 0.004 |
| Relative wall thickness | 0.47 ± 0.1 | 0.47 ± 0.1 | 0.441 | 0.46 ± 0.1 | 0.47 ± 0.1 | 0.216 | 0.46 ± 0.1 | 0.47 ± 0.1 | 0.583 |
| LA volume (mL/m2) | 29 ± 8 | 37 ± 11 | <0.001 | 30 ± 8 | 30 ± 9 | 0.808 | 30 ± 9 | 30 ± 9 | 0.901 |
| LV ejection fraction (%) | 64 ± 6 | 62 ± 7 | 0.035 | 64 ± 5 | 63 ± 6 | 0.079 | 65 ± 5 | 61 ± 7 | <0.001 |
| GLS (%) | 18.6 ± 2.5 | 18.0 ± 2.7 | 0.103 | 19.0 ± 2.4 | 18.3 ± 2.6 | 0.004 | 19.9 ± 1.6 | 15.6 ± 1.6 | <0.001 |
| GCS (%) | 29.4 ± 5.6 | 28.9 ± 5.1 | 0.466 | 29.6 ± 5.6 | 29.2 ± 5.5 | 0.404 | 30 ± 5 | 28 ± 6 | <0.001 |
| Mitral E/A | 0.81 ± 0.21 | 0.81 ± 0.27 | 0.983 | 0.95 ± 0.13 | 0.73 ± 0.23 | <0.001 | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.821 |
| DecT (ms) | 249 ± 49 | 258 ± 54 | 0.265 | 231 ± 41 | 261 ± 51 | <0.001 | 252 ± 49 | 247 ± 52 | 0.374 |
| E/e' (average) | 8.8 ± 2.5 | 9.9 ± 3.1 | 0.003 | 8.29 ± 1.76 | 9.29 ± 2.88 | <0.001 | 8.9 ± 2.6 | 9.1 ± 2.5 | 0. 44 |
| Diastolic strain (%) | 0.42 ± 0.14 | 0.37 ± 0.16 | 0.026 | 0.48 ± 0.12 | 0.28 ± 0.14 | <0.001 | 0.43 ± 2.62 | 0.38 ± 0.16 | <0.001 |
| Diastolic strain ate (1/s) | 0.97 ± 0.25 | 0.87 ± 0.22 | 0.007 | 1.06 ± 0.25 | 0.89 ± 0.23 | <0.001 | 1.02 ± 0.2 | 0.82 ± 0.2 | <0.001 |
| Echo characteristics categorical | |||||||||
| LV hypertrophy | — | — | — | 15 (9.5) | 43 (14.9) | 0.105 | 35 (12) | 23 (16) | 0.167 |
| Dilated LA (cut‐off 34) | 101 (26) | 32 (55) | <0.001 | 42 (27) | 91 (32) | 0.296 | 90 (30) | 43 (31) | 0.832 |
| Abnormal E/e' (>13) | 42 (11) | 12 (21) | 0.031 | 3 (2) | 51 (18) | <0.001 | 36 (12) | 18 (13) | 0.792 |
| Abnormal GLS (<18%) | 119 (31) | 23 (40) | 0.167 | 43 (27) | 99 (34) | 0.126 | — | — | |
| Diastolic dysfunction > I | 246 (63) | 43 (74) | 0.105 | — | — | — | 190 (62) | 99 (70) | 0.126 |
ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin receptor blocker; DecT, deceleration time; DD, diastolic dysfunction; ECG, electrocardigram; GCS, global circumferential strain; GLS, global longitudinal strain; LA, left atrium; LV, left ventricle; LVH, left ventricular hypertrophy; minSTmV5V6, minimal ST deviation at m point of leads V5 and V6; PTFV1, P‐wave terminal force measured at lead V1; SL, Sokolow‐Lyon.
Data expressed as mean ± SD or median (interquartile range) for continuous variables. n, (%) for categorical variables.
Presence of more than grade I diastolic dysfunction.
Impaired GLS: GLS <18%.
Using continuous measures, Cornell‐V and Cornell‐P were significantly higher in groups with Echo‐LVH, DD, and impaired GLS (P ≤ 0.023). SLV showed no differences among the groups. The overall prevalence of ECG evidence of LVH (ECG‐LVH) by the conventional criteria was the greatest by Cornell‐P—detected in 8.6% of Echo‐LVH, 4.5% of DD, and 6.3% of impaired GLS. By SL voltage and Cornell‐V, only 5.2% and 3.4% in Echo‐LVH were abnormal, respectively. The 75th percentile gender specific cut‐off of Cornell‐P for LVH from the current cohort was 1442 mm · ms for men and 1518 mm · ms in women; this cut‐off detected 45% of those with Echo‐LVH but also detected 22% of those with no Echo‐LVH as being abnormal. The 75th percentile cut‐off for minSTmV5V6 was 20 μV. This cut‐off showed no differences among the groups. As ECG markers are gender dependent, we further assessed their correlation with each SBHF feature stratified by gender ( Table A1 ). In general, correlation between men was better than women. There was significant correlation of Cornell‐V and Cornell‐P with LVMi, with better correlation using Cornell product than voltage. Correlation with GLS and e’ were similarly better with Cornell‐P. SLV showed insignificant correlation. MinSTmV5V6 showed significant correlation with LVMi and GLS. The overall discriminative ability of four ECG markers for SBHF features is displayed by receiver operating characteristic curve in Figure 1.
Figure 1.
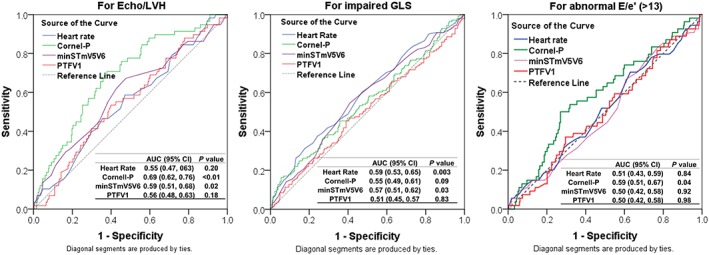
Receiver operating characteristic curve of common ECG markers for descriminative characteristics for echocardiographic LVH, impaired global longitidunal strain (GLS), and for abnormal E/e' (cut‐off 13).
The independent associations of ECG markers with SBHF features are summarized in Table 2. Cornell‐P and resting heart rate were associated with Echo‐LVH, DD, and impaired GLS independent of age, gender, SBP, BMI, Charlson comorbidity score, and other ECG markers. One SD of the mean increased Cornell‐P (635 mm · ms) was associated with an odds ratio that was 1.48 for Echo‐LVH, 1.38 for DD, and 1.37 for impaired GLS (P < 0.012) independent of clinical variables. In multivariable analysis with all four ECG markers, the independent association of Cornell‐P and increased resting heart rate remained significant, with similar effect size (P < 0.047) (Table 2).
Table 2.
Multivariable logistic regression for association and prediction of echocardiographic features of stage B heart failure
| Left ventricular hypertrophy | Diastolic dysfunction | Impaired GLS (<18%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 | OR (95% CI) | P value | R 2 | OR (95% CI) | P value | R 2 | OR (95% CI) | P value | |
| Models with each of following:a | |||||||||
| Heart rate (11 bpm) | 0.083 | 0.73 (0.54, 0.99) | 0.044 | 0.181 | 1.41 (1.13, 1.76) | 0.003 | 0.136 | 1.54 (1.24, 1.91) | <0.001 |
| Cornell product (635 mm · ms) | 0.101 | 1.48 (1.14, 1.90) | 0.003 | 0.175 | 1.38 (1.08, 1.78) | 0.012 | 0.116 | 1.37 (1.11, 1.69) | 0.003 |
| minSTmV5V6 (39 μV) | 0.080 | 0.75 (0.55, 1.01) | 0.058 | 0.157 | 0.92 (0.74, 1.13) | 0.420 | 0.098 | 0.84 (0.68, 1.04) | 0.843 |
| PTFV1 (3532 μVms) | 0.075 | 1.24 (0.94, 1.64) | 0.136 | 0.156 | 0.93 (0.75, 1.15) | 0.521 | 0.091 | 0.98 (0.79, 1.21) | 0.866 |
| Model with all the following: | |||||||||
| Heart rate (11 bpm) | 0.134 | 0.72 (0.53, 0.99) | 0.047 | 0.199 | 1.39 (1.10, 1.75) | 0.005 | 0.158 | 1.52 (1.22, 1.90) | <0.001 |
| Cornell product (635 mm · ms) | 1.49 (1.13, 1.96) | 0.004 | 1.39 (1.05, 1.84) | 0.021 | 1.31 (1.04, 1.65) | 0.021 | |||
| minSTmV5V6 (39 μV) | 0.87 (0.63, 1.19) | 0.379 | 1.02 (0.81, 1.29) | 0.872) | 0.92 (0.73, 1.16) | 0.492 | |||
| PTFV1 (3532 μVms) | 1.27 (0.92, 1.67) | 0.166 | 0.98 (0.78, 1.23) | 0.853 | 1.06 (0.85, 1.33) | 0.606 | |||
CI, confidence interval; GLS, global longitudinal strain; minSTmV5V6, minimal ST deviation at m point of leads V5 and V6; OR, odds ratio; PTFV1, P‐wave terminal force measured at lead V1.
Each model contains age, gender, heart rate, SBP, BMI, Charlson comorbidity score; LIFE: using cut‐offs from LIFE study as stated in methods.
Value as per standard deviation.
Predefined cut‐offs of the four abnormal ECG markers were assessed for diagnostic characteristics for echo features of SBHF. The diagnostic characteristics including sensitivity and positive predictive value for detection of echocardiographic LVH, DD, and impaired GLS are summarized in Table 3. Sensitivity was overall low using single marker, which improved slightly using combined markers with the expected loss of specificity from including multiple variables.
Table 3.
Diagnostic characteristics—comparison of ECG markers using conventional cut‐offs, gender specific upper quartile cut‐offs for detection of stage B heart failure
| Left ventricular hypertrophy | Impaired global longitudinal strain | Diastolic dysfunction (≥ stage I) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| #LVH/total# at risk (PPV) | #LVH/total# LVH (sensitivity) | P | #AbnGLS/total# at risk (PPV) | #AbnGLS/total# AbnGLS (sensitivity) | P | #DD/total# at risk (PPV) | #DD/total# DD (sensitivity) | P | |
| Single ECG marker and cut‐off | |||||||||
| Cornell product 75th (m > 1442, f > 1518) | 26/111 (23%) | 26/58 (45%) | <0.001 | 42/111 (38%) | 42/142 (30%) | 0.113 | 85/111 (77%) | 85/289 (29%) | 0.002 |
| Abnormal PTFV1 (≤−4000 μV·ms) | 17/156 (11%) | 17/58 (29%) | 0.338 | 48/156 (31%) | 48/142 (34%) | 0.740 | 105/156 (67%) | 105/289 (36%) | 0.390 |
| Abnormal minSTmV5V6(≤−20 μV) | 19/119 (16%) | 19/58 (32%) | 0.257 | 45/119 (38%) | 45/142 (32%) | 0.098 | 80/119 (67%) | 80/289 (28%) | 0.493 |
| Abnormal heart rate(≥80 bpm) | 5/57 (9%) | 5/58 (9%) | 0.312 | 26/57 (46%) | 26/142 (18%) | 0.016 | 45/57 (79%) | 45/289 (16%) | 0.016 |
| Combined ECG markers | |||||||||
| Presence of ≥1 abnormal ECG | 44/296 (15%) | 44/58 (76%) | 0.096 | 96/296 (32%) | 96/142 (68%) | 0.672 | 205/296 (69%) | 205/289 (71%) | 0.004 |
| Presence of ≥2 abnormal ECG | 17/115 (15%) | 17/58 (29%) | 0.503 | 48/115 (42%) | 48/142 (34%) | 0.008 | 83/115 (72%) | 83/289 (29%) | 0.050 |
| Presence of ≥3 abnormal ECG | 5/29 (17%) | 5/58 (9%) | 0.480 | 15/29 (52%) | 15/142 (11%) | 0.017 | 24/29 (83%) | 24/289 (8%) | 0.035 |
DD, diastolic dysfunction; ECG, electrocardiogram; GLS, global longitudinal strain; LVH, left ventricular hypertrophy; PPV, positive predictive value.
# = number of participants.
Association of electrocardiographic markers with primary outcome
After a median interval of 14 ± 4 months, 40 individuals were lost to follow‐up or alive but unable to attend follow‐up. This group was no different from the remaining 407 individuals who completed follow‐up ( Table A2 ). New HF symptoms developed in 47 patients and 4 died (2 of CV causes). Of the 47 who developed symptoms, none was preceded by an acute coronary event. The primary composite endpoint of new onset of HF and CV death occurred in 49 (12%) of the entire cohort—an annualized event rate of 10%. Medication status (angiotensin‐converting‐enzyme inhibitor/angiotensin receptor blocker, beta‐blocker, and calcium antagonists) was not associated with outcome (P > 0.09). The diagnosis of HF was a clinical one, but the nature of HF was identified by echocardiography—only 1 of 47 patients with new‐onset HF had HFrEF, 4 had HFmrEF, and 42 had preserved EF.
In the Kaplan–Meier analysis of the four abnormal ECG markers, log‐rank testing showed only individuals with abnormal Cornell‐P (upper 75th percentile), which was associated with primary outcome (P = 0.04) and not those with minSTmV5V6 (P = 0.15) nor with abnormal PTFV1 (P = 0.26) and abnormal heart rate (P = 0.59). Of the entire cohort, 66% had at least one abnormal ECG marker, 29% had two, and 7% had ≥3. Figure 2 shows adverse outcome that was proportional to the number of abnormal ECG markers.
Figure 2.
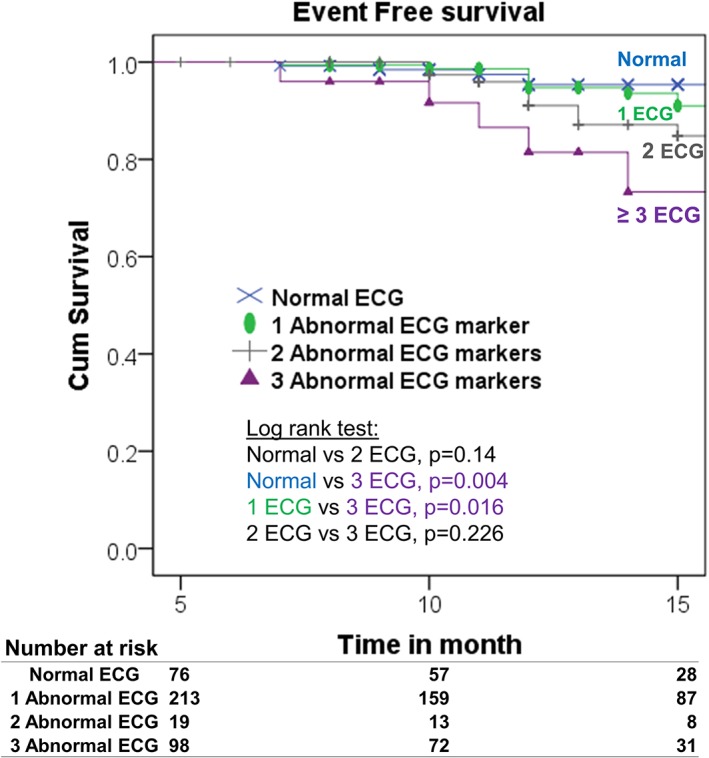
Kaplan–Meier plot of event free survival of those upper quartile of Cornell product. ECG, electrocardiogram.
The independent and incremental predictive value of common ECG and echocardiographic markers for primary outcome was examined using continuous (per SD) in univariable as well as series of multivariable Cox regression models. In univariable analysis, Cornell‐V (not SLV), Cornell‐P, minSTmV5V6 (not PTFV1), and LVMi and GLS (not DD) were significant predictors for outcome (P < 0.026). The 75th percentile of Cornell‐P showed predictive value, and this association remained significant after adjusting for age, gender, and Charlson comorbidity score (Table 4).
Table 4.
Cox regression association of ECG and echocardiographic markers for outcome
| Univariable cox regression | Models Ia (clinical + each ECG and echo marker) | Model IIb | Model IIIc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P | C‐statistic | HR (95% CI) | P | C‐statistic | HR (95% CI) | P | C‐statistic |
| Age (years) | 1.07 (1.01, 1.13) | 0.015 | — | — | — | — | — | — | — | — | — |
| Male, n (%) | 1.41 (0.80, 2.49) | 0.234 | — | — | — | — | — | — | — | — | — |
| Charlson score | 1.21 (1.10, 1.33) | <0.001 | — | — | — | 1.23 (1.12, 1.36) | <0.001 | — | 1.22 (1.11, 1.35) | <0.01 | — |
| ECG markers (per SD) | |||||||||||
| Heart rate (per 11 bpm) | 0.81 (0.59, 1.11) | 0.185 | 0.85 (0.61, 1.18) | 0.335 | 0.704(P = 0.92) | 0.74 (0.53, 1.03) | 0.075 | — | — | — | — |
| SL voltage (mm) | 0.97 (0.93, 1.01) | 0.131 | — | — | — | — | — | — | — | — | — |
| Cornell voltage (mm) | 1.07 (1.01, 1.13) | 0.017 | — | — | — | — | — | 0.701 (P = 0.72) | — | — | 0.727 (P = 0.71) |
| Cornell product (per 635 mm · ms) | 1.41 (1.10, 1.82) | 0.007 | 1.37 (1.06, 1.76) | 0.017 | 0.715 (P = 0.93) | 1.33 (1.01, 1.77) | 0.045 | — | 1.15 (0.85, 1.56) | 0.37 | — |
| minSTmV5V6 (per 39 μV) | 0.69 (0.51, 0.96) | 0.026 | 0.69 (0.49, 0.94) | 0.02 | 0.695 (P = 0.89) | 0.78 (0.57, 1.09) | 0.144 | — | 0.80 (0.58, 1.12) | 0.19 | — |
| PTFV1 (per 3532 μVms) | 0.97 (0.72, 1.29) | 0.834 | 0.87 (0.64, 1.18) | 0.368 | 0.695 (P = 0.33) | 0.90 (0.66, 1.22) | 0.504 | — | — | — | — |
| 75th Cornell‐P(m ≥ 1442;f ≥ 1581) | 1.84 (1.00, 3.85) | 0.049 | 1.89 (1.03, 3.51) | 0.041 | 0.704 (P = 0.85) | — | — | — | — | — | — |
| Echo markers (per SD) | |||||||||||
| LV mass (per 17 g/m2) | 1.68 (1.31, 2.16) | <0.001 | 1.63 (1.26, 2.12) | <0.01 | 0.724 (P = 0.36) | — | — | — | 1.61 (1.22, 2.11) | 0.001 | — |
| Abnormal GLS (per 2.6%) | 0.64 (0.49, 0.83) | 0.001 | 0.74 (0.56, 0.97) | 0.029 | 0.761 (P = 0.03) | — | — | — | — | — | — |
| Diastolic dysfunction ≥ grade I | 1.47 (0.76, 2.84) | 0.253 | 1.15 (0.58, 2.28) | 0.694 | 0.703 (P = 0.23) | — | — | — | — | — | — |
ECG, electrocardiogram; CI, confidence interval; GLS, global longitudinal strain; HR, hazard ratio; minimal ST deviation at m point of leads V5 and V6; PTFV1, P‐wave terminal force measured at lead V1; SD, standard deviation; SL, Sokolow‐Lyon.
Model I, each line is a model with Clinical (age, gender, and Charlson comorbidity score) and each ECG, and echo marker. C‐statistic for clinical = 0.699. All number in bracket after other C‐statistics values were P values compare with C‐statistic of clinical = 0.699.
Model II contains all four ECG markers: heart rate, Cornell‐P, minSTmV5V6, and PTFV1 with Charlson score.
Model III contains Charlson score, Cornell product, minSTmV5V6, and LV mass.
The four ECG markers (Cornell‐P, minSTmV5V6, PTFV1, and abnormal heart rate ≥80 bpm) were moderately correlated (correlation coefficient: −0.01 to −0.42). When they were entered into the models together with age, gender, and Charlson comorbidity score, Cornell‐P and minSTmV5V6 remained to be significantly association with outcome. In the subsequent analyses with echocardiographic markers entered into models, the association of either Cornell‐P or minSTmV5V6 became insignificant with the presence of either LVMi or GLS, indicating a much stronger association of echocardiographic features with outcome (Table 4).
The incremental value of ECG markers over clinical measures (with and without echocardiographic features) was examined using NRI analysis. Addition of abnormal ECG to clinical information (model I), clinical + any one echo marker (model II), and any two echo markers did not demonstrate any significant incremental value for outcome with better performance of adding two ECG markers than one (NRI = −0.01 to 0.11, P > 0.065) ( Table A3 ).
Figure 3 demonstrates the association of abnormal ECG (ECG+) with outcome in the presence of one (Figure 3 A) or more than one (Figure 3 B) abnormal echo markers. Results showed that in patients with mild cardiac abnormalities (one abnormal echo), the presence of abnormal ECG is significantly associated with outcome (hazard ratio: 2.2, 1.04–4.68, P = 0.04) regardless of echo status.
Figure 3.
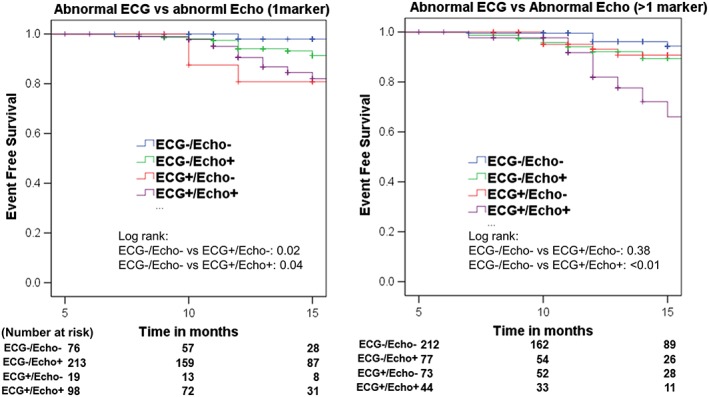
The presence of echo features of stage B heart failure with/without abnormal electrocardiogram (ECG) and associated outcome. Abnormal ECG was defined as presence of any two abnormal ECG marker (Cornell‐P, minSTmV5V6, PTFV1, and baseline HR). Abnormal echo was defined as the presence of any one (A) or >1 (B) of LVH, impaired GLS (18% cut‐off), and diastolic dysfunction. Abnormal ECG and normal echo (ECG+/Echo−, coded red) is associated with worst outcome in mild SBHF by presence of 1 echo marker (A). More cardiac impairment (defined by >1 echo marker) is associated with worse outcome regardless of their ECG status (B).
Abnormal ECG appeared to have prognostic value in those with mild disturbances of cardiac structure and function by echocardiography, although generally more prognostic information appeared to be obtainable from echocardiography. Abnormal ECG did not add incremental value to clinical and echocardiographic assessment (Figure 4).
Figure 4.
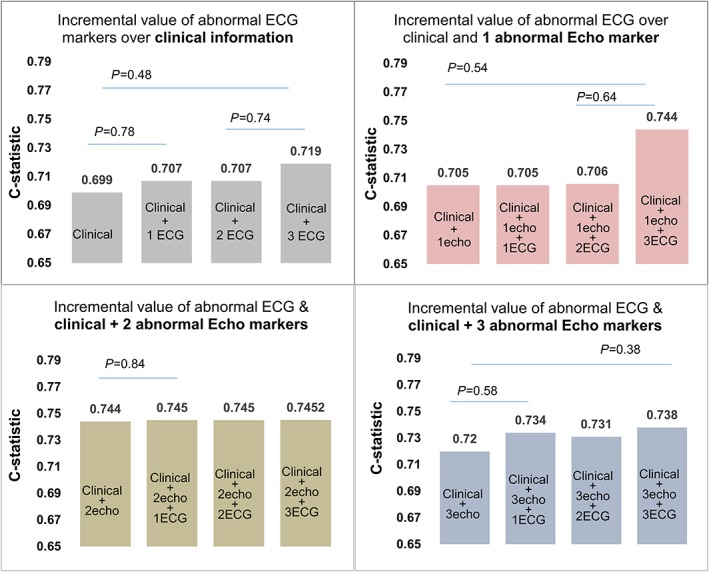
Incremental prognostic value of abnormal electrocardiogram (ECG) over clinical and abnormal echocardiographic markers of stage B heart failure. Clinical includes age, gender, and Charlson comorbidity score; abnormal ECG was defined as the presence of any one of (75th percentile of Cornell product; minSTmV5V6, PTFV1, and heart rate); Abnormal echo was defined as the presence of any one, two, or all three of LVH, impaired GLS (18% cut‐off), and diastolic dysfunction. This figure shows that the presence of more abnormal ECG markers had relative incremental prognostic value over clinical information only and when only one abnormal echo marker was present.
Discussion
In this heterogeneous, community cohort with known non‐ischaemic HF risks and preserved EF, we did not find ECG markers to be of value in screening for SBHF because of low prevalence, low sensitivity, and low predictive value, compared with echocardiographic features of SBHF. However, a number of associations between ECG and new indices of LV dysfunction and outcome were identified. Cornell‐P and increased resting heart rate were independently associated with echocardiographic SBHF features. Cornell‐P and minSTmV5V6 were associated with primary outcome independent of clinical measures but not independent of or incremental to echocardiographic measures.
Stage B heart failure is defined as a condition with asymptomatic structural and/or functional changes in the heart. The clinical recognition of early HF can be difficult, and the prevalence of incident HF may vary broadly depending on the diagnostic criteria.28, 29 A recent meta‐analysis reported that incident HF diagnosis in 8 out of 15 included studies was based on a non‐standardized clinical description.30 Differences in the diagnostic criteria for HF may have impact on the outcome assessment in these studies. Among four commonly used HF diagnostic criteria (Framingham, Boston, Gothenburg, and European Society of Cardiology criteria),31 there were significant differences in predicting clinically relevant outcomes including incident hospital admission. The Framingham criteria seems to correlate best with echocardiography, which is the gold standard to diagnose HF.31
Accordingly, we used the Framingham HF criteria to select subjects with HF. Echocardiography was performed in the subjects with HF to evaluate LVEF. Although we excluded any known and possible HF at baseline, the annualized rate of incident HF was 10%. A higher proportion of stage C1 at baseline may partially explain this.32 Individuals in stage C1 have a significantly worse outcome than SBHF. A high incidence rate was observed in another community study of a cohort with combined diabetes and hypertension,33 in whom E/e' >15 (detected in 23%) was used to categorize SBHF. In our cohort, the prevalence of increased E/e' was lower in entire cohort (12%) but was similar in those with both hypertension and T2DM (20%).
In this study, we provided a comprehensive assessment of early markers of myocardial dysfunction (DD and strain imaging) in addition to assessment of structural cardiac changes. In the non‐ischaemic population with preserved LVEF, impaired GLS and DD, and LVH have a comparable effect on functional capacity.2 The current guidelines have recommended that strain could be used in asymptomatic subjects at risk of HF for early detection of preclinical myocardial dysfunction.27 Indeed, this is feasible in the community—a number of community‐based studies have used strain, including the Northern Manhattan study,34 Framingham study,35 the CARDIA study,36 and others. Previous studies in a different population, with a significant proportion of ischaemic disease have demonstrated the association of ECG changes of LVH with DD.13 The association of ECG features of LVH with systolic function is based on LV midwall shortening, which is likely to be affected by LV geometry.14 Using speckle‐tracking echocardiography, a sensitive imaging marker for early myocardial damage, which has been linked to outcomes.5 This study confirmed the association of ECG markers with early systolic changes by impaired GLS, and these associations were independent of clinical measures including blood pressure, BMI, and comorbidities such as diabetes and hypertension. The potential mechanisms linking abnormal ECG markers and depressed systolic function are multiple. Ischaemia could be an important contributor and is hard to exclude in a cohort with a high prevalence of hypertension and diabetes.14 Myocardial interstitial fibrosis is another possible and important link.
Screening for SBHF in the non‐ischaemic population is challenging, because of a lack of feasible and effective markers. LVH is widely used as an important feature of SBHF and can be diagnosed by ECG or echocardiography. The association of ECG‐LVH with risk of incident HF has been widely recognized in a recent meta‐analysis.30 ECG‐LVH and echocardiographic LVH were found to be equally predictive of incident HF in a community study after follow‐up of 12 years.12 Thus, ECG‐LVH has been used as established risk component in two widely used HF risk scores.37, 38 Other studies have proposed an independent and incremental prognostic significance of ECG‐LVH over echocardiography.39, 40 However, the prevalence of ECG‐LVH is known to be low, varies from 0.6–40%, with an average of 18% only if using combined multiple diagnostic criteria.41 In the process of screening, a single ECG marker may be insufficiently effective because of its low sensitivity and low positive predictive value.42 In a community‐based study, Gencer et al. studied predictive value of combined multiple ECG makers. He found combined abnormal ECG markers were present in up to 34% of population and were significantly incremental to clinical measures.10 Given its safety, low cost, and wide availability and a first‐line routine examination, the ECG has an important role in the primary care. Computerized measurement facilitated a comprehensive and multi‐marker approach for the purpose of screening. In our study, a combination of four commonly used ECG measures had only slightly improved screening sensitivity over one marker. Besides, its prognostic value showed benefit only in those with early cardiac changes, compared with echocardiography. Thus, the value of ECG as a diagnostic and prognostic marker in SBHF is still controversial.
An effective screening programme needs more than a feasible screening test. Screening at the primary care level faces major challenges relating to the feasibility. First, the approach to screening for SBHF is influenced by the scope of target for prevention. The intervention strategy for non‐ischaemic SBHF has not been well defined. It is unknown whether the presence of increased risk would justify intervention without evidence of HF. Second, traditional SBHF based on structural remodelling (LVEF and LVH) needs to be supplemented by more functional parameters,2 which are more sensitive and can detect myocardial impairment prior to the onset of structural remodelling. Although clinical risk‐based and ECG could serve to select higher risk individuals, echocardiography is still needed for guiding intervention. Third, the use of biochemical marker and hand‐held ultrasound (HHU) devices. The sensitivity of BNP may be a particular issue in screening of non‐ischaemic HF, due to the effects of obesity on BNP levels.43 Plasma natriuretic peptides have been better markers for systolic HF than they are for HF with preserved EF or preclinical HF as they reflect cardiac wall stress, which can be expected to be normal until there is an increment of filling pressure. In asymptomatic individuals, findings from studies have been heterogeneous. The sensitivity and positive predictive values of natriuretic peptides have been low—for example, the sensitivity was reported to be 30% against LVH by cardiac magnetic resonance imaging.44 Despite this inverse relationship, NT‐proBNP was reported to provide significant prognostic information in a population study with 21 years of follow‐up.45 Given the limited availability and relative cost of standard echocardiography, an HHU system may able to provide a potential substitute. HHU can play an important role in structural cardiac evaluation. Although there has been growing interest in its role as a screening tool in the community, the main limitations relate to its imaging capabilities—other than assessing LVEF, the current HHU system does not provide assessment of DD or GLS.
The present study was a community‐based clinical trial. There are several limitations. First, because the follow‐up period was short, the outcome assessment may be limited. Second, relatively high rate of incident HF in this cohort may suggest the presence of unrecognized HF at baseline. As previously reported, the possibility of high prevalence of stage C1 in this cohort may explain their rapid progress to new HF.32 Third, the lack of protection of clinical outcome by treatment may indicate confounding by indication that is the most at risk patients were treated in primary care but were more likely to have events. Fourth, we did not perform a new echocardiography in all of the study participants at follow‐up, only in subjects with clinical HF nor did we obtain biomarkers (e.g. BNP), as previous work showed these were more effective in symptomatic rather than asymptomatic dysfunction.46 Moreover, the test performance of BNP is may be constrained in this setting by increasing patient age, obesity, and insulin resistance,43, 46 although recently published data showed controversial results.45 Fifth, the concomitant presence of CAD was not investigated. Atherosclerosis may co‐exist with diabetic cardiomyopathy and hypertensive heart disease and may cause LV dysfunction because of CAD. We sought to exclude patients with a history consistent with CAD, but we cannot exclude an ischaemic contribution to the reported cardiac functional changes. Recruitment was partly through newspaper advertising, which may have led to a population selection bias.
Conclusions
Although standard ECG markers showed low sensitivity and low positive predictive value for SBHF, Cornell‐P and abnormally increased heart rate were independently associated with LVH, impaired GLS, and DD. Cornell‐P and ST changes showed prognostic value for clinical HF, and death of CV causes independent of clinical measures but were not incremental to echocardiography. However, ECG abnormalities were associated with poor outcome (clinical HF and death of CV causes) in those with early and mild echocardiographic features of impairment.
Conflict of interest
None declared.
Funding
This study was partially supported by Tasmanian Community Fund, Hobart and Diabetes Australia, Melbourne and Siemens Healthcare Australia, Melbourne, Australia. None of these agencies had any role in design, analysis, or interpretation of this study. H.Y. is supported by a Health Professional Scholarship from the National Heart Foundation of Australia (100307). K.N. is supported by an award from the Select Foundation. None of these agencies had any role in design, analysis, or interpretation of this study.
Acknowledgements
The authors gratefully acknowledge the contribution of our tireless volunteer coordinators, Diane Binns, Jasmine Prichard, and Jane Mitchell.
Table A1.
Correlation between electrocardiographic markers and cardiac structural and functional measures in men and women
| LVMi | GLS | e' | E/e' | Diastolic strain | Diastolic strain rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho | P value | Rho | P value | Rho | P value | Rho | P value | Rho | P value | Rho | P value | |
| Male | ||||||||||||
| Cornell voltage | 0.39 | <0.01 | −0.22 | 0.002 | −0.17 | 0.01 | 0.05 | 0.47 | −0.20 | 0.004 | −0.30 | <0.01 |
| SL voltage | 0.05 | 0.50 | 0.09 | 0.16 | 0.002 | 0.97 | −0.03 | 0.69 | 0.10 | 0.16 | 0.02 | 0.73 |
| Cornell product | 0.40 | <0.01 | −0.19 | 0.01 | −0.19 | 0.01 | 0.05 | 0.50 | −0.20 | 0.01 | −0.27 | <0.01 |
| minSTmV5V6 | −0.30 | <0.01 | −0.15 | 0.03 | 0.11 | 0.10 | −0.06 | 0.43 | 0.07 | 0.35 | 0.13 | 0.07 |
| PTFV1 | 0.10 | 0.16 | −0.06 | 0.39 | −0.08 | 0.23 | −0.01 | 0.87 | 0.05 | 0.44 | −0.10 | 0.17 |
| Female | ||||||||||||
| Cornell voltage | 0.18 | 0.004 | −0.13 | 0.04 | −0.25 | <0.01 | 0.05 | 0.47 | −0.14 | 0.03 | −0.13 | 0.04 |
| SL voltage | 0.02 | 0.78 | 0.02 | 0.79 | 0.042 | 0.52 | 0.01 | 0.85 | 0.12 | 0.07 | 0.12 | 0.07 |
| Cornell product | 0.26 | <0.01 | −0.16 | 0.01 | −0.21 | 0.001 | 0.08 | 0.22 | −0.11 | 0.09 | −0.13 | 0.05 |
| minSTmV5V6 | −0.18 | 0.01 | 0.12 | 0.06 | 0.07 | 0.30 | 0.10 | 0.13 | 0.13 | 0.05 | 0.17 | 0.01 |
| PTFV1 | 0.03 | 0.69 | 0.09 | 0.13 | 0.13 | 0.04 | −0.02 | 0.73 | 0.21 | 0.001 | 0.12 | 0.07 |
GLS, global longitudinal strain; LVMi, left ventricular mass index; minSTmV5V6, minimal ST deviation at m point of leads V5 and V6; PTFV1, P‐wave terminal force measured at lead V1; SL, Sokolow‐Lyon.
Table A2.
Baseline characteristics in those completed vs. those unable to complete follow‐up
| Completed follow‐up | Unable to follow‐up | ||
|---|---|---|---|
| (n = 407) | (n = 40) | P value | |
| Age (year) | 71 ± 5 | 71 ± 5 | 0.627 |
| Gender male | 196 (48) | 12 (30) | 0.028 |
| Body mass index (g/m2) | 30 ± 5 | 31 ± 6 | 0.234 |
| Type 2 diabetes mellitus | 218 (54) | 23 (58) | 0.634 |
| Obese (BMI ≥30 g/m2) | 182 (45) | 21 (53) | 0.346 |
| Hypertension | 333 (82) | 29 (73) | 0.152 |
| Previous chemotherapy | 36 (9) | 5 (12) | 0.445 |
| Family history | 147 (36) | 13 (33) | 0.649 |
| Previous heart condition | 29 (7) | 6 (15) | 0.077 |
| Charlson comorbidity score | 1 (0–2) | 1 (0–2) | 0.595 |
| LV ejection fraction | 64 ± 6 | 64 ± 7 | 0.770 |
| GLS | 18.6 ± 2.5 | 18.0 ± 2.9 | 0.404 |
| Mitral E/A | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.752 |
| Mitral e' (cm/s) (averaged) | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.399 |
| E/e' (averaged) | 8.9 ± 2.6 | 9.0 ± 2.5 | 0.768 |
| Left atrium volume (mL/m2) | 30 ± 9 | 30 ± 9 | 0.431 |
| LV mass (g/m2) | 84 ± 18 | 82 ± 16 | 0.521 |
| Diastolic dysfunction | 265 (65) | 24 (60) | 0.519 |
| Abnormal E/e'13 | 49 (12) | 5 (13) | 0.932 |
| LV hypertrophy (echo) | 53 (13) | 5 (13) | 0.925 |
| LA enlargement34 | 124 (31) | 9 (23) | 0.289 |
| Abnormal GLS, cut‐off 18 | 129 (32) | 13 (33) | 0.917 |
| Cornell voltage (mm) | 9.9 (7.0–13.7) | 8.2 (4.6–12.9) | 0.115 |
| Sokolow‐Lyon voltage (mm) | 17.9 (13.9–22.9) | 18.9 (14.3–22.5) | 0.763 |
| Cornell product (mm · ms) | 1093 (783–1513) | 1036 (807–1409) | 0.627 |
| minSTmV5V6 (μV) | 2.2 ± 39 | 11.6 ± 35 | 0.234 |
| PTFV1 (μVms) | −2856 ± 3539 | −3546 ± 3438 | 0.178 |
| LV hypertrophy by SL voltage | 7 (2) | 0 (0) | 0.403 |
| LV hypertrophy by Cornell voltage | 7 (2) | 2 (5) | 0.159 |
GLS, global longitudinal strain; LA, left atrium; LV, left ventricle; PTFV1, P‐wave terminal force measured at lead V1.
Continuous variable as mean ± SD or median (interquartile range). Categorical variable as n (%).
Table A3.
Net reclassification improvement examine the incremental value of ECG markers over clinical (model I), ECG marker over clinical +1 echo (model II), and ECG marker over clinical +2 echo markers (model III)
| Model I (clinical + ≥1 abnormal ECG marker) | Increased risk | Decreased risk | Net correctly reclassified % | |||||
|---|---|---|---|---|---|---|---|---|
| Composite endpoints (n = 49) | Quartile 1 (<6.24%) | Quartile 2 (6.24–9.57%) | Quartile 3 (9.57–15.6%) | Quartile 4 (≥15.69%) | n | n | % | |
| (Clinical) | ||||||||
| Quartile 1 (<6.24%) | 5 | 0 | 0 | 0 | 6 | 1 | 10.2 | |
| Quartile 2 (6.24–9.57%) | 1 | 6 | 0 | 0 | ||||
| Quartile 3 (9.57–15.6%) | 0 | 0 | 10 | 6 | ||||
| Quartile 4 (≥15.69%) | 0 | 0 | 0 | 21 | ||||
| No event (n = 358) | ||||||||
| Quartile 1 (<6.24%) | 81 | 13 | 0 | 0 | 38 | 44 | 0.3 | |
| Quartile 2 (6.24–9.57%) | 16 | 66 | 14 | 0 | ||||
| Quartile 3 (9.57–15.6%) | 0 | 18 | 61 | 11 | ||||
| Quartile 4 (≥15.69%) | 0 | 0 | 10 | 67 | ||||
| Net reclassification improvement = 0.11 | 0.105 | |||||||
| P = 0.065 | ||||||||
| Model II (clinical +1 echo + ≥1 abnormal ECG) | Increased risk | Decreased risk | Net correctly reclassified % | |||||
|---|---|---|---|---|---|---|---|---|
| Composite endpoints (n = 49) | Quartile 1 (<6.32%) | Quartile 2 (6.32–9.42%) | Quartile 3 (9.42–15.87%) | Quartile 4 (≥15.99%) | n | n | % | |
| (Clinical + 1echo) | ||||||||
| Quartile 1 (<6.32%) | 4 | 0 | 0 | 0 | 4 | 0 | 8.2 | |
| Quartile 2 (6.32–9.42%) | 3 | 5 | 0 | 0 | ||||
| Quartile 3 (9.42–15.87%) | 0 | 0 | 11 | 4 | ||||
| Quartile 4 (≥15.99%) | 0 | 0 | 0 | 22 | ||||
| Reclassified | ||||||||
| Increased risk | Decreased risk | Net correctly reclassified % | ||||||
| No event (n = 358) | Quartile 1 (<6.32%) | Quartile 2 (6.32–9.42%) | Quartile 3 (9.42–15.87%) | Quartile 4 (≥15.99%) | n | n | % | |
| Quartile 1 (<6.32%) | 83 | 11 | 0 | 0 | 37 | 38 | 0.3 | |
| Quartile 2 (6.32–9.42%) | 18 | 64 | 14 | 0 | ||||
| Quartile 3 (9.42–15.87%) | 0 | 12 | 68 | 12 | ||||
| Quartile 4 (≥15.99%) | 0 | 0 | 8 | 62 | ||||
| Net reclassification improvement = 0.085 | 0.085 | |||||||
| P = 0.074 | ||||||||
| Model II (clinical +2 Echo + ≥1 abnormal ECG) | Increased risk | Decreased risk | Net correctly reclassified % | |||||
|---|---|---|---|---|---|---|---|---|
| Composite endpoints (n = 49) | Quartile 1 (<5.69%) | Quartile 2 (5.69–9.42%) | Quartile 3 (9.42–15.76%) | Quartile 4 (≥15.76%) | n | n | % | |
| (Clinical+ 2 Echo) | ||||||||
| Quartile 1 (<5.69%) | 5 | 0 | 0 | 0 | 1 | 4 | −6.1 | |
| Quartile 2 (5.69–9.42%) | 1 | 5 | 0 | 0 | ||||
| Quartile 3 (9.42–15.76%) | 0 | 1 | 7 | 1 | ||||
| Quartile 4 (≥15.76%) | 0 | 0 | 2 | 27 | ||||
| Reclassified | ||||||||
| Increased Risk | Decreased Risk | Net correctly reclassified % | ||||||
| No event (n = 358) | Quartile 1 (<5.69%) | Quartile 2 (5.69–9.42%) | Quartile 3 (9.42–15.76%) | Quartile 4 (≥15.76%) | n | n | % | |
| Quartile 1 (<5.69%) | 84 | 11 | 0 | 0 | 23 | 40 | 4.7 | |
| Quartile 2 (5.69–9.42%) | 15 | 75 | 8 | 0 | ||||
| Quartile 3 (9.42–15.76%) | 0 | 17 | 71 | 4 | ||||
| Quartile 4 (≥15.76%) | 0 | 0 | 8 | 63 | ||||
| Net reclassification improvement = −0.014 | −0.014 | |||||||
ECG, electrocardiogram.
Clinical = age, gender, Charlson score.
Yang, H. , Marwick, T. H. , Wang, Y. , Nolan, M. , Negishi, K. , Khan, F. , and Okin, P. M. (2017) Association between electrocardiographic and echocardiographic markers of stage B heart failure and cardiovascular outcome. ESC Heart Failure, 4: 417–431. doi: 10.1002/ehf2.12151.
References
- 1. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Journal of the American College of Cardiology. 2009; 53: e1–e90. [DOI] [PubMed] [Google Scholar]
- 2. Kosmala W, Jellis CL, Marwick TH. Exercise limitation associated with asymptomatic left ventricular impairment: analogy with stage B heart failure. J Am Coll Cardiol. 2015; 65: 257–266. [DOI] [PubMed] [Google Scholar]
- 3. Investigators TS. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD investigators. N Engl J Med. 1992; 327: 685–691. [DOI] [PubMed] [Google Scholar]
- 4. Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM, on Behalf of the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N Engl J Med. 1992; 327: 669–677. [DOI] [PubMed] [Google Scholar]
- 5. Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10‐year outcomes in type 2 diabetes mellitus. Heart. 2015; 101: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non‐ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016; 18: 1331–1339. [DOI] [PubMed] [Google Scholar]
- 7. Dhingra R, Pencina MJ, Wang TJ, Nam BH, Benjamin EJ, Levy D, Larson MG, Kannel WB, D'Agostino RB Sr, Vasan RS. Electrocardiographic QRS duration and the risk of congestive heart failure: the Framingham Heart Study. Hypertension. 2006; 47: 861–867. [DOI] [PubMed] [Google Scholar]
- 8. Okin PM, Kamel H, Kjeldsen SE. Devereux RB. Electrocardiographic left atrial abnormality and risk of incident stroke in hypertensive patients with electrocardiographic left ventricular hypertrophy. J Hypertens. 2016; 34: 1831–1837. [DOI] [PubMed] [Google Scholar]
- 9. Okin PM, Roman MJ, Lee ET, Galloway JM, Best LG, Howard BV, Devereux RB. Usefulness of quantitative assessment of electrocardiographic ST depression for predicting new‐onset heart failure in American Indians (from the Strong Heart Study). Am J Cardiol. 2007; 100: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gencer B, Butler J, Bauer DC, Auer R, Kalogeropoulos A, Marques‐Vidal P, Applegate WB, Satterfield S, Harris T, Newman A, Vittinghoff E, Rodondi N. Association of electrocardiogram abnormalities and incident heart failure events. Am Heart J. 2014; 167: 869–875 e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olesen LL, Andersen A. ECG as a first step in the detection of left ventricular systolic dysfunction in the elderly. ESC Heart Failure. 2016; 3: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almahmoud MF, O'Neal WT, Qureshi W, Soliman EZ. Electrocardiographic versus echocardiographic left ventricular hypertrophy in prediction of congestive heart failure in the elderly. Clin Cardiol. 2015; 38: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krepp JM, Lin F, Min JK, Devereux RB, Okin PM. Relationship of electrocardiographic left ventricular hypertrophy to the presence of diastolic dysfunction. Ann Noninvasive Electrocardiol. 2014; 19: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okin PM, Wachtell K, Gerdts E, Dahlof B, Devereux RB. Relationship of left ventricular systolic function to persistence or development of electrocardiographic left ventricular hypertrophy in hypertensive patients: implications for the development of new heart failure. J Hypertens. 2014; 32: 2472–2478 discussion 2478. [DOI] [PubMed] [Google Scholar]
- 15. Okin PM, Roman MJ, Lee ET, Galloway JM, Best LG, Howard BV, Devereux RB. Usefulness of quantitative assessment of electrocardiographic ST depression for predicting new‐onset heart failure in American Indians (from the Strong Heart Study). Am J Cardiol. 2007; 100: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okin PM, Devereux RB, Fabsitz RR, Lee ET, Galloway JM, Howard BV. Quantitative assessment of electrocardiographic strain predicts increased left ventricular mass: the Strong Heart Study. J Am Coll Cardiol. 2002; 40: 1395–1400. [DOI] [PubMed] [Google Scholar]
- 17. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 18. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949; 37: 161–186. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 20. Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006; 92: 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 22. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 23. Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta‐analysis. Journal of the American Society of Echocardiography 2013; 26: 185–191. [DOI] [PubMed] [Google Scholar]
- 24. Ishii K, Imai M, Suyama T, Maenaka M, Nagai T, Kawanami M, Seino Y. Exercise‐induced post‐ischemic left ventricular delayed relaxation or diastolic stunning: is it a reliable marker in detecting coronary artery disease? J Am Coll Cardiol. 2009; 53: 698–705. [DOI] [PubMed] [Google Scholar]
- 25. Brooks D, Solway S, Gibbons WJ. ATS statement on six‐minute walk test. Am J Respir Crit Care Med. [Comment Letter]. 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 26. Kalon K. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993; 22(Supplement A): 6A–13A. [DOI] [PubMed] [Google Scholar]
- 27. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 28. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992; 20: 301–306. [DOI] [PubMed] [Google Scholar]
- 29. Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, Oliveira A. Prevalence of chronic heart failure in Southwestern Europe: the EPICA Study. Eur J Heart Fail. 2002; 4: 531–539. [DOI] [PubMed] [Google Scholar]
- 30. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta‐analysis. Open heart. 2015; 2 e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Bari M, Pozzi C, Cavallini MC, Innocenti F, Baldereschi G, De Alfieri W, Antonini E, Pini R, Masotti G, Marchionni N. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol. 2004; 44: 1601–1608. [DOI] [PubMed] [Google Scholar]
- 32. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007; 115: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 33. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre‐clinical diastolic dysfunction a population‐based study. J Am Coll Cardiol. 2010; 55: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Race‐ethnic differences in subclinical left ventricular systolic dysfunction by global longitudinal strain: a community‐based cohort study. Am Heart J. 2015; 169: 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Age‐ and sex‐based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013; 6: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, Isogawa A, Lewis CE, Wu C, Jacobs DR Jr, Liu K, Lima JA. Association of insulin resistance and glycemic metabolic abnormalities with LV structure and function in middle age: the CARDIA Study. JACC Cardiovasc Imaging. 2017; 10: 105–114. [DOI] [PubMed] [Google Scholar]
- 37. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999; 159: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 38. Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008; 1: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leigh JA, O'Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged ≥65 Years. Am J Cardiol. 2016; 117: 1831–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001; 103: 2346–2351. [DOI] [PubMed] [Google Scholar]
- 41. Cuspidi C, Rescaldani M, Sala C, Negri F, Grassi G, Mancia G. Prevalence of electrocardiographic left ventricular hypertrophy in human hypertension: an updated review. J Hypertens. 2012; 30: 2066–2073. [DOI] [PubMed] [Google Scholar]
- 42. Casiglia E, Schiavon L, Tikhonoff V, Bascelli A, Martini B, Mazza A, Caffi S, D'Este D, Bagato F, Bolzon M, Guidotti F, Haxhi Nasto H, Saugo M, Guglielmi F, Pessina AC. Electrocardiographic criteria of left ventricular hypertrophy in general population. Eur J Epidemiol. 2008; 23: 261–271. [DOI] [PubMed] [Google Scholar]
- 43. Cheng S, Fox CS, Larson MG, Massaro JM, McCabe EL, Khan AM, Levy D, Hoffmann U, O'Donnell CJ, Miller KK, Newton‐Cheh C, Coviello AD, Bhasin S, Vasan RS, Wang TJ. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am J Cardiol. 2011; 108: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta S, Rohatgi A, Ayers CR, Patel PC, Matulevicius SA, Peshock RM, Markham DW, de Lemos JA, Berry JD, Drazner MH. Risk scores versus natriuretic peptides for identifying prevalent stage B heart failure. Am Heart J. 2011; 161: 923–930 e922. [DOI] [PubMed] [Google Scholar]
- 45. Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E. N‐terminal pro‐brain natriuretic peptide and heart failure risk among individuals with and without obesity: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016; 133: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ewald B, Ewald D, Thakkinstian A, Attia J. Meta‐analysis of B type natriuretic peptide and N‐terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008; 38: 101–113. [DOI] [PubMed] [Google Scholar]


