Abstract
Aims
Cachexia is a severe complication of cancer that adversely affects the course of the disease and is associated with high rates of mortality. Patients with cancer manifest symptoms, such as fatigue, shortness of breath, and impaired exercise tolerance, which are clinical signs of chronic heart failure. The aim of this study was to evaluate cardiac muscle wasting in cancer individuals.
Methods and results
We retrospectively analysed 177 individuals who died of cancer, including 58 lung, 60 pancreatic, and 59 gastrointestinal (GI) cancers, and 42 cancer‐free controls who died of other, non‐cardiovascular reasons. Cancer cachexia (CC) was defined based on clinical and/or pathological diagnosis, body mass index (BMI) <20.0 kg/m2 and/or oedema‐free body weight loss of 5.0% during the previous year or less. The pathology reports were analysed for BMI, heart weight (HW), and left and right ventricular wall thicknesses (LVWT and RVWT, respectively). The analysis of clinical data included recording of biochemical parameters and medication data of study patients. CC was detected in 54 (30.5%) subjects. Individuals with CC had a significantly lower HW than non‐cachectic subjects (363.1 ± 86.2 vs. 447.0 ± 128.9 g, P < 0.001) and control group (412.9 ± 75.8 g, P < 0.05). BMI correlated with HW in cases with GI cancer (r = 0.44, P < 0.001), lung cancer (r = 0.53, P < 0.0001), and pancreatic cancer (r = 0.39, P < 0.01).
Conclusions
Body weight loss in individuals with lung, pancreatic, and GI cancers is accompanied by a decrease in HW. In patients with CC who receive cancer treatment, screening for cardiac muscle wasting may have clinical importance.
Keywords: Cancer cachexia, Weight loss, Heart weight, Cardiac function
Introduction
Cancer cachexia (CC) is a multifactorial paraneoplastic syndrome characterized by anorexia, body weight loss, and loss of adipose tissue and skeletal muscle.1 Its prevalence ranges from 50 to 80% in cancer patients,2 and remarkably about 20% of cancer‐related mortalities derive from cachexia rather than direct tumour burden.3 The progression of the disease varies between cancer types, with cachexia being more prevalent within pancreatic, colon, or non‐small‐cell lung malignancies.4 Patients with pancreatic or gastric cancer experience the highest frequency of weight loss, where patients can lose up to 30% of their pre‐illness weight.5 This wasting condition also lowers responsiveness to chemotherapy and radiotherapy and increases the risk of postoperative complications6 contributing to poor prognosis and a depreciating quality of life.7
The pathogenesis of CC includes anorexia, inflammation, metabolic disturbances, and enhanced muscle proteolysis.6 The loss of skeletal muscle mass results from a decrease in protein synthesis, an increase in protein degradation, or a combination of both.8 Muscle hypercatabolism depends on the activation of calcium‐dependent proteases, on calpains, and on the hyperactivation of the ATP‐ubiquitin‐dependent proteolytic pathways.9, 10 Muscle wasting is the most important phenotypic feature of CC and the principal cause of function impairment, fatigue, and respiratory complications.11 The loss of muscle tissue and fat mass leads to weight loss, which is strongly associated with poor outcomes from the earliest disease stages through to advanced cancer.12 Systemic inflammation also plays a significant role in cachexia‐associated wasting. Pro‐inflammatory cytokines, including interleukin (IL)‐1, IL‐6, and tumour necrosis factor alpha (TNF‐α) induce myofibrillar breakdown by activation of the ubiquitin proteasome pathway, via nuclear transcription factor kappa B (NF‐κB)‐dependent and NF‐κB‐independent mechanisms.13, 14, 15 Cytokine‐mediated release of cortisol and adrenergic hormones can also lead to increased fat oxidation and fat atrophy, insulin resistance, hypermetabolism, anaemia, and fatigue.13 In addition, tumour‐derived catabolic factors, such as lipid‐mobilizing factor and proteolysis‐inducing factor may play a role in the development of cancer anorexia and cachexia by acting directly on adipose tissue and skeletal muscle, without affecting food intake.16
The mechanisms of skeletal muscle wasting in CC have been extensively described in previous studies.5, 15 The theory on whether the process of weight loss is accompanied by a loss of cardiac muscle tissue has not been studied in detail previously. Therefore, the aim of the study was to assess whether weight loss in cancer patients is also accompanied by tissue loss of the heart muscle.
Methods
Study population
We retrospectively analysed 177 deceased cancer individuals who were treated and died from lung, pancreatic, and gastrointestinal (GI) tumours at Charité Medical School from 2002 to 2009. The individuals were randomly chosen from pathology reports at the Virchow Institute of Pathology of Charité Campus Mitte, between October 2009 and January 2010. Individuals who died of cancer but had also reported cardiovascular (CV) disease prior to developing cancer were excluded from this analysis. Forty‐two cases that had no medical history of CV disease and cancer were selected as the control group. The control patients did not have any chronic disease that could have caused the development of cachexia. The causes of death of control individuals were sepsis (only if death occurred within 3 days), multiorgan failure, cerebral coma, acute peritonitis, intracerebral haemorrhage, acute necrotizing pancreatitis, pulmonary artery thromboembolism, coagulopathy due to acute liver failure (acute necrotizing hepatitis), sun stroke, aspiration pneumonia, spleen abscess, and traumatic brain injury.
Assessment of pathology data
The pathology reports of cancer cases were examined for the type of cancer, tumour localization and classification, the presence of metastases, and body weight data. The body weight data were carefully recorded by physicians in medical reports of cancer patients upon their admission to different divisions and during treatment at the Charité University Hospital. The weight and height data were recorded upon death, and body mass index (BMI = weight/height2) was calculated. CC was defined based on clinical and/or pathological diagnosis, BMI < 20.0 kg/m2, and/or at least a 5.0% loss of oedema‐free body weight during the previous 12 months or less.17 Based on these criteria, all cancer cases were divided into cachectic and non‐cachectic groups. Cardiac dimensions, including heart weight (HW), relative HW, left ventricular wall thickness (LVWT), and right ventricular wall thickness (RVWT), were obtained. The relative HW was calculated by the formula HW/body weight × 100%. The presence of CV and other co‐morbidities was recorded from pathology reports of study cases.
Assessment of clinical data
The hospital online system of the Charité Medical School was used to obtain medical reports of individuals who were selected at the Virchow Institute of Pathology. Two control cases were excluded due to the previous history of cancer and systemic lupus erythematosus. The medical reports of eight control cases were not available in the hospital database. The body weight data, recorded upon admission to the hospital, laboratory parameters, and medication data were recruited from medical reports. The dynamics of body weight change were taken from medical reports to document weight loss.
Biochemical parameters
Standard laboratory data were recorded from medical reports to reveal the presence of biochemical abnormalities, including full blood count, plasma levels of protein and albumin, inflammatory markers, such as C‐reactive protein, liver enzymes, alanine aminotransferase, aspartate aminotransferase, as well as renal function indicators (creatinine and urea) −4 ± 1 weeks before the death of cancer cases and upon the last hospital admission for control subjects. It was impossible to obtain the data at equal time to death across all groups because the clinical data for control subjects were only available for the last hospital admission, from a week to several months before death. The missing biochemical parameters were not determined during laboratory tests.
Drug treatment
The analysis of medication data included the recording of cancer treatment and CV drug therapy of study individuals, which were completely available in hospital medical reports of study individuals. The treatment data were collected from medical reports −4 ± 1 weeks, −6 ± 1 months, and −12 ± 1 months before death of cancer subjects to evaluate the course of treatment over a year. The treatment received by cancer patients included chemotherapy, radiation treatment, and combined radiochemotherapy. The CV drugs received by all study individuals were also recorded, including angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, Ca‐channel blockers, diuretics, and cardiac glycosides. The CV therapy data for control subjects were collected upon last hospital admission.
Statistical analysis
Numeric values were presented as mean ± standard deviation. Unpaired Student's t‐test, ANOVA with Fisher's post hoc test, and χ2 test were used as appropriate. The Kolmogorov–Smirnov test assessed normal distribution. Normally distributed data were analysed by one‐way ANOVA followed by Tukey's test, while data without normal distribution by Kruskal–Wallis test. The correlation analysis was performed by Pearson's two‐tailed correlation method. A P value ≤0.05 was considered statistically significant. Standard statistical software packages, SPSS 16.0 and StatView 5.0 (SAS Institute, Cary, NC) were used to perform statistical analysis.
Results
We studied 58 lung cancer, 60 pancreatic cancer, 59 GI cancer, and 42 control subjects. The study included 135 male (61.6%) and 84 female cases. The age of all individuals ranged from 21 to 95 years (mean: 62.9 ± 12.4 years). Cases were subdivided according to whether or not CC was present, and a total of 54 (30.5%) subjects met these criteria. Individuals with CC were predominately men and were of similar age as non‐cachectic subjects (P = 0.74, Table 2). Baseline characteristics of study cases are shown in Tables 1 and 2.
Table 1.
Pathology data of cancer individuals for three cancer groups and controls at baseline
| Control (n = 42) | Lung cancer (n 1 = 58) | Pancreatic cancer (n2 = 60) | GI cancer (n 3 = 59) | ||||
|---|---|---|---|---|---|---|---|
| Cachectic (n = 20) | Non‐cachectic (n = 38) | Cachectic (n = 16) | Non‐cachectic (n = 44) | Cachectic (n = 18) | Non‐cachectic (n = 41) | ||
| Age (years) | 56.2 ± 16.0 | 65.0 ± 11.2* | 63.7 ± 9.8* | 67.2 ± 11.1* | 64.1 ± 8.9* | 62.0 ± 10.0 | 68.9 ± 10.1*** |
| Sex (M/W) | 24/18 | 16/4 | 24/14 | 10/6 | 25/19 | 10/8 | 26/15 |
| Height (m) | 1.71 ± 0.13 | 1.72 ± 0.08 | 1.72 ± 0.10 | 1.74 ± 0.09 | 1.70 ± 0.08 | 1.71 ± 0.10 | 1.73 ± 0.12 |
| Weight (kg) | 84.52 ± 21.6 | 64.48 ± 11.12***, # | 78.39 ± 15.7 | 64.91 ± 18.6**, ## | 82.16 ± 11.2 | 63.62 ± 14.05** | 86.84 ± 18.1### |
| BMI (kg/m2) | 28.47 ± 5.91 | 21.94 ± 3.93***, ## | 26.37 ± 4.25 | 21.73 ± 4.8***, ### | 28.47 ± 4.21 | 21.7 ± 3.7***, ### | 29.3 ± 5.94 |
| HW (g) | 412.9 ± 75.8 | 376.25 ± 92.3# | 451.03 ± 124.6 | 361.06 ± 75.5 | 405.8 ± 101.7 | 350.3 ± 90.6### | 486.6 ± 146.8* |
| Relative HW (%) | 0.50 ± 0.11 | 0.59 ± 0.14 | 0.58 ± 0.16 | 0.55 ± 0.19 | 0.51 ± 0.10 | 0.56 ± 0.13 | 0.57 ± 0.16 |
| LV WT (mm) | 13.85 ± 1.84 | 14.05 ± 2.78 | 14.58 ± 2.50 | 15.00 ± 2.48 | 14.63 ± 2.35 | 13.61 ± 3.15 | 15.2 ± 2.94* |
| RV WT (mm) | 4.54 ± 0.95 | 4.58 ± 1.30 | 4.84 ± 1.65 | 4.3 ± 1.1 | 4.7 ± 1.2 | 4.17 ± 1.29*, a | 4.73 ± 1.1 |
BMI, body mass index; GI, gastrointestinal; HW, heart weight; LV WT, left ventricular wall thickness; RV WT, right ventricular wall thickness.
Kruskal–Wallis test GI cancer vs. control groups.
P < 0.05,
P < 0.01,
P < 0.001 vs. control group;
P < 0.05,
P < 0.01,
P < 0.001 cachectic vs. non‐cachectic groups.
Table 2.
Baseline biochemical data of cachectic, non‐cachectic, and control cases
| Control cases (n = 32) | Cachectic cases (n = 54) | Non‐cachectic cases (n = 123) | |
|---|---|---|---|
| Age (years) | 56.3 ± 15.8 | 64.7 ± 10.8** | 63.9 ± 16.1** |
| Sex (M/W) | 24/18 | 36/18 | 75/48 |
| BMI (kg/m2) | 28.41 ± 5.86 | 21.81 ± 4.03*** | 27.82 ± 5.60††† |
| Sodium (mmol/L) | 138.17 ± 6.58 (28) | 134.12 ± 5.08 (40)* | 136.21 ± 5.36 (61) |
| Potassium (mmol/L) | 4.13 ± 0.65 (29) | 3.99 ± 0.57 (40) | 4.1 ± 0.62 (63) |
| Creatinine (mg/dL) | 1.52 ± 1.15 (31) | 1.04 ± 0.97 (44)## | 1.18 ± 0.90 (78) |
| Urea (mg/dL) | 72.32 ± 53.11 (31) | 52.6 ± 31.6 (34) | 65.6 ± 58.87 (61) |
| Uric acid (mg/dL) | 5.56 ± 4.81 (8) | 4.57 ± 3.58 (13) | 6.41 ± 3.06 (15) |
| Bilirubin total (mg/dL) | 2.43 ± 5.86 (24) | 3.62 ± 5.18 (24) | 2.57 ± 3.89 (55) |
| Albumin (g/dL) | 2.38 ± 0.82 (22) | 2.95 ± 0.62 (22)* | 3.14 ± 0.67 (34)*** |
| Protein (g/dL) | 4.93 ± 1.46 (24) | 6.78 ± 1.03 (20)*** | 6.08 ± 1.27 (40)** |
| C‐reactive protein (mg/L) | 12.72 ± 12.05 (29) | 10.08 ± 7.35 (34) | 8.35 ± 7.34 (61) |
| Alanine transaminase (U/L) | 408.36 ± 1295.23 (28) | 41.37 ± 37.7 (38) | 106.79 ± 334.6 (61) |
| Aspartate transaminase (U/L) | 770.62 ± 2488.11 (26) | 72.7 ± 95.79 (40) | 96.82 ± 130.78 (62) |
| Thyroid‐stimulating hormone (mU/L) | 1.23 ± 1.20 (20) | 1.59 ± 1.39 (16) | 1.19 ± 1.18 (27) |
| Haemoglobin (g/dL) | 10.78 ± 2.60 (32) | 10.73 ± 1.97 (45) | 11.05 ± 1.90 (76) |
| Haematocrit (L/L) | 0.33 ± 0.071 (32) | 0.32 ± 0.06 (45) | 0.34 ± 0.052 (76) |
| Erythrocytes (pL−1) | 3.58 ± 0.71 (32) | 3.64 ± 0.69 (45) | 3.76 ± 0.63 (76) |
| Leucocytes (nL−1) | 14.62 ± 13.99 (32) | 11.46 ± 6.12 (45) | 11.07 ± 5.44 (76) |
| Thrombocytes (nL−1) | 166.78 ± 109.59 (32) | 297.84 ± 176.1 (45)** | 258.18 ± 151.12 (76)* |
| Red blood cell distribution width (%) | 15.66 ± 3.50 (31) | 15.94 ± 2.35 (20)# | 17.1 ± 2.98 (29) |
| Gamma‐glutamyl transferase (U/L) | 132.96 ± 200.00 (24) | 216.8 ± 251.09 (18) | 324.4 ± 239.91 (18)* |
P values refer to ANOVA between three groups. All data are presented as mean ± SD.
P < 0.05,
P < 0.01,
P < 0.001 vs. controls, rest = non‐significant;
P < 0.05,
P < 0.01 between cachectic, non‐cachectic, and control groups (Kruskal–Wallis test);
P < 0.001 cachectic vs. non‐cachectic groups.
From all 177 cancer individuals, in only 72 (40.7%) patients were premorbid weight data obtained, whereas in 45 (25.4%), 28 (15.8%), 13 (7.3%), and 11 (6.2%) patients, two, three, four, and five body weight measurements were recorded, respectively. The body weight data were available for a few weeks to a year before death for the majority of cancer patients and a few years before death for some cancer patients.
The BMI values were significantly lower in cachectic individuals than in non‐cachectic subjects for three cancer types (all P < 0.01), as well as compared with the control group (all P < 0.001, Table 1). The HW of cachectic cases with lung and GI cancers were significantly lower compared with that of non‐cachectic subjects (both P < 0.05, Table 1). The HW of control group cases was significantly lower than that of the GI non‐cachectic group (P < 0.05, Table 1). The LVWT was higher in GI non‐cachectic individuals than in the control group (P < 0.05), whereas the RVWT was lower in GI cachectic group than in the control subjects (P < 0.05, Table 1). The relative HW, LVWT, and RVWT did not differ between cachectic and non‐cachectic cases for each cancer type (Table 1).
The cachectic, non‐cachectic, and control groups contained a higher proportion of men than women. The cachectic group contained the same number of women as the control group (n = 18) but less than the non‐cachectic group (n = 48). The cachectic group had a twice higher proportion of men than women (36/18) (see Supporting Information, Table S1 ).
The HW of cachectic individuals was lower than that of non‐cachectic (363.1 ± 86.2 vs. 447.0 ± 128.9 g, P < 0.001; Figure 1 A) and control groups (vs. 412.9 ± 75.8 g, P < 0.05; Figure 1 A). The relative HW was higher in cases with cachexia than in control subjects (0.57 ± 0.15 vs. 0.50 ± 0.11%, P < 0.05; Figure 1 B). The LVWT was higher in non‐cachectic group than in controls (14.8 ± 2.6 vs. 13.9 ± 1.8 mm, P < 0.05; Figure 1 C). No difference was detected in terms of LVWT and RVWT between cachectic and non‐cachectic groups.
Figure 1.
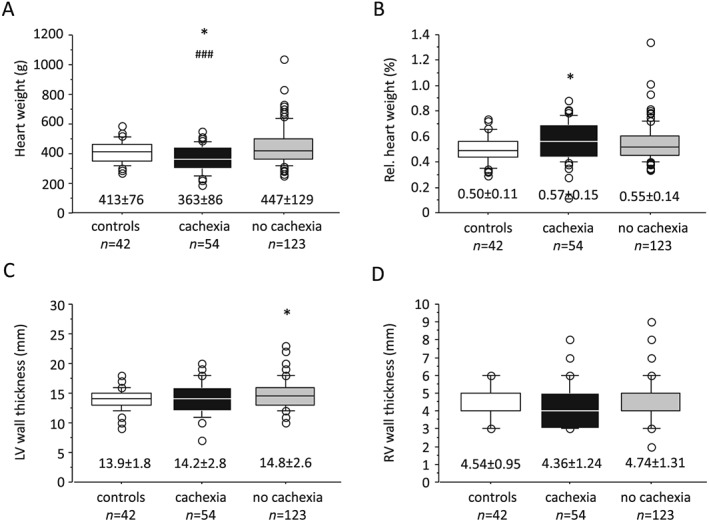
Distribution of (A) heart weight, (B) relative (Rel.) heart weight, (C) left ventricular (LV) wall thickness, and (D) right ventricular (RV) wall thickness in cachectic and non‐cachectic individuals vs. controls.
Correlation analyses
In all study cases, we detected a weak and significant correlation between BMI and HW for cachectic (r = 0.36, P < 0.01), non‐cachectic (r = 0.33, P < 0.001), and control groups (r = 0.40, P < 0.05) (Figure 2 A–C). The linear regression analysis for each cancer type revealed intermediate and significant correlations between BMI and HW for individuals with GI cancer (r = 0.44, P < 0.001), lung cancer (r = 0.53, P < 0.0001), and pancreatic cancer (r = 0.39, P < 0.01) (Figure 3 A–C). The BMI positively correlated with LVWT and RVWT (r = 0.32, P < 0.05 and r = 0.34, P < 0.05, respectively) in cases with GI cancer. A similar correlation was observed between BMI and LVWT in individuals with lung cancer (r = 0.29, P < 0.05) (see Table S1 ). No significant correlation was observed between BMI and RVWT in cases with lung cancer, as well as LVWT and RVWT in cases with pancreatic cancer. The association of BMI with relative HW was also weak and insignificant for three cancer types (see Table S2 ).
Figure 2.
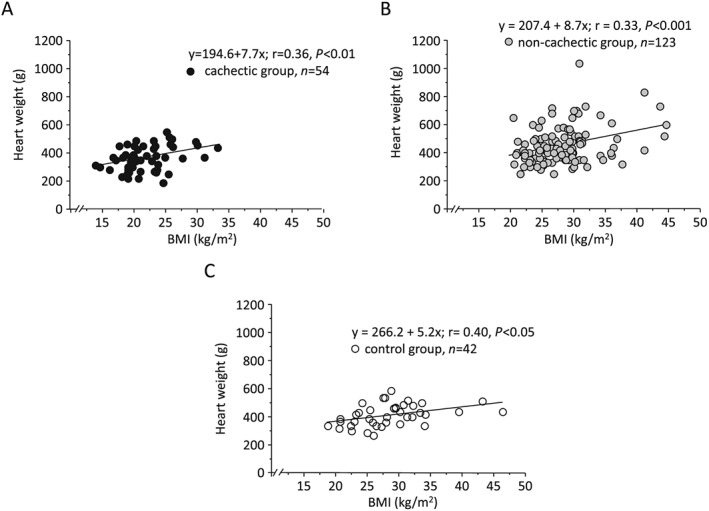
Simple regression analysis of body mass index (BMI) and heart weight for (A) cancer cachectic, (B) non‐cachectic, and (C) control cases.
Figure 3.
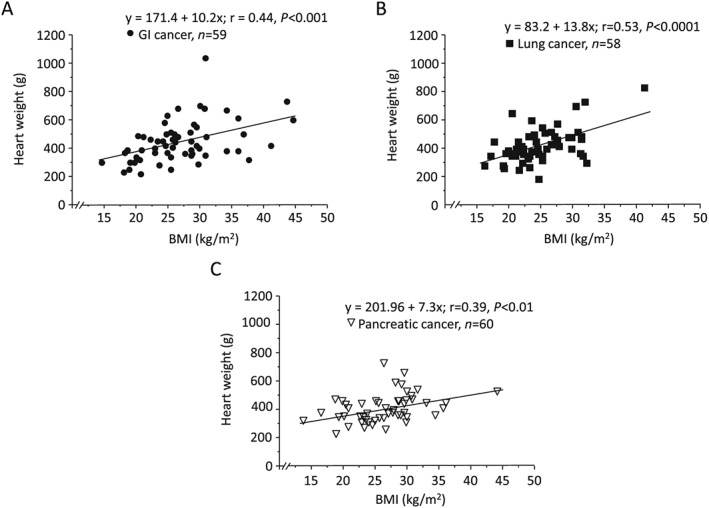
Simple regression analysis of body mass index (BMI) and heart weight for individuals with (A) gastrointestinal (GI) cancer, (B) lung cancer, and (C) pancreatic cancer.
Clinical characteristics of study cohorts
Baseline biochemical parameters for 177 cancer and 32 control cases are shown in Table 2. We have observed abnormal laboratory data characterized by increased C‐reactive protein levels (>5.0 mg/L), anaemia (haemoglobin < 12 g/dL), and hypoalbuminaemia (<3.2 g/dL) in cachectic and non‐cachectic subjects, but these parameters did not differ significantly between the two groups (Table 2).
Cardiovascular medication
The medication data of all study cases is listed in Table S3 .
We detected significant differences between all cancer and control subjects with regard to received CV drugs. In particular, the treatment with beta‐blockers (26.6 vs. 53.1%, P < 0.01), Ca‐channel blockers (10.7 vs. 28.1%, P < 0.01), diuretics (32.2 vs. 81.2%, P < 0.0001), glycosides (7.9 vs. 21.9%, P < 0.05), and glucocorticosteroids (26.6 vs. 78.1%, P < 0.0001) was significantly different between the two groups.
The cachectic and non‐cachectic cases differed from the control group with regard to treatment with beta‐blockers (both P < 0.05), Ca‐channel blockers (P < 0.05), diuretics (P < 0.0001), glucocorticosteroids (P < 0.0001), and α1‐receptor antagonist (P < 0.05). The treatment with glycosides and non‐steroidal anti‐inflammatory drugs was significantly different between the cachectic and control groups (both P < 0.01). No difference was detected between these groups with regard to individuals' CV medication (see Table S3 ).
From all 177 cancer individuals, coronary artery disease was detected in 65 (36.7%), arterial hypertension in 18 (10.2%), heart failure (HF) in 36 (20.3%), pulmonary artery thromboembolism in 20 (11.3%), myocardial infarction in 16 (9.03%), and infective endocarditis in 5 (2.82%) cases.
Cancer treatment
The chemotherapy and radiation treatment of cancer cases is summarized in Table 3 and in Table S4 .
Table 3.
The distribution of anticancer treatment of study cases
| Cancer treatment | All cancer cases (n = 177) | Cachectic cases (n = 54) | Non‐cachectic cases (n = 123) | P valuea |
|---|---|---|---|---|
| Chemotherapy, n (%) | 96 (54.2) | 44 (81.5) | 52 (42.3) | 0.000001 |
| Radiotherapy, n (%) | 39 (22.0) | 18 (33.3) | 21 (17.1) | <0.05 |
| Radiochemotherapy, n (%) | 32 (18.1) | 16 (29.6) | 16 (13.0) | <0.01 |
χ2 P values between cachectic and non‐cachectic groups.
The number of cachectic individuals was significantly higher compared with non‐cachectic subjects with regard to overall chemotherapy (81.5 vs. 42.3%, P < 0.0001), radiotherapy (33.3 vs. 17.1%; P < 0.05), as well as combined radiochemotherapy treatment (29.6 vs. 13.0%; P < 0.01, Table 3).
The number of cachectic cases who received chemotherapy was prevalent over non‐cachectic subjects for each drug class, in particular with regard to treatment with topoisomerase inhibitors (20.4 vs. 7.3%, P < 0.05), nucleoside metabolic inhibitors (44.4 vs. 21.9%, P < 0.01), platinum‐based drugs (55.6 vs. 14.6%, P < 0.0001), taxanes (14.8 vs. 3.3%, P < 0.01), anthracycline topoisomerase inhibitors (9.3 vs. 2.4%, P < 0.05), and somatostatin analogue (5.6 vs. 0.8%, P = 0.05) (see Table S4 ).
Discussion
We have shown in this retrospective study that the reduction of HW was observed in individuals who developed CC independent of cancer type. The HW of cachectic patients with lung, pancreatic, and GI cancers was lower compared to that of non‐cachectic and control subjects. The same was true for all cachectic cases where the HW was significantly reduced compared with non‐cachectic and control groups. The number of men was prevalent in cachectic and non‐cachectic groups. Therefore, the lower HWs in the cachectic group were not due to female sex but were the result of wasting in cachexia.
The progressive decrease in body weight of cancer individuals was accompanied by wasting of cardiac muscle. This was represented by a weak and significant correlation between BMI and HW of cachectic and non‐cachectic subjects. The association of BMI with HW was stronger and significant for cases with lung, pancreatic, and GI cancers. Moreover, we showed a correlation of BMI with LVWT and RVWT in individuals with GI cancer and with LVWT in cases with lung cancer.
The diagnosis of patients with CC was primarily made at the beginning stages of the disease when these patients could not have managed to develop a substantial weight loss. In contrast, the majority of non‐cachectic patients (63.4%) was diagnosed at the later stages of the disease (from 1 to 6 months before death), and/or they died early after the original manifestation of the disease. In case of late diagnosis, these patients could have supposedly developed weight loss prior to hospitalization. However, the body weight data before admission to the hospital were not available, so it was impossible to get an idea about the dynamics of previous weight loss. Although the diagnosis of cancer was made late in most non‐cachectic patients, the decrease in body weight after hospitalization until death was not significant enough (<5.0%) so that these patients could be considered cachectic. One of the reasons could be that they died early due to complications arising from cancer. The earliest possible body weight data were recorded from medical reports to document weight loss in cancer patients during hospital treatment.
The analysis of laboratory data of cancer individuals revealed increased C‐reactive protein levels, anaemia, and hypoalbuminaemia, which corresponded to the clinical definition of cachexia. However, the laboratory parameters were not significantly different between cachectic and non‐cachectic groups.
The hypothesis on whether cardiac wasting develops in cancer patients is still controversial. In support of this theory speaks the evidence that patients with CC manifest symptoms, such as fatigue, shortness of breath, and impaired exercise capacity, that are typical clinical signs of chronic HF.18 This hypothesis was originally tested in a number of animal studies. In previous studies by Springer et al.,18, 19, 20 cardiac function was assessed in the AH‐130 hepatoma rat model of CC. The loss of body weight in tumour‐bearing rats was accompanied by a reduction in absolute HW, left ventricular (LV) mass and cardiac contractility, as assessed by LV ejection fraction (LVEF) and LV fractional shortening.18, 20 The authors found progressive fibrosis in the hearts of tumour‐bearing rats. They showed that the loss of LV mass was higher (>50%) than the loss of lean mass (~35%), indicating that the heart is more susceptible to catabolic stimuli than skeletal muscle.20 They observed a reduction in HW, LVWT, and cardiac and perivascular fibrosis in patients who died of cancer irrespective of cachexia. Hence, the process of cardiac remodelling seems somewhat similar in a rat model and cancer patients who died as a result of CC.
In studies by Tian et al.,21, 22 the effect of CC on heart function and cardiac muscle structure was investigated in male CD2F1 mice inoculated with colon‐26 adenocarcinoma cells. Heart function as measured by fractional shortening in vivo using transthoracic echocardiography, heart rate, and cardiac wall thickness were significantly reduced compared to those of control mice. The authors also found cardiac fibrosis in tumour‐bearing mice and disrupted myocardial structure as revealed by transmission electron microscopy. Cardiac atrophy in mice with CC was manifested by a decreased amount of cardiac myofibrillar proteins, myosin heavy chain (MHC), and troponin I; increased protein ubiquitination; and alteration in the composition of protein levels of MHC as revealed by a decrease in MHCα (adult isoform) and increase in MHCβ (foetal isoform), which is known to be associated with HF. Tian et al.21 observed a gene expression pattern for cardiac remodelling in cachectic mice, including increased brain natriuretic peptide and c‐Fos and decreased peroxisome proliferator‐activated receptor alpha and its responsive gene carnitine palmitoyltransferase 1 beta.
In a similar study by Xu et al., the expression of biomarkers of protein degradation was increased in the hearts of female CD2F1 mice with colon‐26 tumour, which caused systolic dysfunction and reduction in diastolic posterior wall thickness as assessed by echocardiography.23 The heart muscle was affected by tumour growth, and cardiomyocyte function was impaired during cellular contraction and relaxation. Cramer et al.24 reported that the determinants of CV function were impaired in colorectal cancer patients independent of chemotherapy, as assessed by a reduction in exercise capacity, LVEF, lean mass, and heart rate variability compared with the control group.
It has been postulated that CC leads to cardiac atrophy and HF, which by itself can result in cardiac cachexia contributing to the severity of the disease.25 The presence of co‐morbidities and chemotherapy treatment are considered important factors that can contribute to myocardial dysfunction in cachectic patients. Cardiotoxic chemotherapy may additionally result in cardiac dysfunction and HF in some cancer patients.25 In this case, the impairment of cardiac function results from both cachexia and cardiotoxicity induced by chemotherapy. Radiation therapy, which is also frequently used in the treatment of cancer, has cardiotoxic effects and can potentially compound the cardiotoxicity of chemotherapeutic agents.26
The clinical manifestations of cardiotoxicity vary depending on the type of chemotherapeutic drug used. Congestive HF and LV dysfunction are associated with use of anthracyclines, a cumulative‐dose reaction, in those with previous cardiac diseases and after mediastinal irradiation.27 Cardiotoxicity has also been reported after 5‐fluorouracil administration,28 which may induce myocardial ischaemia and electrocardiogram alteration of the repolarization phase.29 Antimicrotubule molecules, such as vinca alkaloids or taxanes, may produce cardiac HF, rhythm and conduction disturbances, and ischaemia.27 However, the most characteristic side effect of chronic cardiotoxicity is asymptomatic systolic and/or diastolic LV dysfunction resulting in severe congestive cardiomyopathy that may eventually lead to death.30
In our study, the majority of patients with CC had increased length of survival after cancer diagnosis and managed to develop cachexia as a result of malignancy. As expected, more cachectic patients received chemotherapy and radiation treatment compared with the non‐cachectic group. However, in some of these patients, cachexia developed faster due to the rapid progression of the disease and a significant weight loss over a relatively short time interval. This category of patients did not manage to receive a long‐term cancer treatment. It is reasonable to assume that survival in cachectic patients had increased due to a systematic treatment over at least a year, which could have affected cardiac function. In contrast, non‐cachectic patients received therapy for a shorter time, and more than half of them (52.03%) did not receive treatment at all, mostly due to the late diagnosis of cancer (70.3%), and died earlier before developing CC. Therefore, the side effects of treatment and tumour on cardiac function could not be so profound.
The cancer patients were mostly hospitalized for receiving cycles of chemotherapy and/or radiation treatment for cancer. In between cancer treatment, the minority of them was hospitalized in other clinical divisions due to decompensation of accompanying conditions, such as CV diseases. Acute illnesses were rare in cancer patients, and the loss of body weight caused by them was not significant enough so that it could have affected the extent of weight loss caused by cancer in these patients.
Since cachectic patients have been described as more susceptible to anticancer agent‐induced toxicity,31 this could have resulted in a more extensive cardiotoxic damage of the heart in addition to cachexia‐induced myocardial dysfunction. In our study, this was revealed by more pronounced cardiac wasting and reduction in ventricular wall thickness in cachectic individuals compared with non‐cachectic and control subjects. Unfortunately, diagnostic tests, such as echocardiography and magnetic resonance imaging, were not available for cancer cases, which could have provided information about the dynamics of cardiac structural and functional alterations over the course of treatment.
The cancer individuals were of old age (>60 years) and had minor CV co‐morbidities, which could have additionally contributed to the impairment of myocardial function in cancer patients. It has been reported that CV risk factors, as well as pre‐existing HF, can strengthen cardiac susceptibility against cachexia and increase the rate of cardiac cachexia.25 We observed significant differences between cancer cases and healthy controls with regard to treatment with CV drugs. The percentage of medications received by control patients was significantly higher compared with those received by cachectic and non‐cachectic subjects. This was due to the fact that most of the control patients received symptomatic treatment with CV drugs and steroids during the last hospital treatment about a month before death, which could not have a profound effect on cardiac function. On the contrary, cancer patients received a long‐term drug therapy for accompanying heart disease, which could have also protected cardiac damage due to developing CC. The cachectic and non‐cachectic individuals did not differ with regard to treatment with CV medication.
The findings of the present study suggest that CC is associated with cardiac muscle wasting, which can contribute to disease symptoms, compromise the clinical status, and increase the mortality of patients. This creates a necessity for a continuous assessment of cardiac function in cancer patients, particularly those with various co‐morbidities and risk factors whose quality of life could be significantly compromised if their heart condition remained untreated.25 However, considering a large variation in normal values for cardiac function and size in cancer patients, it will be difficult to identify changes in these parameters during chemotherapy. In certain clinical situations, the decrease in cardiac size is not always associated with the development of myocardial dysfunction. This phenomenon was described in a study that included doxorubicin‐treated childhood survivors who developed restrictive cardiomyopathy more than 15 years after exposure to cancer treatment. Although LVEF was normal, LV mass and cavity size have decreased, leading to the development of a ‘Grinch syndrome’ in these patients.32
The results of previous animal studies have shown that several therapies, including NF‐κB inhibitors, activin receptor antagonists, and β2‐adrenoceptor agonists, have been effective in attenuating cardiac cachexia in preclinical cancer models.33, 34, 35 In particular, medications used in the treatment of HF, such as spironolactone, bisoprolol, and simvastatin, reduced the wasting of skeletal muscle and LV mass, attenuated cardiac dysfunction, and myocardial fibrosis, as well as improved survival in animals with CC.20, 36 The beneficial effects of exercise training for treating skeletal and cardiac muscle cachexia in cancer still need to be resolved.37 It can be assumed that a multimodal approach, including nutritional support, pharmacological intervention, and exercise training, will lead to the best therapeutic outcomes.37, 38 Future clinical investigations should be directed to the study of the efficacy of these interventions in preserving cardiac function in a human model of CC and evaluation of clinical relevance of cardiac structural and functional alterations in the prognosis of cancer.
Conclusions
The results of the study have shown that body weight loss in individuals with lung, pancreatic, and GI cancers is accompanied by wasting of cardiac muscle. Literature data show that cardiac dysfunction in preclinical cancer models is associated with myocardial fibrosis, atrophy, and altered ultrastructure. Patients with CC should undergo diagnostic screening for cardiac muscle wasting while receiving chemotherapy and radiation treatment. Further research needs to discover novel treatment options to prevent cardiac cachexia, improve quality of life, and enhance survival for patients with cancer.
Conflict of interest
None declared.
Funding
German Academic Exchange Service (DAAD) A/08/76145.
Supporting information
Table S1. Pathology data of cachectic, non‐cachectic, and control male and female subgroups at baseline.
Table S2. Simple regression analysis displaying the correlation of BMI with cardiac parameters according to cancer type.
Table S3. The cardiovascular medication data of cancer and control group cases.
Table S4. The chemotherapy data of study patients received during the course of cancer treatment.
Barkhudaryan, A. , Scherbakov, N. , Springer, J. , and Doehner, W. (2017) Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Failure, 4: 458–467. doi: 10.1002/ehf2.12184.
References
- 1. Bossola M, Pacelli F, Tortorelli A, Doglietto GB. Cancer cachexia: it's time for more clinical trials. Annals of Surgical Oncology 2006; 14: 276–285. [DOI] [PubMed] [Google Scholar]
- 2. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014; 14: 754–762. [DOI] [PubMed] [Google Scholar]
- 3. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002; 2: 862–871. [DOI] [PubMed] [Google Scholar]
- 4. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495. [DOI] [PubMed] [Google Scholar]
- 5. Gould DW, Lahart I, Carmichael AR, Koutedakis Y, Metsios GS. Cancer cachexia prevention via physical exercise: molecular mechanisms. J Cachexia Sarcopenia Muscle 2013; 4: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy KT, Lynch GS. Update on emerging drugs for cancer cachexia. Expert Opin Emerg Drugs 2009; 14: 619–632. [DOI] [PubMed] [Google Scholar]
- 7. Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res 2007; 13: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 8. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009; 89: 381–410. [DOI] [PubMed] [Google Scholar]
- 9. Costelli P, Baccino FM. Mechanisms of skeletal muscle depletion in wasting syndromes: role of ATP‐ubiquitin dependent proteolysis. Curr Opin Clin Nutr Metab Care 2003; 6: 407–412. [DOI] [PubMed] [Google Scholar]
- 10. Costelli P, De Tullio R, Baccino FM, Melloni E. Activation of Ca2+‐dependent proteolysis in skeletal muscle and heart in cancer cachexia. Br J Cancer 2001; 84: 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bossola M, Pacelli F, Doglietto GB. Novel treatments for cancer cachexia. Expert Opin Investig Drugs 2007; 16: 1241–1253. [DOI] [PubMed] [Google Scholar]
- 12. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez‐Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med 2000; 160: 861–868. [DOI] [PubMed] [Google Scholar]
- 13. Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options—a mini‐review. Gerontology 2014; 60: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hinch EC, Sullivan‐Gunn MJ, Vaughan VC, McGlynn MA, Lewandowski PA. Disruption of pro‐oxidant and antioxidant systems with elevated expression of the ubiquitin proteosome system in the cachectic heart muscle of nude mice. J Cachexia Sarcopenia Muscle 2013; 4: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palus S, von Haehling S, Springer J. Muscle wasting: an overview of recent developments in basic research. J Cachexia Sarcopenia Muscle 2014; 5: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mantovani G. The current management of cancer cachexia In Mantovani G, Anker SD, Inui A, Morley JE, Fanelli FR, Scevola D, Schuster MW, Yeh SS, editors. Cachexia and wasting: a modern approach. Milan: Springer; 2006. p. 563–579. [Google Scholar]
- 17. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 18. Springer J, Tschirner A, Grzesiak A, Kaschina E, Von Haehling S, Anker S. Cancer cachexia therapy: a matter of helping the heart? J Cardiac Fail 2010; 16:Suppl: S12. [Google Scholar]
- 19. Springer J, Palus S, Rauchhaus M, Anker S. Experimental cancer cachexia severely impairs heart function. J Cardiac Fail 2008; 14:Suppl: S18. [Google Scholar]
- 20. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Pötsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argiles JM, Thum T, Földes G, Doehner W, Hilfiker‐Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014; 35: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian M, Asp ML, Nishijima Y, Belury MA. Evidence for cardiac atrophic remodeling in cancer‐induced cachexia in mice. Int J Oncol 2011; 39: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 22. Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer‐induced cachexia in mice. Int J Oncol 2010; 37: 347–353. [DOI] [PubMed] [Google Scholar]
- 23. Xu H, Crawford D, Hutchinson KR, Youtz DJ, Lucchesi PA, Velten M, McCarthy DO, Wold LE. Myocardial dysfunction in an animal model of cancer cachexia. Life Sci 2011; 88: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T, Scharnagl H, Riess H, Anker SD, von Haehling S. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol 2014; 64: 1310–1319. [DOI] [PubMed] [Google Scholar]
- 25. Kazemi‐Bajestani SM, Becher H, Fassbender K, Chu Q, Baracos VE. Concurrent evolution of cancer cachexia and heart failure: bilateral effects exist. J Cachexia Sarcopenia Muscle 2014; 5: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan TC, Scherrer‐Crosbie M. Cardiac complications of chemotherapy: role of imaging. Curr Treat Options Cardiovasc Med 2014; 16: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis and management. Circulation 2004; 109: 3122–3131. [DOI] [PubMed] [Google Scholar]
- 28. Balloni L, Porta C, Rossi S, Gola A, Pugliese P, Ferrari S, Bovio A, Danova M, Riccardi A. Left ventricular function in colon cancer patients receiving adjuvant fluoro‐folate chemotherapy: an echocardiographic study. Oncol Rep 2000; 7: 887–890. [DOI] [PubMed] [Google Scholar]
- 29. Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio‐oncology and cardio‐oncological prevention. J Natl Cancer Inst 2010; 102: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimos AK, Stougiannos PN, Trikas AG. “First, do no harm”: chemotherapy or healthy heart? Hellenic J Cardiol 2012; 53: 127–136. [PubMed] [Google Scholar]
- 31. Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose‐limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010; 21: 1594–1598. [DOI] [PubMed] [Google Scholar]
- 32. Lipshultz SE, Scully RE, Stevenson KE, Franco VI, Neuberg DS, Colan SD, Silverman LB, Moslehi JJ, Cheng S, Sallan SE. Hearts too small for body size after doxorubicin for childhood ALL: Grinch syndrome. J Clin Oncol 2014; 32: 10021 [abstract]. [Google Scholar]
- 33. Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010; 142: 531–543. [DOI] [PubMed] [Google Scholar]
- 34. Wysong A, Couch M, Shadfar S, Li L, Rodriguez JE, Asher S, Yin X, Gore M, Baldwin A, Patterson C, Willis MS. NF‐kB inhibition protects against tumor‐induced cardiac atrophy in vivo. Am J Pathol 2011; 178: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toledo M, Springer J, Busquets S, Tschirner A, Lopez‐Soriano FJ, Anker SD, Argilés JM. Formoterol in the treatment of experimental cancer cachexia: effects on heart function. J Cachexia Sarcopenia Muscle 2014; 5: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palus S, von Haehling S, Flach VC, Tschirner A, Doehner W, Anker SD, Springer J. Simvastatin reduces wasting and improves cardiac function as well as outcome in experimental cancer cachexia. Int J Cardiol 2013; 168: 3412–3418. [DOI] [PubMed] [Google Scholar]
- 37. Murphy KT. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am J Physiol Heart Circ Physiol 2016; 310: H466–H477. [DOI] [PubMed] [Google Scholar]
- 38. Argilés JM, Busquets S, Lopez‐Soriano FJ, Costelli P, Penna F. Are there any benefits of exercise training in cancer cachexia? J Cachexia Sarcopenia Muscle 2012; 3: 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pathology data of cachectic, non‐cachectic, and control male and female subgroups at baseline.
Table S2. Simple regression analysis displaying the correlation of BMI with cardiac parameters according to cancer type.
Table S3. The cardiovascular medication data of cancer and control group cases.
Table S4. The chemotherapy data of study patients received during the course of cancer treatment.


