Abstract
Management of adults with failing Fontan physiology poses many challenges, especially as transplantation offers the only realistic alternative to palliative care. We present the first combined heart and liver transplant performed in Europe, for a late survivor of single ventricle palliation with the Fontan circulation. In addition to the conventional medical and surgical challenges posed, we highlight the management of the associated multi‐organ failure with focus on the liver and novel strategies for assessment and optimization.
Introduction
Since 1971, Fontan surgery has transformed the outlook for single ventricle patients.1 This affects around 16 per 100 000 children with most now surviving to adulthood.2
The circulation depends on low pulmonary vascular resistance facilitating adequate preload to the systemic ventricle.3 However, even the well‐functioning circuit eventually fails as chronic non‐pulsatile pulmonary flow and elevated systemic venous pressures accelerate multi‐organ failure. Management of the failing Fontan is not limited to replacing the pump but also addressing the extra‐cardiac problems associated with the declining circulation.
Transplantation, at present, provides the only alternative to palliative care but has high early mortality and poorly defined referral criteria. The present case highlights the complexity of the situation, particularly with respect to liver involvement.
Case report
A 50‐year‐old man with small tricuspid valve, hypoplastic double‐outlet right ventricle, peri‐membranous ventricular septal defect, and malposed great vessels underwent a modified atriopulmonary Fontan (homograft valves at right atrial junction with caval veins and pulmonary artery) at 9 years old.4 He remained well, working full‐time, until his 30s, when he developed arrhythmia. In his late 40s, he began to experience exertional symptoms and abdominal swelling because of ascites.
Cardiovascular magnetic resonance (CMR) showed a large (90 × 74 mm) right atrium, no atrioventricular valve regurgitation or Fontan pathway narrowing, and preserved systolic ventricular function (ejection fraction 59%). Mean right atrial pressure was 22 mmHg. He had marked thoracic veno‐venous collaterals, amiodarone‐associated thyroid disease, iron deficiency, and impaired renal function (creatinine 266 μmol/L).
Despite preserved synthetic liver function (bilirubin 14 μmol/L, albumin 46 g/L), computerized tomography showed cirrhosis and portal hypertension (recanalized umbilical vein and splenomegaly). Novel CMR sequences5 optimized for high resolution cardiac fibrosis detection highlighted coarse nodular liver architecture implying extensive fibrotic change (Figure 1 A and B). There were no liver regenerative nodules. Child Pugh score was 7, Childs grade B, MELD‐XI 24, and VAST 3. Progressive liver disease and risk of hepatic decompensation justified combined listing for heart and liver transplantation.
Figure 1.
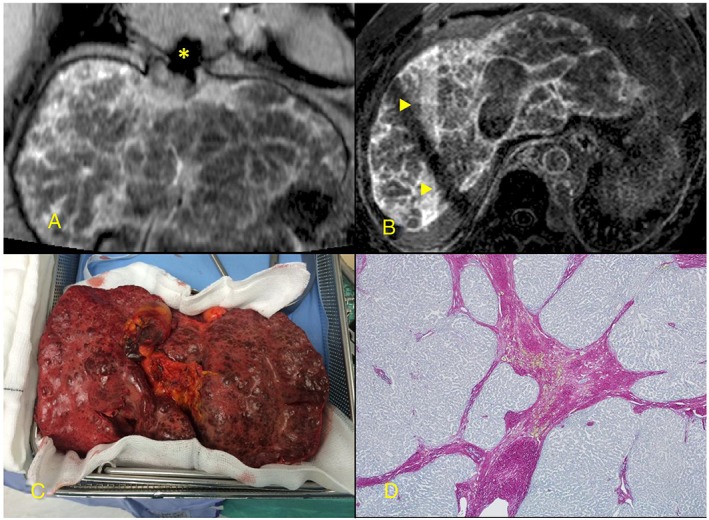
(A) and (B) Coarse, nodular, and fibrotic appearance of liver late after gadolinium contrast injection. (C) Gross appearance of explanted liver. (D) Histology (collagen stain) showing dense collagenous septa. Artefacts: Asterisk indicates inferior vena caval metallic stent artefact in (A) and the triangles indicate respiratory navigator cross bars in (B).
The patient was admitted for diuresis, milrinone infusion, and serial abdominal paracentesis to optimize haemodynamics and end‐organ function. This was complicated by two episodes of sepsis (secondary to an ascitic tap and an intravenous cannula). Between admission and transplantation (7 months), 6–10 L of ascites was removed bi‐weekly, creatinine improved from 292 to 130 μmol/L, and bilirubin from 19 to 10 μmol/L. At transplantation, MELD‐XI score was 11.
Operative course
The organs were procured from a single donor following donor–recipient crossmatch. The patient had been prioritized locally for a matched block. Heart transplant was performed via redo‐median sternotomy with bicaval cannulation and the abdomen closed. Caval vein and pulmonary artery anastomoses were completed with the aortic clamp removed, and the heart was reperfused. After weaning bypass, the chest was left open, and liver transplant was performed using standard caval replacement technique. The allograft ischaemic time was 147 min. The whole operation was performed on cardio‐pulmonary bypass with a bypass time of 727 min and a total operative time of 1102 min.
Liver histopathology
The explanted liver weighed 1.58 kg and was irregular with multiple tan nodules (1 to 5 mm) (Figure 1 C). Histology, consistent with CMR, showed extensive broad sclerotic fibrous bands surrounding small nodules (Figure 1 D). There was marked sinusoidal dilatation, significant atrophy, and focal parenchymal necrosis. The portal vein showed muscular hypertrophy and intimal thickening consistent with portal hypertension.
Immunosuppression and monitoring
Methylprednisolone 10 mg/kg was administered at anaesthetic induction and tapered off over months to a maintenance dose. Basiliximab and ciclosporin A were given on Day 1 but replaced with Tacrolimus on Day 2; this was switched back to ciclosporin A on Day 87 because of clinical neurotoxicity. Mycophenolate mofetil was given for a short period at 3 months because of fluctuating ciclosporin levels but discontinued because of concomitant confusion.
Rejection was assessed by echocardiography and hepatic biochemical function. Endomyocardial biopsies performed at 3, 5, and 7 weeks and 3, 4, and 6 months showed no evidence of rejection. Augmentation (three doses of methylprednisolone 10 mg/kg) was given for clinical liver rejection on Day 4. Liver biopsy at 2 weeks showed cholestasis and possible features of sepsis or drug‐induced liver injury but no acute rejection. Liver biopsy at 4 months showed moderate–severe acute rejection, and augmentation was repeated.
Post‐operative recovery
The length of stay on intensive care was 57 days with four readmissions for sepsis, graft rejection, and tacrolimus neurotoxicity. Morbidity is shown in the Table 1. The patient required continuous haemofiltration for 1 month, and then, variable periods of intermittent haemodialysis finally discontinued at 7 months. Notably, he had persistent right pleural effusion, ascites (drained twice), and peripheral oedema for several months following transplantation. The total length of stay was 14 months (7 months post‐transplant). Discharge creatinine was 180 μmol/L. Following discharge, the patient enrolled in intensive rehabilitation and returned to full‐time work. He is presently New York Heart Association functional class I with good cardiac function on echo, normal liver function, and moderate renal impairment managed without dialysis.
Table 1.
Post‐transplantation morbidity
| System | Event | Post‐operative day |
|---|---|---|
| Respiratory | Tracheostomy (failed extubation ×2) | Day 12 |
| Hospital acquired pneumonia | Day 111a | |
| Persistent right pleural effusion | ||
| Sepsis | Gangrenous umbilicus debrided | Day 8 |
| Percutaneous drainage of abdominal abscess | Day 29 | |
| Mediastinitis debrided | Day 48a | |
| Liver | Infarcted one segment of graft | |
| Biliary anastomotic leak: stented via ERCP | Day 32 | |
| Renal | Continuous renal replacement therapy | 4 weeks |
| Variable periods of intermittent renal replacement therapy | 6 months | |
| Neurological | Presumed neurotoxicity from Tacrolimus | Day 87a |
| Gut and nutrition | Nasogastric and jejunal feeding/total parental nutrition | 2 months |
| Persistent ascites |
ERCP, endoscopic retrograde cholangiopancreatogram.
Denotes event necessitating readmission to intensive care.
Discussion
The adult Fontan population is rapidly expanding, and this case is significant for physicians counselling these patients and planning future care. To our knowledge, our patient is the first in Europe to undergo combined heart and liver transplant (CHLT) for advanced failing Fontan physiology. Recently, CHLT in this setting has been reported in the USA with good outcomes; it should be noted that our patient, in comparison, was older and at the advanced end of the Fontan failure spectrum with renal dysfunction, portal hypertension, and significant chest wall collaterals.6 Though feasible, the approach demands huge investment from the patient, their family, and health professionals.
Isolated heart transplantation in the failing Fontan is complicated by anatomical complexity, potentially high levels of preformed human leucocyte antibodies, susceptibility to infection, and high risk of bleeding or multi‐organ failure.7 The degree of hepatic and renal impairment needs case‐by‐case assessment by a multi‐disciplinary team, as multi‐organ transplantation may be required. While cirrhosis adversely affects outcome following cardiac surgery, it is not known at what point liver dysfunction may preclude isolated cardiac transplantation in this group.8
The spectrum of liver disease is wide and presently unpredictable and includes hyper‐vascular nodules, hepato‐cellular carcinoma, portal hypertension, and ascites. Liver synthetic function is often preserved despite significant reduction in liver volume and/or extensive fibrosis on histology. In our case, non‐invasive CMR tissue characterization suggested extensive scarring of the liver. Extending the field of view of novel fibrosis sequences to intentionally include the liver alongside the heart may offer earlier detection of disease progression. This however requires further evaluation. At present, our practice is that patients with cirrhosis but with normal synthetic function, normal hepatic venous anatomy, good liver volume, and no significant portal hypertension or hepato‐cellular carcinoma may be considered for heart‐only transplantation.
The CHLT is suggested to have an immuno‐protective effect attributed to the transplanted liver, and long‐term outcomes in congenital patients are favourable.9, 10 In the recent CHLT series in Fontan patients, indicated for cirrhosis, 1 year survival was 100% in contrast to around 65% reported for heart alone transplant in a similar group.6, 7 There is, however, a recognized shortage of organ donors. Concerns may reasonably be raised regarding the advisability of utilizing two organs in a high‐risk complicated transplant when patients with more straightforward anatomy requiring single organs are potentially dying on the waiting list. The prolonged waiting time can be utilized, however, to optimize end‐organ function prior to transplantation, and we have found this strategy, reported elsewhere, to be effective in the Fontan setting.11 Nevertheless, this may be complicated by hospital‐acquired infection and prolongation of stay. Expectant anaesthetic management of complex perioperative issues, including haemorrhage and coagulaopathy, may also help support good outcomes.12
Our case highlights the emerging problem of liver dysfunction in the older Fontan patient. It demonstrates that although a good outcome from multi‐organ transplantation can be obtained, resource implications are such that this treatment can only be relied on for a few. Other strategies, including novel diagnostic and therapeutic approaches, will need to be developed and validated.
Conflict of interest
None declared.
Duong, P. , Coats, L. , O'Sullivan, J. , Crossland, D. , Haugk, B. , Babu‐Narayan, S. V. , Keegan, J. , Hudson, M. , Parry, G. , Manas, D. , and Hasan, A. (2017) Combined heart–liver transplantation for failing Fontan circulation in a late survivor with single‐ventricle physiology. ESC Heart Failure, 4: 675–678. doi: 10.1002/ehf2.12202.
References
- 1. Fontan F, Mounicot FB, Baudet E, Simonneau J, Gordo J, Gouffrant JM. “Correction” of tricuspid atresia. 2 cases “corrected” using a new surgical technique. Ann Chir Thorac Cardiovasc 1971; 10: 39–47. [PubMed] [Google Scholar]
- 2. Coats L, O'Connor S, Wren C, O'Sullivan JJ. The single‐ventricle patient population: a current and future concern a population‐based study in the North of England. Heart 2014; 100: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 3. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart 2016; 102: 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yacoub MH, Radley‐Smith R. Use of a valved conduit from right atrium to pulmonary artery for “correction” of single ventricle. Circulation 1976; 54(6 Suppl): 1163–1170. [PubMed] [Google Scholar]
- 5. Keegan J, Jhooti P, Babu‐Narayan SV, Drivas P, Ernst S, Firmin DN. Improved respiratory efficiency of 3D late gadolinium enhancement imaging using the continuously adaptive windowing strategy (CLAWS). Magn Reson Med 2014; 71: 1064–1074. [DOI] [PubMed] [Google Scholar]
- 6. D'Souza BA, Fuller S, Gleason LP, Hornsby N, Wald J, Krok K, Shaked A, Goldberg LR, Pochettino A, Olthoff KM, Kim YY. Single center outcomes of combined heart and liver transplantation in the failing Fontan. Clin Transplant 2017; 31. [DOI] [PubMed] [Google Scholar]
- 7. Murtuza B, Hermuzi A, Crossland DS, Parry G, Lord S, Hudson M, Chaudhari MP, Haynes S, O'Sullivan JJ, Hasan A. Impact of mode of failure and end‐organ dysfunction on the survival of adult Fontan patients undergoing cardiac transplantation. Eur J Cardiothorac Surg 2017; 51: 135–141. [DOI] [PubMed] [Google Scholar]
- 8. Hsieh WC, Chen PC, Corciova FC, Tinica G. Liver dysfunction as an important predicting risk factor in patients undergoing cardiac surgery: a systematic review and meta‐analysis. Int J Clin Exp Med 2015; 8: 20712–20721. [PMC free article] [PubMed] [Google Scholar]
- 9. Pinderski LJ, Kirklin JK, McGiffin D et al. Multi‐organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant 2005; 24: 1828–1833. [DOI] [PubMed] [Google Scholar]
- 10. Bradley EA, Pinyoluksana KO, Moore‐Clingenpeel M, Miao Y, Daniels C. Isolated heart transplant and combined heart‐liver transplant in adult congenital heart disease patients: insights from the United Network of Organ Sharing. Int J Cardiol 2017; 228: 790–795. [DOI] [PubMed] [Google Scholar]
- 11. Stevenson LW. Clinical use of inotropic therapy for heart failure: looking backward or forward? Part II: chronic inotropic therapy. Circulation 2003; 108: 492–497. [DOI] [PubMed] [Google Scholar]
- 12. Navaratnam M, Ng A, Williams GD, Maeda K, Mendoza JM, Concepcion W, Hollander SA, Ramamoorthy C. Perioperative management of pediatric en‐bloc combined heart‐liver transplants: a case series review. Paediatr Anaesth 2016; 26: 976–986. [DOI] [PubMed] [Google Scholar]


