Abstract
Aims
The multicentric TranslatiOnal Registry for CardiomyopatHies (TORCH) of the German Centre for Cardiovascular Research aims to recruit 2300 patients with non‐ischemic cardiomyopthies.
Methods and results
The investigations were performed after standard operating procedures. The data are collected in standardized electronic case report forms provided by the data holding of the central data management of the German Centre for Cardiovascular Research using secuTrial (interActive Systems GmbH, Berlin, Germany). The personal‐identifying data and informed consent are collected, stored, and quality‐checked by the independent Trusted Third Party in Greifswald. The quality management of the medical data is performed by the data and quality centre Greifswald. In December 2014, the recruitment for TORCH has started. Currently, data and biomaterial from about 1397 patients and more than 74 500 biomaterial aliquots were collected. Regular study centre‐specific quality reports address completeness and plausibility of data and provide detailed information about current missing or implausible data entries to improve the data quality by using a query management in addition.
Conclusions
A regular quality control and reporting improve the data quality in TORCH and will support high‐quality data analysis and the translation of research results into routine care.
Keywords: Cardiomyopathy, Registry, Central data management, Quality management
Introduction
The German Centre for Cardiovascular Research (DZHK) is one of the six German Centres for Health Research. Scientists in the DZHK investigate causes and pathogenesis of an array of cardiovascular diseases. Cardiovascular diseases are the most frequent main diagnosis for hospitalizations in Germany.1 One aim of the DZHK is to develop new therapies to improve the individualized therapy, the poor prognosis, and the quality of life of patients with cardiomyopathies. The funding of the first three projects (one registry, one interventional study, and one cohort) started in 2012. One of these projects is the cardiomyopathy registry TranslatiOnal Registry for CardiomyopatHies (TORCH). The aims of this German multicentre registry are a detailed phenotype data collection and the establishment of a federated biobank to investigate the pathogenesis, the diagnostics, and therapies for cardiomyopathy patients.
The data collection is standardized and harmonized within all studies, cohorts, or registries within the DZHK. The quality management is standardized and study centre specific and performed by the data and quality centre in Greifswald. It enables high data quality and is the basis for correct data analysis. The design of the registry and endpoints of TORCH are described in Seyler et al.1
Methods
Study infrastructure
The TORCH registry has a non‐interventional design. Patients' clinical data investigations at baseline and after 1 year of follow‐up are collected, and their biomaterial is stored at the TORCH recruiting study centres. The multicentre recruitment in TORCH is performed in several recruiting centres in Germany (DZHK centres and external partner sites). DZHK centres are the seven partner sites of the DZHK: Berlin, Goettingen, Greifswald, Hamburg/Kiel/Luebeck, Heidelberg/Mannheim, Munich, and Rhine‐Main, which have a proven expertise regarding cardiovascular research cooperation. However, the external non‐DZHK partner sites (are further German university and non‐university hospitals) may also recruit for the TORCH registry.
The coordination of the registry TORCH is composed of two partners (main study centre): the scientific and clinical steering centre in Heidelberg and the data and quality centre in Greifswald. The scientific and clinical steering centre in Heidelberg is responsible for clinical and scientific aspects, preconditions to receive a positive ethic vote including the study protocol, the organization and the support with the local ethics committees, regular teaching, and the payment per included patient of the recruiting centres. The data and quality centre in Greifswald is responsible for the data quality management and data monitoring.
The recruiting study centres are responsible for the recruitment, the storage of biomaterial, and the data capture of medical and biomaterial data in secuTrial.
The Central Data Management (CDM) is organized as joint research project consisting of three partner sites: (i) the independent Trusted Third Party (TTP) Greifswald for the storage of identifying data (IDAT), consent management, pseudonymisation, and revocation; (ii) the Data Holding (DH) Goettingen for medical data (MDAT) as findings and diagnostic measurement data; and (iii) the IT‐laboratory information system and IT‐infrastructure (ITLab) Berlin, which coordinates the central laboratory information management system (LIMS) and the image data management system.
The ethics project in Munich harmonizes the informed consent forms for all DZHK studies, registries, and cohorts (SRC) and supported TORCH in ethical aspects.
The DZHK main office is charged with the harmonization of the SRC and the coordination between them and the CDM (Figure 1 ).
Figure 1.
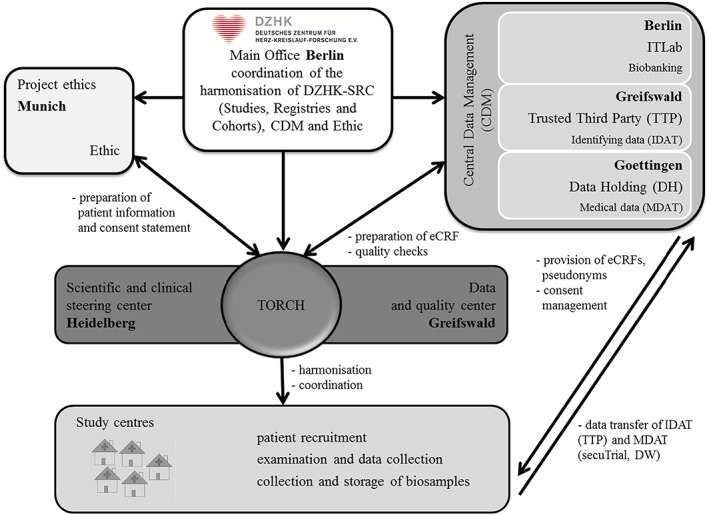
Responsibilities in the registry TranslatiOnal Registry for CardiomyopatHies (eCRF, electronic case report forms; ITLab, information technology and laboratory information system).
Further, all DZHK studies use harmonized informed consents, standardized electronic case report forms (eCRFs), and the same DZHK standard operation procedures (SOPs) for the clinical investigations and biomaterial extraction.
Ethical aspects—informed consent and revocation
Inclusion and exclusion criteria for the registry TORCH are described in Seyler et al.1 All eligible patients are informed by the clinical staff in the participating clinical recruiting study centres about TORCH and asked to give their written informed consent to participate in TORCH. The ethical aspects of the TORCH registry conform to the latest version of the Declaration of Helsinki. TORCH received the first positive ethic vote in October 2014 from the ethic committee in Heidelberg (S‐344/2014). The study protocol together with patient information and informed consent was submitted to the ethics committees of each participating study centre to obtain legal advice prior to study entry. Only patients with a positive ethics vote will be included.
In Table 1, the dates of the positive ethic vote, the initiation visit by the main study centre, and the start of recruitment are presented for each recruiting study centre.
Table 1.
Initiation dates and beginning of recruitment of the TORCH study centres (ordered by date of ethic vote)
| TORCH study centre | Type of study centre | Date of ethic vote | Date of initiation visit | Date of recruitment start |
|---|---|---|---|---|
| University Hospital Heidelberg | Main study centre and DZHK centre | 29.10.2014 | — | 01.12.2014 |
| University Medicine Mannheim | DZHK centre | 20.01.2015 | 16.09.2015 | 01.10.2015 |
| Charité University Medicine Berlin, Campus Mittea | DZHK centre | 11.02.2015 | 08.05.2015 | 19.05.2015 |
| Charité University Medicine Berlin, Campus Benjamin Franklina | DZHK centre | 11.02.2015 | 08.05.2015 | 13.05.2015 |
| Charité University Medicine Berlin, Campus Virchowa | DZHK centre | 11.02.2015 | 07.07.2015 | 08.07.2015 |
| Heart Center Berlin, Department of Heart Surgerya | DZHK centre | 11.02.2015 | 07.07.2015 | 10.06.2015 |
| Heart Center Berlin, Department of Cardiologya | DZHK centre | 11.02.2015 | 15.10.2015 | 27.09.2016 |
| University Medicine Greifswald | Main study centre and DZHK centre | 16.02.2015 | — | 18.02.2015 |
| University Medicine Goettingen | DZHK centre | 25.02.2015 | 27.10.2015 | 06.11.2015 |
| Heart Center Munich | DZHK centre | 21.04.2015 | 24.04.2015 | 19.05.2015 |
| University of Hamburg‐Eppendorf | DZHK centre | 23.04.2015 | 02.10.2015 | 24.11.2015 |
| University Medicine Mainz | DZHK centre | 26.05.2015 | 07.10.2015 | 20.11.2015 |
| Hannover Medical School | external centre | 29.05.2015 | 23.09.2015 | 19.04.2016 |
| University Hospital Frankfurt | DZHK centre | 08.06.2015 | 29.09.2015 | 10.12.2015 |
| University Medical Center‐UKSH, Campus Luebeck | DZHK centre | 06.07.2015 | 22.09.2015 | 27.10.2015 |
| University Medical Center‐UKSH, Campus Kiel | DZHK centre | 21.07.2015 | 22.09.2015 | 20.10.2015 |
| Ludwig‐Maximilian University Hospital Munich, Campus City | DZHK centre | 31.07.2015 | 25.11.2015 | 11.01.2016 |
| Ludwig‐Maximilian University Hospital Munich, Campus Großhadern | DZHK centre | 31.07.2015 | 29.03.2016 | 29.03.2016 |
| Technical University Hospital Munich | DZHK centre | 14.08.2015 | 25.11.2015 | 25.01.2016 |
| Kerckhoff Clinic Bad Nauheim | DZHK centre | 02.03.2016 | 15.06.2016 | 13.07.2016 |
DZHK, German Centre for Cardiovascular Research; TORCH, TranslatiOnal Registry for CardiomyopatHies.
Ethic vote of Heidelberg was accepted by Berlin Ethics Commission.
First, the study physicians inform the patients who fulfil the inclusion criteria about the process and the aim of the registry, the personal benefit, and the possible risks for the patients. Participation in the registry TORCH is on a voluntary basis. This is taken into account and emphasized in the patient's informed consent. The consent form concludes individual opt‐out modules for a highly individualized registry agreement. Patients were asked to sign the informed consent including consent to the extraction of biomaterial samples and clinical investigations concerning the routine care. The scan of the signed consent document is transferred encrypted to the TTP for central quality management and electronic traceability of consented and revoked policies. The original document stays in the clinical record of the patient. A revocation of the participation of a patient, which means that he or she cancels his/her consent to TORCH, is possible at any time because of the principles of informational self‐determination2 without giving reasons and without any disadvantages for further medical care. At revocation (cancellation of participation) by the patient, already obtained biomaterial will be destroyed, and medical data will be anonymized. Anonymization is achieved by deletion of the assignment of the Master Person Index identifier to the pseudonyms (MDAT and biomaterials) within the TTP. The destruction of biomaterials is initiated in the study centres. In the DH, medical data are disabled for export to the transfer party. Each step (anonymization of data and destruction of biomaterials) will be documented.
Of non‐participants'—patients who meet the inclusion criteria but have refused to participate in TORCH—sociodemographic data (age, sex, and nationality), the reason of non‐attendance, the cardiomyopathy diagnosis, and the left ventricular ejection fraction will be filled in an anonymized questionnaire by the physician in order to identify possible systematic differences between participants. This is transmitted to the data and quality centre of Greifswald, where it will be documented, recorded in a non‐participant database, and analysed with regard to noticeable deviation in a study centre.
Data management
The separation of identifying (IDAT) and medical data (MDAT) is performed both organizationally and locally aligned to the concept of ‘informational separation of powers’ from the guideline for data protection, which is provided by the Technology, Methods, and Infrastructure for Networked Medical Research (TMF).3 In the TTP in Greifswald, the IDATs are managed and stored using the technical capabilities of the ID management, the consent management, and the generic service for pseudonymization. In the DH in Goettingen, a study database using secuTrial was constructed and is operated in collaboration with the main study centre of TORCH. There MDATs are stored and linked to the pseudonyms provided by the TTP. The biomaterials are stored in the respective study centre.
Data protection
Data protection aspects for the data capture, storage, and transfer are descripted in detail in a concept document of the CDM,4 which was positively evaluated in December 2013 (TTP Greifswald) and June 2014 (DH Goettingen) by the appropriate federal state agency, respectively. The data protection concept illustrates the technical implementation of the data protection aspects in all DZHK studies, registries, and cohorts including TORCH. The protection consists of firewall‐protected servers, closed zones, IP‐address verification for data capture, encryption algorithms, HTTPS protocolling, and an individual authorized login access into the secuTrial application with username and password.
The TORCH data protection concept is based on the template of the MOSAIC project.5, 6 It regulates individual TORCH‐specific aspects to be considered in the main study centre and the individual recruiting study centres regarding data protection and safety. The concept was positively evaluated by the TMF in March 2015. All clinical staff has to comply with the medical confidentiality and medical professional discretion. The access to the paper‐based clinical record and to the digital database via secuTrial is limited to authorized physicians and nurses (i.e. the clinical staff can only access the data of the patients who were recruited in their own institution).
Data capture
If a person agrees to participate in TORCH, the IDATs are transmitted and encrypted to the TTP, where the process of pseudonymisation is performed. This is only possible on personal computers with known internet protocol address and equipped with a client certificate provided by the TTP as well as after a successful login in secuTrial. The transmission of the IDATs to the TTP is realized using a Transport Layer Security encryption (TLS version 1.2). In order to create a system‐wide unique identifier within the DZHK, an ID is generated. To this ID, pseudonyms can be assigned, which are generated based on the study and content (e.g. for medical data, biomaterial).
All data including medical history, clinical investigations, and laboratory values are documented in eCRFs in secuTrial. The transmission of data is carried out by the study centres on an input screen of the web‐based data capture tool secuTrial. The access is restricted by user‐specific usernames and passwords. This denotes a restricted access to the online application of the medical and IDATs to authorized staff. An inactivity for at least 60 min in secuTrial results in an automatic logout to guarantee a high degree of patients' data safety.
All variables are completed with further information about units and missing data to facilitate the data capture for the study nurses and clinical staff. Information about clinical results is collected as one or multiple answers allowing standardizing data collection. Individual aspects and additional information about diagnostic characteristics or measurement methods can be documented as comments in text form for every item.
Data storage
All IDATs are stored in a database of the TTP. At the moment, the database of the DH consists of the medical data and both patients' pseudonyms for medical and biomaterial data, which were generated by the TTP in Greifswald. A high compatibility to all supported operating systems (Microsoft Windows, Apple, and Linux) and the most frequently used web browser systems (Internet Explorer, Firefox, and Chrome) is ensured by the CDM. The servers are maintained regularly.
The automatic audit trail of secuTrial documents all accesses and data modification. The software secuTrial has its own right and role system to enable data security and to prevent data manipulation.
Biomaterial management
In TORCH, the following biomaterials are collected and stored in a biomaterial database: in the TORCH set serum, ethylenediaminetetraacetic acid plasma, citrate plasma, urine, and endomyocardial biopsies. In the DZHK basis set, serum, ethylenediaminetetraacetic acid plasma, citrate, buffy coat, and urine are stored. The centrifuged aliquot vials are stored in vials at −80°C. The freezers in each recruiting study centre are either connected to the local fall‐back systems as emergency power aggregates or have a warning audio signal to guarantee long‐term storage conditions. The clinical staff was informed and trained about the security requirements regarding the TORCH biomaterial storage. The labels of the vials are subject to DZHK standards to enable a homogenous storage and documentation system. The patient‐related one‐dimensional barcode on the primary vials' surfaces consists of 10 digits. The vials of the aliquots are labelled with the type of biomaterial and a unique number. The racks where the aliquots are sorted provide a two‐dimensional binary data matrix code. The documentation of all information of the vials as the storage position is organized by the recruiting study centres. During the sample preparation process, all information about material type, time‐dependent processes, and the sample condition are documented on special forms. Only authorized clinical staff has access to these storage rooms.
Information related to the biomaterial (number of vials, vial position in the freezer, abnormalities of the biomaterial, etc.) is documented in the web‐interface in secuTrial. When the central LIMS will be fully connected to the CDM structure, the documentation will be performed in the central LIMS utilizing a computer connected barcode scanner to reduce any transmission failures.
Quality management
In the course of DZHK‐wide harmonization of data and biomaterial collection, an agreed basic data set of selected eCRF modules was harmonized across all studies, registries, and cohorts. This allows analyses of medical examination results of the agreed basic data over all DZHK clinical studies.
The CDM of DZHK provides a uniform and transparent data structure for TORCH. It is also responsible for the centralized data backup and data protection as well as data storage. For all eCRF modules, the data management provides data dictionaries including the description of the variable labels and values.
Before study starts, a comprehensive user test in collaboration between the data and quality centre Greifswald, the scientific and clinical steering centre Heidelberg, the TTP, and the DH was performed. Each TTP form, each eCRF, and each variable were tested systematically.
For all users, all processes starting with patient enrolment, conducting medical investigations as well as data capture and transfer are described in the study protocol of TORCH, clinical SOPs, or a secuTrial user guide. The clinical SOPs (e.g. electrocardiogram, echocardiography, and spirometry) were harmonized DZHK‐wide. The TTP provides SOPs for the consent (data capture, ticket‐system for uploading and downloading documents, and revocation). For data capture in secuTrial, a user manual is available by the DH. For the data collection in TORCH, TORCH‐specific SOPs were provided (quality of life, biomaterial management, depression, and non‐responders).
In an initial quality training (08.10.2014), both the investigators and the clinic responsible employee of the study centres were trained on all topics regarding general aspects of the DZHK, inclusion and exclusion criteria in TORCH, ethical aspects and informed consent, data collection, data capture in the web‐interface secuTrial and the TTP, biomaterial storage, and the DZHK SOPs about clinical investigations and biomaterial. Before a study centre will start with patient recruiting, an initiation visit will be performed locally organized by the main study centre of Heidelberg and Greifswald at which the staff of the study centre will be trained.
Automatic plausibility and completeness checks worked out together with the main study centre is implemented in secuTrial to allow a high data quality during the data capture process. Implausible data entries result in an immediate warning or error message. In addition, the data and quality centre Greifswald performs complex quality controls on a database export per recruiting study centre in about 2‐month intervals for all patients who are marked with a so‐called review right. The quality control includes baseline and follow‐up data. The aim is to identify missing entries, input errors, and implausible values by comparison with the reference ranges or units. A review right on a patient is set by the study centre and means that this patient is completely documented and has to be included in the quality control by the data and quality centre Greifswald. During this data quality process, all recruiting study centres receive a regular feedback regarding their data quality. Therefore, a comprehensive quality report (about 500 pages) including descriptive statistics of all data is provided to each study centre including their own collected and inserted data. Additionally, the data and quality centre Greifswald sends a short form of the quality report with implausible data and queries only with concrete demand for action. Additionally, for missing or implausible data, the study centre receives a query in secuTrial to proof or correct the entry based on the source data. During this process, error sources or misunderstood questions are often identified and can be supplemented with more details to facilitate data capture and to support a higher data quality.
Clinical monitoring
Monitoring and site visits started in 2016 in every recruiting study centre to monitor the quality of the clinical examinations, the documentation, and the biomaterial storage and to provide appreciative feedback and hints. After the site visit, the recruiting study centre receives a monitoring report about the proofed quality aspects and, if needed, some instructions.
Download area, newsletters, and standard operation procedures
All TORCH documents including the study protocol, all TORCH newsletters, the DZHK SOPs for anamnesis, electrocardiogram, echocardiography, 6‐minute walk test, heart catheter, magnetic resonance tomography, spiroergometry, biomaterial extraction, and biomaterial preparation can be downloaded from the TORCH download area by all TORCH members and the CDM. Further, the TORCH‐specific SOPs for quality of life questionnaire, non‐participation questionnaire, TORCH‐specific biomaterial storage and documentation, and depression screening can be downloaded as well as all documents for IT connection to the CDM. All documents in the download area are proofed regularly and updated if needed.
Every 2 months, a newsletter designed by the data and quality centre Greifswald and enhanced with clinical expertise by the scientific and clinical steering centre Heidelberg is sent to the recruiting study centres to inform about news, hints for the data capture, the current recruitment status, a frequently asked question part, and a link to the TORCH download area.
Use and access
The ownership issues and the use of data and biomaterials are regulated in the DZHK use and access regulations. All data and biomaterials, which are collected by the registry TORCH, are subject to the regulations of the DZHK. The TORCH study coordinator has a right to use the data and biomaterials with respect to a specific research question. Temporary, dedicated, non‐exclusive, and non‐transferable usage rights are granted to the DZHK partners. The use of data and biomaterial requires a positive assessment of the research question of a previously submitted application in accordance with the ‘Use and Access Committee’.
When providing the data for research purposes to DZHK scientists, it will be ensured that only variables relevant for the research question were transferred. The DH in Goettingen takes on the task of the transfer unit for the MDAT. For the provision of data to DZHK scientists, the medical data are extracted in cooperation with the TTP by a record linkage after verifying the consent of each patient. Data were provided after the generation of a second pseudonym for each research project.
Each data provision is documented by the DH of the CDM. In a contract, the time of use and that the data and/or biomaterials may only be used for the proposed research question as well as that is not allowed to hand them over are covered. To build a database of results, it is planned to transfer results determined from biomaterials back to the DH, which can be made available to other research projects afterwards. So a sustainable use and conservation of biomaterials are possible.
Results
The cooperation project TORCH is composed of the main study centre, the TTP Greifswald, the DH Goettingen, the project ethics in Munich, and the DZHK main office in Berlin (Figure 1 ). In 2014, TORCH was registered in clinicaltrials.gov (identifier NCT02187263) and in the German Clinical Trials Register (identifier DRKS00008017).
TORCH was the first recruiting study in the clinical research group of the DZHK and therefore a start of unique national multicentre registry and biobank. Since December 2014, N = 1397 patients (mean 54.1 ± 14.6 years) in 20 study centres (status: 12.12.2016) were recruited. In April 2016, the first non‐DZHK centre started the recruitment.
In Table 2, the eCRFs including the number of items and the current completeness status for all included patients at baseline and the 1‐year follow‐up are listed. It is shown that only about 55% of the eCRFs are approved for quality control by the study centres by setting review right as described previously. Up to now, a total number of 126 non‐participant sheets were sent to the data and quality centre. Personal reason (60.3%) was named as main reason for refusal from non‐participants (mean age: 57.3 ± 16.4 years, 62% men). In December 2016, more than 74 500 biomaterial aliquots of TORCH patients were collected (Table 3).
Table 2.
The eCRFs used in TORCH including the number of items and the completeness of eCRFs at baseline and 1‐year follow‐up (status: 12.12.2016)
| Electronic case report form (eCRF) | Number of items (total N = 844) | Patients with review right at baseline (total patients N = 1397) (N, (%)) | Patients with review right at baseline (total patients N = 355) (N, (%)) | Standard operation procedure |
|---|---|---|---|---|
| Anamnesis | 115 | 806 (57.7) | 110 (31.0) | Anamnesis and clinical diagnostics |
| Diagnosis of cardiomyopathy | 37 | 870 (62.3) | 111 (31.3) | Anamnesis and clinical diagnostics |
| Medication | 36 | 852 (61.0) | 110 (31.0) | None |
| Echocardiography | 90 | 846 (60.6) | 96 (27.0) | Echocardiography |
| Electrocardiography | 59 | 869 (62.2) | 108 (30.4) | Electrocardiography |
| Heart catheter | 85 | 859 (61.5) | 111 (31.3) | Heart catheter |
| Laboratory tests | 71 | 870 (62.3) | 111 (31.3) | None |
| 6‐minute walk test | 23 | 888 (63.6) | 113 (31.8) | 6‐minute walk test |
| Magnetic resonance imaging | 165 | 847 (60.6) | 111 (31.3) | Magnetic resonance imaging |
| Spiroergometry | 46 | 881 (63.1) | 112 (31.5) | Spiroergometry |
| Disease‐specific quality of life questionnaire (Minnesota Living With Heart Failure Questionnaire) | 43 | 872 (62.4) | 112 (31.5) | Quality of life questionnaire |
| X‐ray thorax | 6 | 885 (63.4) | 111 (31.3) | None |
| Depression | 9 | 732 (52.4) | 97 (27.3) | Depression |
| Biomaterial | 53 | 712 (51.0) | None | Biomaterial handling |
| Vital status | 6 | 20 (1.4) | None | None |
eCRFs, electronic case report forms; TORCH, TranslatiOnal Registry for CardiomyopatHies.
Table 3.
Collected biomaterial aliquots in TORCH (as of 12.12.2016)
| Blood/urine | Material | TORCH set | DZHK basis set | Total |
|---|---|---|---|---|
| Blood | Serum | 10 937 | 10 519 | 21 456 |
| Blood | EDTA | 11 908 | 11 550 | 23 458 |
| Blood | Citrate | 4977 | 4894 | 9871 |
| Blood | Buffy coat | — | 2206 | 2206 |
| Urine | Urine | 8919 | 8891 | 17 810 |
| Total | — | 36 741 | 38 060 | 74 801 |
EDTA, ethylenediaminetetraacetic acid; DZHK, German Centre for Cardiovascular Research; TORCH, TranslatiOnal Registry for CardiomyopatHies.
Since first patient in regular intervals, newsletters were sent to the study centres to inform them about news and document updates. Up to now, a total of six newsletters were published including a frequently asked question area with a total of 30 pieces of advice regarding clinical definitions as well as advice on recruitment and the data capture in secuTrial.
Since the first quality controls by the data and quality centre Greifswald, data quality was improved, and several mistakes could be corrected (e.g. missing or implausible data, false units, or implausible dates). At this time all in all, 5109 queries were used to ask the study centre for checking or correction of the data capture for 771 patients including in quality control.
Discussion
A prospective national cardiomyopathy registry and standardized medical data are the basis of fundamental research and analyses of the pathogenesis, diagnostics, and translation in medical practice. By longitudinal follow‐up of patients, the role of genetic, epigenetic, and molecular biomarkers can be investigated in TORCH for a high sample size of 2300 cardiomyopathy patients.
In TORCH, data and biomaterial are collected in routine care; therefore, no further investigations have to be performed, and the high data benefit outweighs the comparably less effort of data collection. The biomaterial and corresponding medical data are available for many scientists and research issues. The Minnesota Living with Heart Failure Questionnaire7, 8 is collected additionally to routine care to allow relations to self‐report quality of life of cardiomyopathy patients.
The harmonization of the DZHK infrastructure and CDM is state of the art and is also used by the German Center for Diabetes Research9 and the German Center for Neurodegenerative Diseases. The DZHK uses all current standards and recommendations of the good epidemiological practice,10 first of all, standardized IT infrastructure and fulfilment of German data protection regulations.
In CDM, all aspects of requirements, such as data acquisition, standardized storage, integration of decentralized data capture, use and access, and data export for scientific analyses are combined and can be used sustainably.6, 11 Advantages of CDM are the shared usage of harmonized IT infrastructures, the data security, the possibility to check and improve data quality, and standardized data exports.11 The CDM infrastructure and DZHK‐wide harmonization and standardization enable a high data security. High data quality is the basis for good clinical and epidemiological research.5, 10
All in all, the approval for quality control for about 55% of the entered data is given by the recruiting study centres via the review right. For the other data, the documentation is not completed, and the quality control cannot be applied. With a regular, standardized, and systematic quality management, possible sources of error can be identified and further errors possibly avoided. A continuous control of data capture allows a timely feedback to (and correction by) the recruiting study centres to improve completeness and quality of the data.
In the next step, regular site visits will be scheduled by the data and quality centre, which include the inspection of the actual document versions, the comparison of source data with entered data, and the documentation of the decentralized stored biomaterial. The comparison of data will be performed by a monitor using standardized digital checklists, which are currently prepared in Cardiff TeleForm® (ElectricPaper, Lüneburg, Germany). Subsequently, the recruiting study centre will receive a detailed monitoring report by the monitor with timely and specific information to enhance the data quality of the registry.
The quality management based on completeness and plausibility checks has actively helped to improve data quality in the recruiting epidemiologic DZHK registry TORCH and will support the translation of research results into routine care.
Conflict of interest
None declared.
Funding
This work was supported by the German Centre for Cardiovascular Research (grant number 81X1400102) and the German Ministry of Education and Research.
Acknowledgement
We thank Erich Wichmann for comprehensive support for all ethical aspects.
Schwaneberg, T. , Weitmann, K. , Dösch, A. , Seyler, C. , Bahls, T. , Geidel, L. , Stahl, D. , Lee, M. , Kraus, M. , Katus, H. A. , and Hoffmann, W. (2017) Data privacy management and data quality monitoring in the German Centre for Cardiovascular Research's multicentre TranslatiOnal Registry for CardiomyopatHies (DZHK‐TORCH). ESC Heart Failure, 4: 440–447. doi: 10.1002/ehf2.12168.
[Correction added after online publication on 26 July 2017: affiliation addresses corrected].
References
- 1. Seyler C, Meder B, Weis T, Schwaneberg T, Weitmann K, Hoffmann W, Katus HA, Dösch A. TranslatiOnal Registry of CardiomyopatHies (TORCH) – rationale and first results. ESC Heart Failure 2017. https://doi.org/10.1002/ehf2.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Statistisches Bundesamt . Gesundheit – Diagnosedaten der Patienten und Patientinnen in Krankenhäusern (einschl. Sterbe‐ und Stundenfälle). 2012. 16.12.2013.
- 3. Pommerening K, Drepper J, Helbing K, Ganslandt T. Leitfaden zum Datenschutz in medizinischen Forschungsprojekten. Generische Lösungen der TMF 2.0: Medizinisch Wissenschaftliche Verlagsgesellschaft; 2014. [Google Scholar]
- 4. Havemann C, Bahls T, Bialke M, Hoffmann W, Quade M, Mauß T. Verfahrensbeschreibung und Datenschutzkonzept des Zentralen Datenmanagements des DZHK. 2014. 24.03.2014.
- 5. Bialke M, Penndorf P, Wegner T, Bahls T, Havemann C, Piegsa J, Hoffmann W. A workflow‐driven approach to integrate generic software modules in a Trusted Third Party. J Transl Med 2015; 13: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bialke M, Bahls T, Havemann C, Piegsa J, Weitmann K, Wegner T, Hoffmann W. MOSAIC–A Modular Approach to Data Management in Epidemiological Studies. Methods Inf Med 2015; 54: 364–371. [DOI] [PubMed] [Google Scholar]
- 7. Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure Questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993; 71: 1106–1107. [DOI] [PubMed] [Google Scholar]
- 8. Rector TS, Francis GS, Cohn JN. Patients' self‐assessment of their congestive heart failure. Part 1: patient‐perceived dysfunction and its poor correlation with maximal exercise tests. Heart Failure 1987; 3: 192–196. [Google Scholar]
- 9. Infrastrukturen DZD. DZD Deutsches Zentrum für Diabetesforschung; 2016. 1.
- 10. Hoffmann W, Latza U, Terschüren C. Leitlinien und Empfehlungen zur Sicherung von Guter Epidemiologischer Praxis (GEP) – überarbeitete Fassung nach Evaluation. Gesundheitswesen 2005; 67: 217–225. [DOI] [PubMed] [Google Scholar]
- 11. Meyer J, Ostrzinski S, Fredrich D, Havemann C, Krafczyk J, Hoffmann W. Efficient data management in a large‐scale epidemiology research project. Comput Methods Prog Biomed 2012; 107: 425–435. [DOI] [PubMed] [Google Scholar]


