Abstract
Aims
Renal dysfunction (RD) is associated with increased morbidity and mortality in heart failure (HF). At present, no specific treatment for patients with RD, to prevent progression of HF, has been developed. How different hormone axes—and thereby potential treatment options—are affected by RD in HF warrants further investigations.
Methods and results
Patients with left ventricular ejection fraction (LVEF) <0.45% were enrolled prospectively from an outpatient HF clinic. Glomerular filtration rate was estimated by the Chronic Kidney Disease Epidemiology Collaboration equation (eGFR), and patients were grouped by eGFR: eGFR group I, ≥90 mL/min/1.73 m2; eGFR group II, 60–89 mL/min/1.73 m2; and eGFR group III, ≤59 mL/min/1.73 m2. Multivariate linear regression models were developed to evaluate the associations between eGFR groups and hormones. A total of 149 patients participated in the study. Median age was 69 [interquartile range (IQR): 64–73] and 26% were female; LVEF was 33% (IQR: 27–39), 78% were in functional class II–III, median eGFR was 74 (54–89) mL/min/1.73 m2, and median N‐terminal pro‐brain natriuretic peptide was 1303 pg/mL (IQR: 441–2740). RD was associated with increased aldosterone, parathyroid hormone (PTH), and copeptin concentrations (P < 0.05 for all) after adjustment for traditional confounders and medical treatment.
Conclusions
RD is associated with increased concentrations of aldosterone, PTH, and copeptin in systolic HF outpatients. Our results underscore the importance of treatment with mineralocorticoid receptor antagonist in systolic HF in particular in patients with RD and suggest that vasopressin and parathyroid receptor antagonism are potential treatment options in HF patients with known RD.
Keywords: Systolic heart failure, Renal dysfunction, Cardiorenal, Aldosterone, Parathyroid hormone, Copeptin
Introduction
Treatment with angiotensin converting enzyme inhibitors/angiotensin II receptor blockers (ACE‐I/ARB), beta‐blockers, mineralocorticoid receptor antagonists (MRAs), and devices have improved outcome in systolic heart failure (HF).1 But treatment of the high‐risk subgroup of patients with renal dysfunction (RD) remains a challenge in daily clinical practice.2 RD is a frequent comorbidity in HF and associated with an increased risk of re‐admission, sudden cardiac death, and all‐cause mortality, but so far, no specific treatment for patients with RD has been developed.3, 4 Whether RD in outpatients with systolic HF is associated with differential or increased activation of different hormonal axes, which are traditionally evaluated in patients with primary renal disease, has not been described previously. This could be of interest, as these hormone axes represent treatment targets in other populations of patients with RD and might be targets in HF patients as well.5, 6
This prospective study tested the hypothesis that RD in outpatients with systolic HF is associated with an increased activity in hormones and prohormones reflecting aldosterone status, calcium metabolism, and vasopressin activity.
Methods
Patient population
Patients for the study were enrolled prospectively at their first visit to the HF clinic at Northzealand Hospital, Denmark. Data was collected from January 2011 to November 2012. All patients were known to have systolic HF and left ventricular ejection fraction (LVEF) <45% and were referred to the clinic for uptitration of guideline‐recommended therapy.7, 8 For enrolment in the study, it was required that plasma creatinine had been stable (±10 μmol/L) for a period of 60 days and the patient is in stable condition, defined as out of hospital for a minimum of 60 days. The only exclusion criterion was inability to provide informed consent.
At an additional visit to the HF clinic, the following were obtained: New York Heart Association (NYHA) classification, medical history, current medication, symptoms, weight, height, and electrocardiogram. Description of coronary angiography was retrieved when available, for categorizing the patients as having non‐ischemic or ischemic heart disease (IHD).9 Patients collected a 24 h urine sample, starting on the day before the exam. Fasting venous blood samples were drawn, and patients underwent echocardiography. Additional samples for aldosterone and renin were drawn after 30 min of supine rest. The study complies with the Declaration of Helsinki and approved by the local ethics committee (H‐1‐2010‐074); all patients provided informed, written, and oral consent.
Renal function
Renal function was estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation incorporating age, race if Black, gender, and plasma creatinine concentration, which has been validated in HF and found to be more accurate than other creatinine‐based equations.10, 11, 12 Patients were divided into groups according to estimated glomerular filtration rate (eGFR): eGFR group I, ≥90 mL/min/1.73 m2; eGFR group II, 60–89 mL/min/1.73 m2; and eGFR group III, ≤59 mL/min/1.73 m2. Intervals for eGFR groups were chosen, so they matched CKD stages as specified by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines.13
Biochemical analyses
After >8 h overnight fast, venous blood samples were obtained and directly analysed for haemoglobin, creatinine, sodium, and potassium. Additional samples for measurement of plasma aldosterone and renin were taken after 30 min rest in the supine position. Albuminuria was defined by an albumin excretion rate ≥ 30 mg/24 h. For later analysis, plasma was collected in ethylenediamine tetracetic acid vials and centrifuged at 4°C (3000 rpm in 10 min), and the plasma was stored at −80°C in aliquots. N‐terminal pro‐brain natriuretic peptide (NT‐proBNP; 1–76) concentration was measured on the Dimension Vista® 1500 from Siemens Medical Solutions Diagnostics using the LOCI® technology (luminescent oxygen channelling assay) (Malvern, PA, USA) according to the manufacturers' procedures.14 Plasma concentrations of copeptin were measured on the Kryptor Compact platform (BRAHMS, Hennigsdorf, Germany). Assay validation has been reported previously.15
Statistical analyses
Baseline clinical and biochemical data are reported as mean for normally distributed variables and medians for skewed distributions and presented with first and third quartiles (Q1–Q3). Basic clinical variables between eGFR groups were compared by χ2 tests for discrete variables and one‐way ANOVA tests (parametric) and Kruskal–Wallis tests (non‐parametric) for continuous variables, as appropriate. Hormones, pro‐hormone, and vitamin D were transformed by the log10 algorithm and tested with simple general linear models in Table 1. As some previous studies of aldosterone in HF have used a definition of excess aldosterone, when plasma aldosterone was >400 pmol/L (>144 pg/mL) and urinary aldosterone excretion was >33.3 nmol/24 h (>12 μg/24 h), the occurrence of abnormal aldosterone concentrations was also evaluated according to this definition and is presented in Table 1.16, 17
Table 1.
Patient characteristics
| Variable | All | eGFR group I | eGFR group II | eGFR group III | P‐value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 149 | 46 | 66 | 47 | ||||||
| Age | years | 69 | (64–73) | 61 | (56–66) | 71 | (67–75) | 73 | (70–79) | <0.001 |
| Sex. female | % | 26 | 31 | 21 | 28 | 0.538 | ||||
| BMI | kg/m2 | 27 | (24–30) | 27 | (24–31) | 27 | (24–30) | 27 | (23–30) | 0.937 |
| Heart rate | 70 | (60–80) | 71 | (60–81) | 68 | (58–79) | 70 | (60–82) | 0.534 | |
| Systolic BP | mmHg | 125 | (112–140) | 130 | (117–152) | 124 | (114–136) | 120 | (108–139) | 0.121 |
| Diastolic BP | mmHg | 77 | (69–85) | 80 | (72–84) | 77 | (69–84) | 71 | (65–79) | 0.016 |
| LVEF | % | 33 | (27–39) | 33 | (28–40) | 34 | (27–40) | 31 | (25–38) | 0.566 |
| NYHA | 0.017 | |||||||||
| I | % | 20 | 22 | 23 | 15 | . | ||||
| II | % | 52 | 72 | 49 | 43 | . | ||||
| III | % | 26 | 6 | 27 | 38 | . | ||||
| IV | % | 2 | 0 | 2 | 4 | . | ||||
| AF | % | 38 | 36 | 39 | 36 | 0.921 | ||||
| Hypertension | % | 62 | 64 | 65 | 57 | 0.632 | ||||
| DM | % | 21 | 17 | 20 | 28 | 0.431 | ||||
| IHD | % | 58 | 45 | 55 | 70 | 0.095 | ||||
| Anaemia | % | 29 | 14 | 26 | 43 | 0.011 | ||||
| Beta‐blockers | % | 87 | 90 | 88 | 83 | 0.657 | ||||
| ACE‐I/ARB | % | 95 | 97 | 96 | 91 | 0.335 | ||||
| MRA | % | 21 | 11 | 21 | 28 | 0.202 | ||||
| Diuretics | % | 72 | 64 | 73 | 79 | 0.324 | ||||
| Vitamin D suppl | % | 12 | 11 | 6 | 23 | 0.025 | ||||
| Sodium | (mmol/L) | 138 | (136–140) | 138 | (135–140) | 138 | (136–140) | 138 | (136–141) | 0.533 |
| Potassium | (mmol/L) | 4.3 | (4.1–4.7) | 4.2 | (3.9–4.5) | 4.3 | (4.1–4.6) | 4.4 | (4.1–4.7) | 0.104 |
| Creatinine | (μmol/L) | 86 | (71–108) | 65 | (53–74) | 81 | (795) | 127 | (110–160) | <0.001 |
| Phosphate | (mmol/L) | 1.18 | (0.99–1.26) | 1.13 | (0.98–1.25) | 1.09 | (0.98–1.16) | 1.23 | (1.06–1.39) | 0.002 |
| Ion‐calcium | (mmol/L) | 1.21 | (1.18–1.23) | 1.22 | (1.19–1.24) | 1.21 | (1.18–1.23) | 1.20 | (1.17–1.24) | 0.299 |
| Hgb | (mmol/L) | 8.5 | (7.9–9.2) | 8.8 | (8.4–9.4) | 8.6 | (7.9–9.3) | 8.2 | (7.4–8.8) | 0.018 |
| eGFR | (mL/min/1.73 m2) | 74 | (54–89) | 96 | (93–101) | 78 | (70–86) | 46 | (36–54) | <0.001 |
| Urine | All | eGFR group I | eGFR group II | eGFR group III | P‐value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| U‐Magnesium excret. | mmol/24 h | 4.0 | (3.0–5.0) | 4.1 | (3.0–5.0) | 4.1 | (3.0–5.0) | 3.7 | (2.0–5.0) | 0.588 |
| U‐Potassium excret. | mmol/24 h | 74.6 | (57.0–90.5) | 74.5 | (54.0–91.0) | 77.8 | (61.0–91.0) | 69.9 | (50.0–88.0) | 0.448 |
| U‐aldosterone excess | % | 22 | 26 | 23 | 17 | 0.707 | ||||
| Albuminuriaa | % | 20 | 20 | 13 | 28 | 0.202 | ||||
| Hormones | All | eGFR group | eGFR group | eGFR group | P‐value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aldosterone excessa | % | 17.3 | 8.8 | 14.3 | 28.6 | 0.053 | ||||
| P‐Aldosterone | (pmol/L) | 154 | (91–309) | 138 | (86–266) | 144 | (80–252) | 194 | (102–484) | 0.033 |
| P‐PTH | (pmol/L) | 7.6 | (5.0–11.8) | 5.7 | (4.1–8.9) | 7.2 | (5.5–11.2) | 10.1 | (6.4–17.2) | 0.001 |
| P‐25‐OH‐vitamin D | (nmol/L) | 66.8 | (47.7–89.5) | 59 | (40.1–74.4) | 62 | (40.3–74.3) | 69 | (47.8–89.5) | 0.334 |
| P‐Cortisol | (nmol/L) | 430 | (358–524) | 397 | (304–482) | 433 | (363–524) | 449 | (383–544) | 0.122 |
| P‐Renin | ×10−3 i.u. | 4.2 | (1.05–12.62) | 4.0 | (0.6–10.8) | 4.5 | (1.0–12.8) | 4.4 | (1.5–28.0) | 0.302 |
| P‐NT‐proBNP | (pg/mL) | 1303 | (441–2740) | 527 | (325–1201) | 1132 | (400–2240) | 3129 | (1514–5367) | <0.001 |
| P‐Copeptin | (pmol/L) | 9.31 | (5.9–17.7) | 6.2 | (4.6–8.2) | 9.0 | (5.9–14.5) | 21.3 | (9.9–43.2) | <0.001 |
AF, atrial fibrillation; ACE‐I/ARB, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; excret, excretion; Hgb, haemoglobin; IHD, ischemic heart disease; LVEF, left ventricle ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association functional class.
Data are presented as mean or median with interquartile ranges. P‐values for the 0‐hypothesis: no difference between groups I–III at a 0.05 significance level (please see section Methods, statistical analyses).
Albuminuria was defined by albumin excretion rate ≥ 30 mg/24 h.
For further analyses, the association between the log‐transformed hormones/pro‐hormone and concentrations of urinary cations (Mg2+ and K+) and eGFR groups were evaluated in multivariate linear models. The multivariate models were all adjusted for the following explanatory variables: eGFR, age, gender, diabetes, LVEF, atrial fibrillation (AF), hypertension, IHD, ACE‐I/ARB, and MRA treatment. To each model, biological relevant explanatory variables were added, as follows: model for parathyroid hormone (PTH) as response variable, P‐calcium and 25‐OH‐vitamin D; model for aldosterone as response variable, renin and potassium; and model for copeptin as response variable, P‐sodium and systolic blood pressure.
We further tested the association between the HF markers, LVEF, NT‐proBNP, NYHA class, and the hormones, in univariate and multivariate analyses with and without eGFR in the models; these results are presented in Table S1 a–c.
To avoid overlooking a moderate effect of eGFR on hormone levels (β: 0.80), at a significance level of 0.05 (α) in a multivariate regression model including eight covariates, n was calculated at 108.18 A total of n = 150 patients were recruited to account for possible missing data. For all analyses, a two‐sided P‐value of <0.05 was considered significant.
Results
Patient characteristics
A total of 230 patients were referred to the clinic: 61 declined to participate, 20 accepted but did not show for the additional visit, and finally, 149 patients participated in the study. Patient characteristics for the entire cohort and stratified according to eGFR group are listed in Table 1.
Median age was 69, and 26% were female; median LVEF was 33%, and 38% had AF at the time of the exam; 58% had IHD defined by coronary angiography descriptions.9 Patients with lower renal function/lower eGFR, that is, eGFR group III, were older and had lower levels of haemoglobin and higher concentrations of aldosterone, PTH, and copeptin in the univariate models; but occurrence of excess aldosterone did not differ significantly (P = 0.053). There were no significant differences in frequencies of AF, hypertension, body mass index, diabetes, IHD, or medication. Notably there was no difference in concentrations of P‐ion‐calcium, P‐25‐OH‐vitamin D, or P‐cortisol.
Owing to discrepancy in occurrence of excess plasma concentration of aldosterone and elevated urinary aldosterone according to eGFR groups, the association between the two variables was evaluated in a univariate linear regression model with log10(urinary aldosterone excretion) as response variable and log10(p‐aldosterone) as explanatory variable, and they were found to be significantly associated (P < 0.001). When eGFR was added as explanatory variable to the model, the association between urinary excretion and plasma concentrations of aldosterone remained significant (P < 0.001). There was no association between log10(urinary aldosterone excretion) and eGFR in a univariate linear model (P = 0.903).
Multivariate linear regression models
In the multivariate linear regression models, aldosterone, copeptin, and PTH concentrations were associated with eGFR group after adjustment for the predefined confounders. There were no significant differences in urinary cations or urinary aldosterone. Beta coefficients for eGFR in the multivariate models for each hormone are presented in Table 2.
Table 2.
The relationship between hormones/pro‐hormone and renal function in multivariate linear regression models
| Model for | β coefficient for eGFR | 95% CI | P‐value |
|---|---|---|---|
| Log10(aldosterone) | −0.0038 | (−0.006 to −0.001) | 0.018 |
| Log10(parathyroid hormone) | −0.0049 | (−0.004 to −0.003) | <0.001 |
| Log10(copeptin) | −0.0118 | (−0.014 to −1.009) | <0.001 |
CI, confidence interval; eGFR, estimated glomerular filtration rate.
All models were adjusted for age, sex, body mass index, left ventricle ejection fraction, atrial fibrillation, history of hypertension and use of mineral corticoid receptor antagonists, and angiotensin converting enzyme inhibitors/angiotensin receptor blockers. Furthermore, the models were adjusted as follows; parathyroid hormone, P‐calcium and P‐25‐OH‐vitamin D; aldosterone, P‐renin and P‐potassium; and copeptin, P‐sodium and systolic blood pressure.
P‐values for the multivariate models for log‐transformed hormones/pro‐hormone according to eGFR group are presented as bar plots in Figure 1 a–c.
Figure 1.
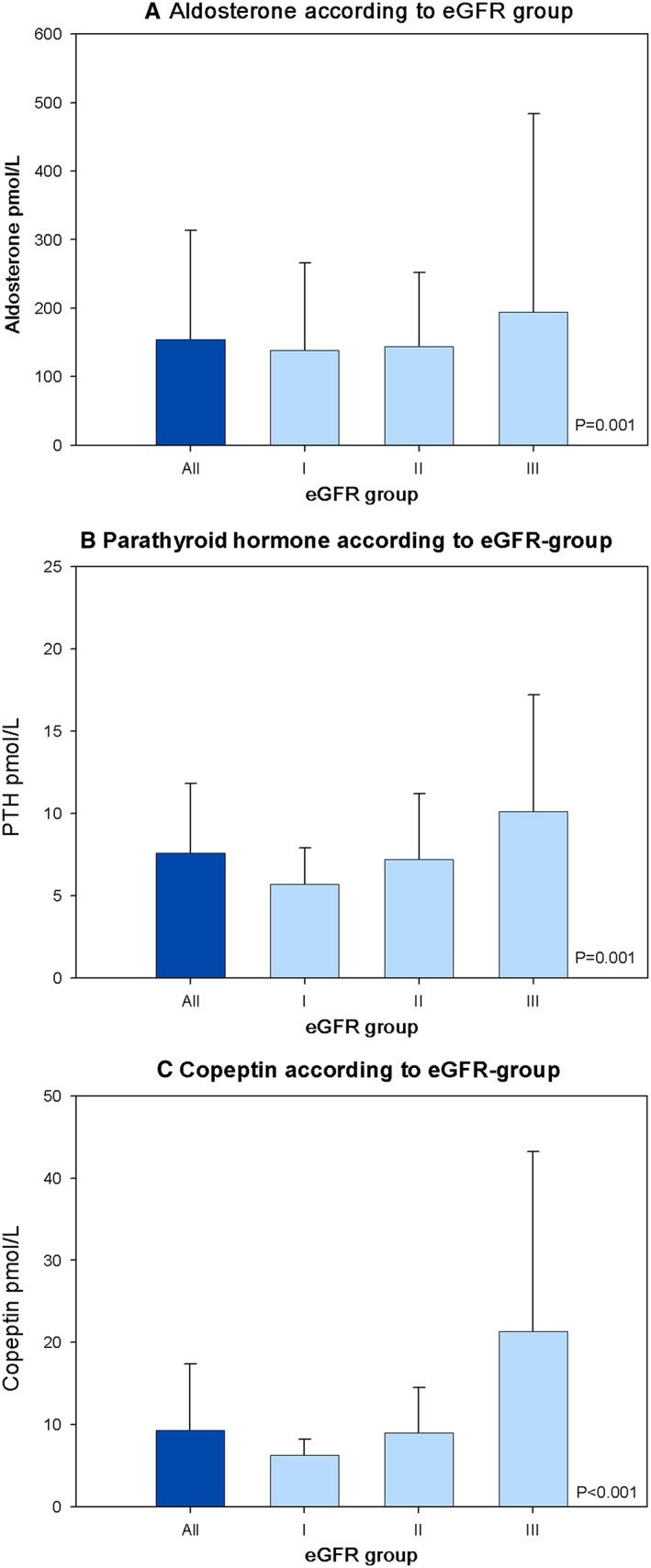
(A–C) Concentrations of hormones according to estimated glomerular filtration rate (eGFR) group. Data bars represent the median level and third quartile of hormones. P‐values for linear regression models adjusted for age, sex, body mass index, left ventricular ejection fraction, atrial fibrillation, history of hypertension, and use of mineralocorticoid receptor antagonists and angiotensin converting enzyme inhibitors/angiotensin receptor blockers. Furthermore, the models were adjusted for parathyroid hormone (PTH), P‐calcium and P‐25‐OH‐vitamin D; aldosterone, P‐renin and P‐potassium; and copeptin, P‐sodium and systolic blood pressure.
We constructed a multivariate logistic regression model for excess aldosterone as a binary variable adjusted for the same explanatory variables as described for the linear models and found a significant association with eGFR groups (P = 0.026) (data not presented in tables).
More patients in the eGFR groups with a low eGFR reported taking an MRA. Despite that the intake did not differ significantly between the groups (P = 0.202; Table 1), it might have resulted in a secondary increase in aldosterone concentrations contributing to the observed association between eGFR and plasma aldosterone; so we repeated the multivariate linear model with log10(aldosterone) as response variable with and without MRA as a covariate, and the association with eGFR group remained significant (without, P = 0.021; with, P < 0.001).
Univariate and multivariate analyses of the hormones and LVEF, NYHA class, and NT‐proBNP
Aldosterone was not associated with LVEF (P = 0.404) or NT‐proBNP (P = 0.126) in univariate analyses, but only when entering eGFR and/or the predefined covariates in multivariate analyses. Aldosterone was associated with NYHA class (P = 0.012) in both univariate and multivariate analyses, with and without eGFR.
PTH was not associated with LVEF (P = 0.586) in univariate analyses but was associated with NYHA class (P = 0.008) and NT‐proBNP (P = 0.004) in univariate models. eGFR was associated with the hormone in all the models.
Copeptin was not associated with LVEF (P = 0.127) in univariate analyses but became significant when adjusted for the covariates (P = 0.032) and was associated with eGFR in all the models (P < 0.05). Copeptin was associated with NYHA class and NT‐proBNP in all models (all P < 0.05).
Please refer to Table S1 a–c for all results.
Discussion
In this cross‐sectional study investigating systolic HF patients with and without RD, we observed that RD was associated with increased plasma concentrations of aldosterone, PTH, and copeptin. These hormone axes represent potential treatment targets in the chronic cardiorenal syndrome, and our analyses underscore the importance of treatment with aldosterone antagonism in HF, in particular in patients with RD.
Aldosterone status
We observed a significant association between RD and aldosterone in the multivariate analyses (Figure 1 a). The increased aldosterone concentrations observed in HF patients with RD are a plausible contributing factor to the increased mortality and morbidity associated with RD in HF.1, 4 Blocking of the mineralocorticoid receptor with the MRAs spironolactone and eplerenone has been a large progress in reducing mortality and morbidity in HF.19, 20 In addition, spironolactone was found to have greater absolute effect on mortality in the subgroup of HF patients with an eGFR < 60 mL/min/1.73 m2, which supports our finding in the multivariate analyses.20
Reduced GFR is mainly due to decreased renal blood flow in HF patients, which also activates the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous systems. Thus, reduced renal blood flow is a possible trigger of the increased aldosterone in the present study.21, 22 However, P‐renin and P‐cortisol were not increased in patients with RD, and the precise mechanism for the observed increased aldosterone concentration cannot be deduced from our data. Urinary excretion of aldosterone did not increase with decreasing eGFR (Table 1). However, as observed in the ALOFT study, we found a significant association between plasma aldosterone concentrations and urinary excretion of aldosterone.17 A possible explanation for the discrepancy between plasma aldosterone and urinary excretion of aldosterone according to eGFR group could be a decrease in renal clearance of aldosterone with declining eGFR.
The finding of increased aldosterone concentrations in HF patients with RD underscores the importance of development for new treatment strategies for blocking of aldosterone‐induced effects, as the guideline‐recommended MRAs are under‐utilized in this group, owing to risk of hyperkalaemia and decrease in renal function.7, 8, 23 In the ARTS‐HF trial, a non‐steroidal MRA, finerenone, is being investigated as an alternative to spironolactone and eplerenone in HF patients with reduced eGFR.24, 25 The results are promising, as the Phase IIb trial showed a similar safety profile as eplerenone but less frequent occurrence of hyperkalaemia and lower events rates of the secondary endpoint of death, hospitalization of worsening of HF in patients with moderate kidney disease with an eGFR of 30 to <59 mL/min/1.73 m2.26
Calcium metabolism
We observed that RD was associated with increased PTH concentrations. Increased plasma concentrations of PTH in HF have been described previously and were in these studies related to HF severity, re‐admission rates, and mortality risk; but an association between RD and PTH concentrations in HF has not been reported previously.27, 28, 29
The exact trigger for increased PTH in HF is unknown.30 A suggested explanation is activation of RAAS with aldosterone‐stimulated faecal and urinary excretion of cations and intracellular storing of Ca2+, resulting in low concentrations of extracellular Ca2+ and secondary hyperparathyroidism to restore extracellular Ca2+.30, 31 As described for aldosterone, reduced renal blood flow in RD is a possible trigger for increased activation of this pathway. However, we did not observe an increased urinary excretion of magnesium or potassium in patients with RD supportive of this explanation (Table 1).
Another possible trigger for the increased concentrations of PTH could be initial hypocalcaemia due to vitamin D deficiency, but we did not observe any difference in P‐25‐OH‐vitamin D concentrations in the present study. This may be due to a difference in 25‐OH‐vitamin D supplementation (Table 1). Furthermore, unmeasured active vitamin D (1,25‐OH‐vitamin D) produced by the kidneys may be decreased in patients with RD despite normal plasma concentrations of inactive 25‐OH‐vitamin D, and the normalization of plasma concentrations of Ca2+ may be the net result of deficiency of active 1,25‐OH‐vitamin D and increased concentrations of PTH. Whether treatment with paricalcitol (active 1,25‐OH vitamin D) or cinacalcet (PTH receptor antagonist) can improve outcome in systolic HF patients with RD and secondary hyperparathyroidism remains to be determined, but an effect on secondary cardiovascular endpoints in patients with chronic kidney disease has been observed in two randomized clinical trials.5, 6 However, treatment with inactive 25‐OH‐vitamin D has no effect on quality of life or functional class in systolic HF patients.32
Vasopressin activity
We observed that copeptin concentrations were increased in patients with RD, in accordance with previous studies.33 The association with RD may be due to arterial underfilling and baroreflex activation.34 Increased copeptin concentrations have been shown to predict mortality risk and risk of re‐admission in HF.33, 35
In the EVEREST trial, treatment with a vaptan failed to improve long‐term clinical outcome in HF.36 However, this result may reflect selection of patients in whom the vasopressin system was not particularly activated, because neither elevated copeptin nor vasopressin nor hyponatraemia was an inclusion criterion. In theory, systolic HF patients with RD and increased copeptin concentrations may benefit from treatment with a vaptan, but this requires further investigation.
Relationship between cardiac function and the hormones
The additional analyses on the relationship between LVEF, NYHA, and NT‐proBNP and the three hormones did not show a specific association with LVEF but did show a specific association to some degree with NYHA class and NT‐proBNP, which again could be argued to reflect the degree of both HF and renal function.
There was only a significant association when eGFR and the clinical covariates were entered in the models, suggesting that the increased levels of the hormones are related to the presence of RD and not to HF alone. As increased levels of these hormones have been shown to be associated with mortality in HF populations,27, 28, 33, 37 our results suggest that targeting the hormone axes in future trials possibly should be limited to HF patients with known moderate RD or increased levels of the hormones.
Limitations
Some methodological strengths and limitations should be discussed. The generalizability of our data is limited to patients with systolic HF, considered eligible for uptitration in neurohormonal blockade and therefore represent a selected population. The results should not be extrapolated to patients with acute, advanced, or terminal HF. The included patients were examined during their first phase of uptitration of HF medication, with no specific criteria regarding status of medication. One could argue that the patients should have been examined either before any medication or after full uptitration to be strictly comparable. As the medication for treating HF is also used for hypertension and post‐myocardial infarction, it is, however, difficult to investigate a HF population not being treated at all. Furthermore, HF is a chronic condition frequently without an index event like in cohorts with a myocardial infarction, which also complicate to determine the optimal experimental day from a strict point of view. Waiting to end uptitration would likely cause some selection bias (results would have been biased towards zero), as some patients especially patients with a low eGFR would have died, but selection bias in the other direction could also be the case in that patients with a low eGFR do not tolerate target doses of medication. Investigation in relation to the baseline visit in the outpatient HF clinic was therefore chosen.
The present three eGFR groups were used owing to the sample size, and it may have resulted in eGFR group I containing both patients with no renal disease, impairment due to age, or mild renal disease. As it has been shown, eGFR is a strong prognostic factor in HF and the three groups are, therefore, considered clinical meaningful. Albuminuria is also a prognostic factor in HF, and more knowledge on this variable is needed. We did not differ between microalbuminuria or macroalbuminuria, and the aetiology of proteinuria cannot be deduced from our results.38
When interpreting our results, it has to be remembered that chronic kidney disease is an inhomogeneous disease, and for example, reduced renal blood flow and parenchymal renal disease may affect hormones differently. We may have underestimated the effect of RD on the different hormone axes owing to misclassification (=bias towards zero) of GFR induced by the CKD‐EPI formula,11 and it may explain why RD and aldosterone excess were only associated significantly in the multivariate analyses. As mentioned in the discussion, increased copeptin levels may represent increased vasopressin production owing to arterial underfilling and renal vasoconstriction and also simply decreased clearance due to RD. Unmeasured confounding due to lack of measurement of 1,25‐OH‐vitamin D may in theory partly explain the association between RD and increased PTH concentrations as previously discussed, but it does not change the conclusion that calcium metabolism is affected in systolic HF patients with RD. Thus, the observed statistical associations are all biological plausible and are observed in cohorts of patients with chronic kidney disease without systolic HF, making our results likely. No measurements of activation of phosphate homeostasis through activation of FGF23–Klotho axis were performed, and whether some of the patients had secondary hyperparathyroidism due to increased FGF23 cannot be determined.
The strengths of the present data are a precise clinical and biochemical characterization of 149 systolic HF patients recruited from daily clinical practice, and we succeeded in collecting 24 h urine samples in this elderly population. Based on our results, Phase II and III trials evaluating antagonism of vasopressin and PTH are intriguing perspectives to improve outcome in the chronic cardiorenal syndrome. A third possibility could be further evaluation of a treatment strategy with non‐steroidal MRA as second option, when withdrawal of the traditional steroidal MRAs is necessary owing to rising potassium or reduced renal function, as was carried out in the CHARM alternative trial.39
Conclusions
RD is associated with increased concentrations of aldosterone, PTH, and copeptin in systolic HF outpatients. Our results underscore the importance of treatment with MRA in systolic HF, in particular in patients with RD, and suggest that vasopressin and parathyroid receptor antagonism are potential treatment options in HF patients with known RD.
Conflict of interest
Assays for analysis of NT‐proBNP were sponsored by Siemens. The authors have no further conflict of interests to report.
Funding
Northzeeland Hospital Research Foundation sponsored the PhD grant for HB.
Supporting information
Table S1a. Aldosterone.
Table S1b. Parathyroid hormone.
Table S1c. Copeptin
Bosselmann, H. , Tonder, N. , Sölétormos, G. , Gaborit, F. , Rossing, K. , Iversen, K. , Goetze, J. P. , Gustafsson, F. , and Schou, M. (2017) Influence of renal impairment on aldosterone status, calcium metabolism, and vasopressin activity in outpatients with systolic heart failure. ESC Heart Failure, 4: 554–562. doi: 10.1002/ehf2.12186.
References
- 1. Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348: 2007–2018. [DOI] [PubMed] [Google Scholar]
- 2. Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from literature. J Am Coll Cardiol 2014; 63: 853–871. [DOI] [PubMed] [Google Scholar]
- 3. Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta‐analysis. J Card Fail 2007; 13: 599–608. [DOI] [PubMed] [Google Scholar]
- 4. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 5. Chertow GM, Block GA, Correa‐Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 367: 2482–2494. [DOI] [PubMed] [Google Scholar]
- 6. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd‐Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307: 674–684. [DOI] [PubMed] [Google Scholar]
- 7. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119: e391–e479. [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 9. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002; 39: 210–218. [DOI] [PubMed] [Google Scholar]
- 10. McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes‐Genis A, Gotsman I, Whalley G, Earle N, Poppe KK, Doughty RN. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease‐Epidemiology Collaboration Group formula. Circulation: Heart Failure 2012; 5: 309–314. [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valente MA, Hillege HL, Navis G, Voors AA, Dunselman PH, van Veldhuisen DJ, Damman K. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail 2014; 16: 86–94. [DOI] [PubMed] [Google Scholar]
- 13. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases 2002; 39: S1–266. [PubMed] [Google Scholar]
- 14. Alehagen U, Dahlström U, Rehfeld JF, Goetze JP. Prognostic assessment of elderly patients with symptoms of heart failure by combining high‐sensitivity troponin T and N‐terminal pro‐B‐type natriuretic peptide measurements. Clin Chem 2010; 56: 1718–1724. [DOI] [PubMed] [Google Scholar]
- 15. Terzic DJ‐FA, Ragnasson O, Gøtze JP, Hammersten O. Evaluation of a sensitive copeptin assay for clinical measurement. Open Clinical Biochemistry Journal 2012; 5: 21–36. [Google Scholar]
- 16. Weber KT. Aldosterone in congestive heart failure. N Engl J Med 2001; 345: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 17. Freel EM, Tsorlalis IK, Lewsey JD, Latini R, Maggioni AP, Solomon S, Pitt B, Connell JM, McMurray JJ. Aldosterone status associated with insulin resistance in patients with heart failure—data from the ALOFT study. Heart 2009; 95: 1920–1924. [DOI] [PubMed] [Google Scholar]
- 18. Jacob Cohen PC. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed. New Jersey: Lawrence Earlbaum Associates; 1983. [Google Scholar]
- 19. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 20. Pitt B, Williams G, Remme W, Martinez F, Lopez‐Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post‐AMI Heart Failure Efficacy and Survival Study. Cardiovascular Drugs and Therapy 2001; 15: 79–87. [DOI] [PubMed] [Google Scholar]
- 21. Smilde TD, Damman K, van der Harst P, Navis G, Westenbrink BD, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure Clinical Research in Cardiology 2009; 98: 121–129. [DOI] [PubMed] [Google Scholar]
- 22. Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9: 872–878. [DOI] [PubMed] [Google Scholar]
- 23. Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circ Heart Fail 2008; 1: 98–106. [DOI] [PubMed] [Google Scholar]
- 24. Pitt B, Filippatos G, Gheorghiade M, Kober L, Krum H, Ponikowski P, Nowack C, Kolkhof P, Kim SY, Zannad F. Rationale and design of ARTS: a randomized, double‐blind study of BAY 94‐8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail 2012; 14: 668–675. [DOI] [PubMed] [Google Scholar]
- 25. Barfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa‐Perez S, Heckroth H, Nitsche A, Erguden JK, Gielen‐Haertwig H, Schlemmer KH, Mittendorf J, Paulsen H, Platzek J, Kolkhof P. Discovery of BAY 94‐8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem 2012; 7: 1385–1403. [DOI] [PubMed] [Google Scholar]
- 26. Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp‐Kirschbaum N, Pieper A, Pitt B. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016; 37: 2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khouzam RN, Dishmon DA, Farah V, Flax SD, Carbone LD, Weber KT. Secondary hyperparathyroidism in patients with untreated and treated congestive heart failure. Am J Med Sci 2006; 331: 30–34. [DOI] [PubMed] [Google Scholar]
- 28. Sugimoto T, Tanigawa T, Onishi K, Fujimoto N, Matsuda A, Nakamori S, Matsuoka K, Nakamura T, Koji T, Ito M. Serum intact parathyroid hormone levels predict hospitalisation for heart failure. Heart 2009; 95: 395–398. [DOI] [PubMed] [Google Scholar]
- 29. Altay H, Zorlu A, Binici S, Bilgi M, Yilmaz MB, Colkesen Y, Erol T, Muderrisoglu H. Relation of serum parathyroid hormone level to severity of heart failure. Am J Cardiol 2012; 109: 252–256. [DOI] [PubMed] [Google Scholar]
- 30. Tomaschitz A, Ritz E, Pieske B, Fahrleitner‐Pammer A, Kienreich K, Horina JH, Drechsler C, Marz W, Ofner M, Pieber TR, Pilz S. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res 2012; 94: 10–19. [DOI] [PubMed] [Google Scholar]
- 31. Kamalov G, Ahokas RA, Zhao W, Zhao T, Shahbaz AU, Johnson PL, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Uncoupling the coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria seen in aldosteronism. J Cardiovasc Pharmacol 2010; 55: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circulation: Heart Failure 2010; 3: 195–201. [DOI] [PubMed] [Google Scholar]
- 33. Bosselmann H, Egstrup M, Rossing K, Gustafsson I, Gustafsson F, Tonder N, Kistorp CN, Goetze JP, Schou M. Prognostic significance of cardiovascular biomarkers and renal dysfunction in outpatients with systolic heart failure: a long term follow‐up study. Int J Cardiol 2013. [DOI] [PubMed] [Google Scholar]
- 34. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia. N Engl J Med 2006; 355: 2099–2112. [DOI] [PubMed] [Google Scholar]
- 35. Balling L, Kistorp C, Schou M, Egstrup M, Gustafsson I, Goetze JP, Hildebrandt P, Gustafsson F. Plasma copeptin levels and prediction of outcome in heart failure outpatients: relation to hyponatremia and loop diuretic doses. J Card Fail 2012; 18: 351–358. [DOI] [PubMed] [Google Scholar]
- 36. Cavalcante JL, Khan S, Gheorghiade M. EVEREST study: Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with tolvaptan. Expert Rev Cardiovasc Ther 2008; 6: 1331–1338. [DOI] [PubMed] [Google Scholar]
- 37. Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Störk S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 2007; 115: 1754–1761. [DOI] [PubMed] [Google Scholar]
- 38. Damman K, Hillege HL, van Veldhuisen DJ. Albuminuria in heart failure: a CHARMing new risk factor? Lancet 2009; 374: 506–508. [DOI] [PubMed] [Google Scholar]
- 39. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet 2003; 362: 772–776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1a. Aldosterone.
Table S1b. Parathyroid hormone.
Table S1c. Copeptin


