Abstract
Studies with angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (ARBs) in patients with heart failure with preserved ejection fraction (HFpEF) have yielded inconsistent results. To conduct a systematic review and meta‐analysis of all evidence for ACE‐I and ARBs in patients with HFpEF, we searched PubMed, Ovid SP, Embase, and Cochrane database to identify randomized trials and observational studies that compared ACE‐I or ARBs against placebo or standard therapy in HFpEF patients. Random‐effect models were used to pool the data, and I 2 testing was performed to assess the heterogeneity of the included studies. A total of 13 studies (treatment arm = 8676 and control arm = 8608) were analysed. Pooled analysis of randomized trials for ACE‐I and ARBs (n = 6) did not show any effect on all‐cause mortality [relative risk (RR) = 1.02, 95% confidence interval (CI) = 0.93–1.11, P = 0.68, I 2 = 0%], while results from observational studies showed a significant improvement (RR = 0.91, 95% CI = 0.87–0.95, P = 0.005, I 2 = 81.5%). In pooled analyses of all studies, ACE‐I showed a reduction of all‐cause mortality (RR = 0.91, 95% CI = 0.87–0.95, P = 0.01). There was no reduction in cardiovascular mortality seen, but in pooled analysis of randomized trials, there was a trend towards reduced HF hospitalization risk (RR = 0.91, 95% CI = 0.83–1.01, I 2 = 0%, P = 0.074). These data suggest that ACE‐I and ARBs may have a role in improving outcomes of patients with HFpEF, underscoring the need for future research with careful patient selection, and trial design and conduct.
Keywords: Heart failure, Preserved ejection fraction, Angiotensin‐converting enzyme inhibitors, Angiotensin receptor blockers, Renin–angiotensin system
Introduction
Patients with heart failure with preserved ejection fraction (HFpEF) represent approximately half of all HF patients.1, 2 Although outcomes for these patients remain poor and similar to patients with HF with reduced ejection fraction (HFrEF), to date, there are no therapies known to improve outcomes in these patients.3, 4 As such, even a therapy for patients with HFpEF that provides only modest benefit may meet an important unmet need. Hypertension, diabetes mellitus, and chronic kidney diseases are highly prevalent and are implicated in development and progression of HFpEF. All these comorbidities benefit from treatment with angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (ARBs). Angiotensin‐converting enzyme inhibitor and ARB also improve outcomes in patients with HFrEF. Thus, it stands to reason that these drugs may benefit HFpEF as well. However, clinical trials5, 6, 7 in HFpEF did not replicate benefits seen in HFrEF with ACE‐I and ARB therapy, and multiple explanations for these discordant results have been proposed. There remains ongoing debate regarding patient selection, crossover rates, low event rates, and open‐label use of investigational agents in these trials; all factors potentially diminishing the power to show a difference.8 In contrast to trial data, observational data, however, suggest potential benefit with ACE‐I and ARB in HFpEF.9, 10 Prior systematic reviews and meta‐analyses studying the role of ACE‐I and ARBs in HFpEF have not included all evidence.11, 12, 13 In an attempt to pool all the evidence quantitatively and qualitatively, we conducted this systematic review and meta‐analysis of randomized clinical trials and observational studies to better understand the effect of ACE‐I and ARBs on outcomes in HFpEF.
Methods
An extensive literature search was conducted utilizing Medline (PubMed and Ovid SP, Embase, and Cochrane Central Register of Controlled Clinical Trials). A variety of search terms as Medical Subject Headings and keywords were employed including ‘heart failure with preserved ejection fraction, HFpEF, diastolic HF, HF with normal ventricular systolic function, persevered cardiac function HF, angiotensin converting enzyme inhibitors, ACE inhibitors, ACE‐I, angiotensin receptor blockers, ARBs, heart failure, enalapril, quinapril, imidapril, delapril, lisinopril, ramipril, perindopril, captopril, Irbesartan, valsartan, candesartan’, and a combination of all these terms. Original research including both prospective observational (prospective cohort and nested case control studies) and randomized controlled trials was selected. The search was conducted from the inception of these databases till January 2016. Only articles in English language were considered. To ensure no article was missed, we also hand searched the references of all pertinent retrieved articles.
Two independent reviewers carefully viewed all the retrieved publications. The exclusion criteria were as follows: (i) patients who had transient symptoms following a recent illness, (ii) studies that did not provide adequate details for clinical endpoints, (iii) report with less than 10 patients, (iv) single‐arm studies, (v) editorials or review articles, (vi) subgroup analysis or interim analysis of landmark articles, and (vii) heart transplant patients. The inclusion criteria included comparison of ACE‐I or ARBs against placebo or standard therapy and a minimum of 3 month follow‐up. Any disagreement was solved by mutual consensus.
Two authors extracted and verified the data. A standardized data collection form was used to extract data from each study. In case of any discrepancy, the original reference article was reviewed again. The following information was extracted: first author's name, year of publication, study design, sample size, name of drug used, baseline patient demographics, primary endpoints, secondary endpoints, and duration of follow‐up.
Quality assessment of studies was performed through Jadad scale.14 Hazard ratios, risk ratios, and odds ratios were assumed to approximate the same measure of relative risk (RR) across studies. Relative risks with 95% confidence interval (CI) were used as the common measures of association. Summary RRs were pooled using a random‐effects meta‐analysis. Statistical heterogeneity across studies was quantified using Cochran χ 2 and the I 2 statistics, respectively. Clinical endpoints used in our analysis were all cause mortality, HF hospitalization, cardiovascular death, total hospitalizations, and composite endpoint of HF hospitalization and all‐cause mortality. We assessed the potential for publication bias through formal tests, namely, Begg's funnel plots and Egger's regression symmetry test.15 All statistical tests were two‐sided and used a significance level of P < 0.05. All data were analysed using STATA 10 (Stata Corporation, Lakeway Drive, College Station, TX, USA).
Results
A total of 13 studies were included,5, 6, 7, 10, 16, 17, 18, 19, 20, 21, 22, 23, 24 cumulatively representing 8676 patients in the treatment and 8608 in the control groups. The literature search strategy is highlighted in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow sheet (Figure 1 ). Seven studies were with ACE‐I,6, 17, 18, 19, 20, 21, 23 four on ARBs,5, 7, 22, 24 and two10, 16 used both. Of these, six10, 17, 18, 20, 21, 24 were observational studies. Yi et al.16 reported data separately for ACE‐I and ARBs making it a total of 14 entries in Table 1. Studies were generally of good quality with a mean Jadad score of 4. No evidence of publication bias was noted as shown by the funnel plot (Figure 2 ). Egger's test value for publication bias was P = 0.69. The demographic and clinical characteristics of all studies are shown in Table 1. Definition of HFpEF varied among studies. The average follow‐up period was 24.8 months (3–72 months). The mean age of the pooled sample was 79 years, and 42% of patients were male.
Figure 1.
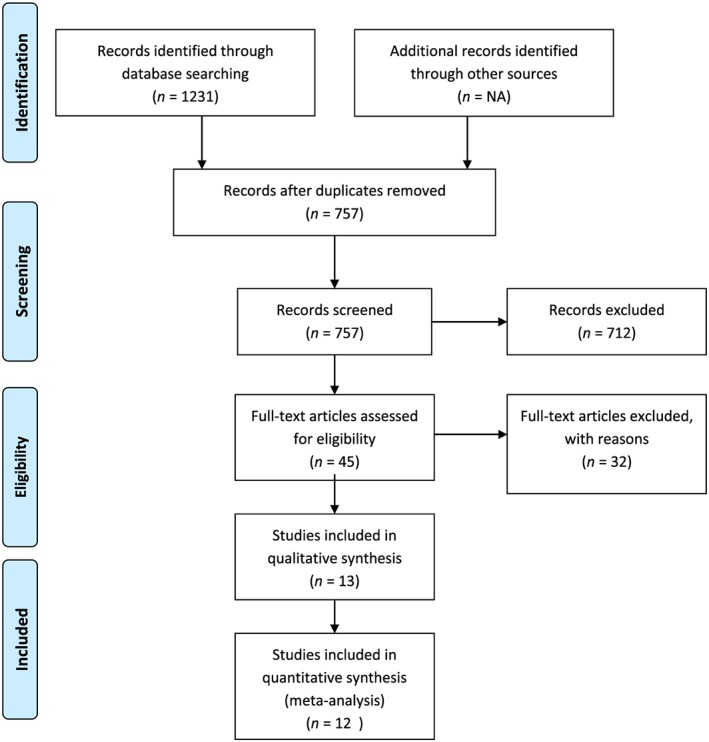
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram showing detailed search strategy.
Table 1.
Baseline characteristics of studies included in the meta‐analysis
| Author | Publication year | Design | Treatment | Sample | Age (years) | Male (%) | Follow‐up (months) | Mean LVEF | Number of deaths | HFpEF definition | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Philbin et al.17 | 1997 | Cohort | ACE‐I | 190/160 | 75 | 43 | 6 | 51 | 53 | 40 | 3 |
| Philbin et al.18 | 2000 | Cohort | ACE‐I | 284/245 | 75 | 45 | 6 | 50.4 | 79 | 40 | 3 |
| Grigorian et al.20 | 2006 | Cohort | ACE‐I | 210/206 | 73 | 49 | 30 | — | 210 | 50 | 3 |
| Tribouilloy et al.21 | 2007 | Cohort | ACE‐I | 120/120 | 76 | 52 | 60 | 62.6 | 131 | 50 | 4 |
| Lund et al.10 | 2012 | Cohort | ACE‐I/ARBs | 3329/3329 | 79 | 47 | 20 | — | 3194 | 40 | 5 |
| Patel et al.24 | 2012 | Cohort | ARBs | 296/296 | 80 | 31 | 72 | 55 | 375 | 40 | 4 |
| Cleland et al.6 | 2006 | RCT | ACE‐I | 424/426 | 75 | 45 | 26 | 64.5 | 109 | 40 | 6 |
| Yip et al.16 | 2008 | RCT | ACE‐I | 45/50 | 73 | 41 | 12 | 65.6 | 3 | 45 | 3 |
| Yip et al.16 | 2008 | RCT | ARBs | 56/50 | 74 | 38 | 12 | 67.4 | 4 | 45 | 3 |
| Kitzman et al.23 | 2010 | RCT | ACE‐I | 35/36 | 69 | 15 | 12 | 65 | — | 50 | 6 |
| Zi et al.19 | 2003 | RCT | ACE‐I | 36/38 | 78 | 44 | 6 | 58.6 | 2 | 40 | 4 |
| Massie et al.7 | 2008 | RCT | ARBs | 2067/2061 | 72 | 40 | 49 | 59.5 | 881 | 45 | 5 |
| Yusuf et al.5 | 2003 | RCT | ARBs | 1514/1509 | 67 | 60 | 8 | 54 | 481 | 40 | 6 |
| Parthasarathy et al.22 | 2009 | RCT | ARBs | 70/82 | 62 | 51 | 3 | 71 | — | 40 | 4 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARBs, angiotensin receptor blockers; HFPEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction; RCT, randomized clinical trial.
Figure 2.
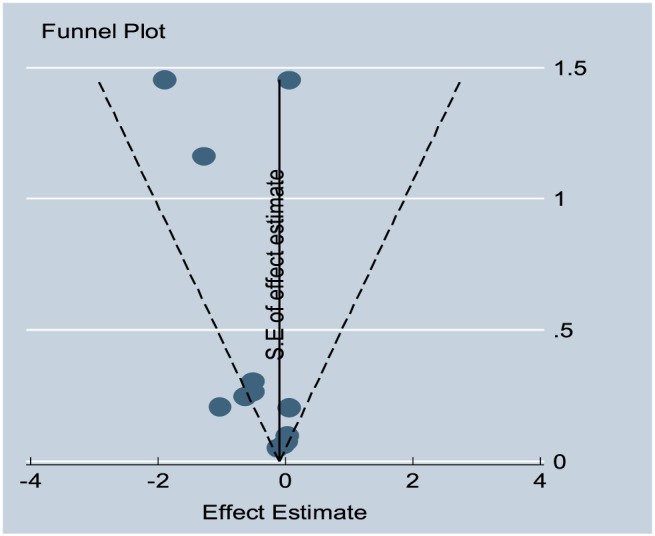
Funnel plot representing publication bias for all‐cause mortality.
Pooled analysis of randomized trials did not show an improvement in all‐cause mortality (RR = 1.02, 95% CI = 0.93–1.11, P = 0.68, I 2 = 0%), while results from observational studies showed a benefit (RR = 0.91, 95% CI = 0.87–0.95, P = 0.005, I 2 = 81.5%). When results of randomized trials and observational studies were pooled (Figure 3 A), ACE‐I and ARBs were found to modestly reduce all‐cause mortality (RR = 0.94, 95% CI = 0.90–0.98, P = 0.01, I 2 = 67.5%). After removing the four studies with ARB, the significant lower mortality with ACE‐I remained (RR = 0.91, 95% CI = 0.87–0.95, P = 0.01, I 2 = 75.1%) (Figure 3 B).
Figure 3.
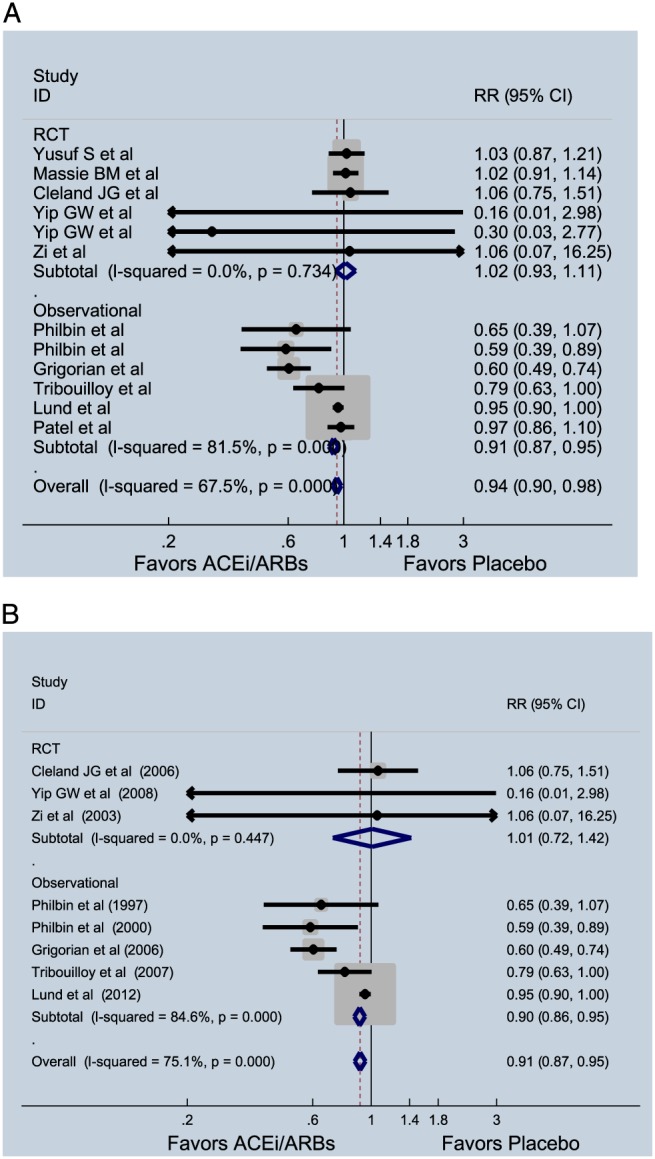
(A) Effect of angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (RCBs) on all‐cause mortality in patients with heart failure with preserved ejection fraction. (B) Effect of ACE‐Is on all‐cause mortality in patients with heart failure with preserved ejection fraction. CI, confidence interval; RCT, randomized clinical trial; RR, relative risk.
Six studies consisting of 8626 patients (treatment = 4332 and control = 4294) reported data on cardiovascular mortality. ACE‐I and ARBs did not benefit cardiovascular mortality (RR = 1.00, 95% CI = 0.90–1.12, P = 0.953, I 2 = 0%) (Figure 4 ). Of these, five were randomized trials that showed similar results (RR = 1.01, 95% CI = 0.90–1.13, P = 0.906, I 2 = 0%). When only ACE‐I studies were considered, no significant reduction in cardiovascular mortality was noted, (RR = 0.90, 95% CI = 0.63–1.28, P = 0.543, I 2 = 0%).
Figure 4.
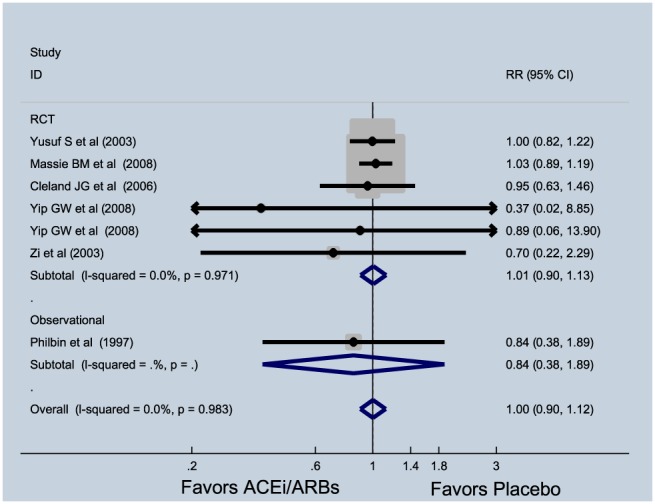
Effect of angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (RCBs) on cardiovascular mortality in patients with heart failure with preserved ejection fraction. CI, confidence interval; RCT, randomized clinical trial; RR, relative risk.
Pooled analysis of randomized trials showed a trend towards reduced HF hospitalization risk (RR = 0.91, 95% CI = 0.83–1.01, I 2 = 0%, P = 0.074). Three observational studies reported data on HF hospitalization and did not show a significant association (RR = 0.99, 95% CI = 0.86–1.13, I 2 = 0%). Combined analyses of randomized and observational data found similar results (RR = 0.93, 95% CI = 0.86–1.01, I 2 = 0%, P = 0.11) (Figure 5 A). The effect of ACE‐I and ARBs on composite endpoint of death and HF hospitalization showed a non‐significant trend (RR = 0.95, 95% CI = 0.91–1.01, P = 0.085, I 2 = 0%) (Figure 5 B). In both cases, removing ARB studies did not meaningfully affect results (HF hospitalization: RR = 0.90, 95% CI = 0.72–1.14, P = 0.39, I 2 = 0%; death or HF hospitalization: RR = 0.93, 95% CI = 0.83–1.05, P = 0.25, I 2 = 0%). Angiotensin‐converting enzyme inhibitor and ARBs did not reduce all‐cause hospitalization (RR = 0.99, CI = 0.96–1.02, P = 0.52, I 2 = 0%).
Figure 5.
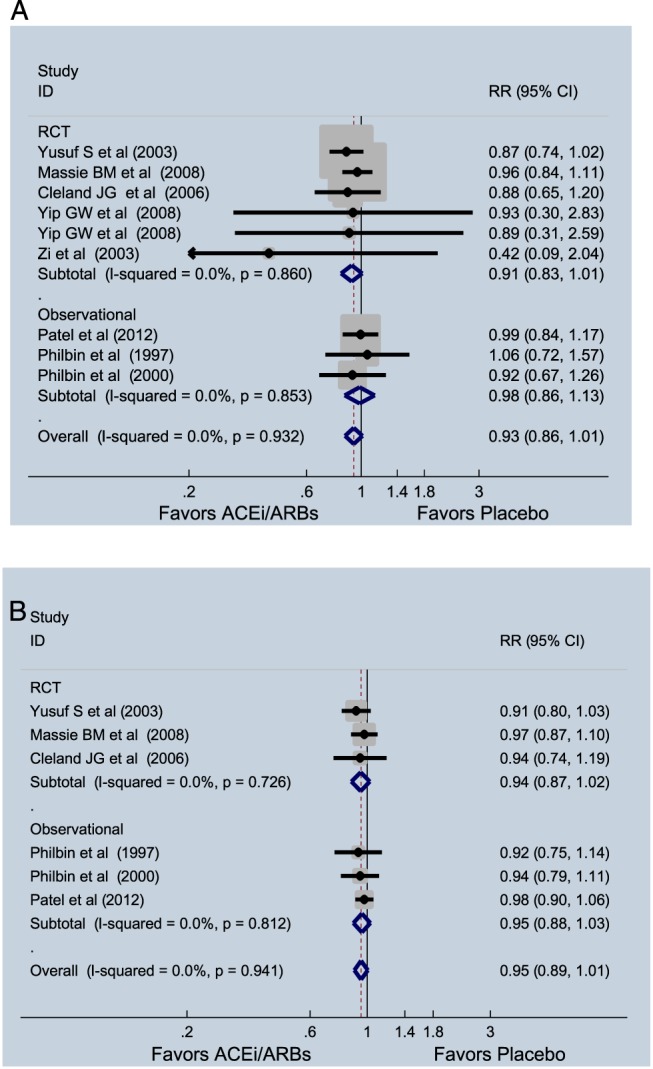
(A) Effect of angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (RCBs) on hospitalizations due to heart failure in patients with heart failure with preserved ejection fraction. (B) Effect of ACE‐I and RCBs on hospitalizations or mortality in patients with heart failure with preserved ejection fraction. CI, confidence interval; RCT, randomized clinical trial; RR, relative risk.
Discussion
Despite pathophysiologic rationale, randomized trials with ACE‐I and ARB in patients with HFpEF failed to improve outcomes. However, multiple issues other than the efficacy of the intervention may have confounded these results. In this systematic meta‐analysis that included both randomized trials and observational studies, we found that ACE‐I and ARBs were associated with a modest, but statistically significant, reduction in all‐cause mortality in HFpEF patients. The magnitude of benefit was larger when analysis was limited to ACE‐I. In randomized trials alone, this effect was not seen. In pooled analysis, although no overall benefit on cardiovascular mortality was seen, data limited to ACE‐I demonstrated a similarly modest reduction in risk, albeit statistically non‐significant and also driven by the results from observational studies. Similar trends were seen for HF hospitalization. While a systematic meta‐analysis does not obviate the aforementioned limitations of trials design and conducts issues with ACE‐I and ARB for patients with HFpEF or the general concerns with observational data, these results suggest that further, more refined approaches to studying these drugs in HFpEF may yield different conclusions.
The only large randomized trial (perindopril in elder people with chronic HF study) of ACE‐I in patients with HFpEF did not suggest any improvement in clinical outcomes.6 However, it is important to recognize the many trial factors that might have caused the therapy to fail. There was a very high dropout rate of almost 40% owing to a prolonged recruitment period. Moreover, almost one‐third of the patients also received open‐label ACE‐I after first year of follow‐up.25 This can potentially explain the lack of ACE‐I effect found in the latter part of the trial, in contrast to the positive signal earlier in the trial.
The two large randomized trials of ARBs (Candesartan in Heart Failure—Assessment of Mortality and Morbidity‐Preserved Trial and Irbesartan in Heart Failure with Preserved Systolic Function‐Preserved Trial) had heterogeneity in the enrolled patient population.5, 7 Both trials used lower EF threshold for HFpEF and neither used diastolic function as an entry criterion. In fact, in the Candesartan in Heart Failure—Assessment of Mortality and Morbidity‐Preserved Echocardiographic Substudy, nearly two‐thirds of patients had no or mild diastolic dysfunction, a feature generally believed as central to the HFpEF condition.26 Stage of the disease may also impact results because ARB was more effective in patients who with lower natriuretic peptide levels.27
Our pooled analysis did not reveal any effect of ACE‐I/ARB on cardiovascular mortality. As suggested by Fu et al.,12 the mortality benefit from renin–angiotensin modulation might arise from non‐cardiac benefit. Their analysis12 showed that ACE‐I did not lower all‐cause mortality in subset of patients >75 years indicating that old age and comorbidities might influence mortality rate in elderly. We also did not find any significant results with regard to all‐cause hospitalizations or readmission due to HF. This is in contrast to Zhang et al.11 who reported that these drugs reduce HF hospitalizations. Their pooled analysis consisted of spironolactone trials as well. Nevertheless, we also noted a trend, and our results trended significance with ACE‐I.
Because of paucity of data, we included non‐randomized studies that had small sample sizes with limited follow‐up. The studies included in our analysis had significant heterogeneity, and many studies did not provide any data on subsets of patients limiting our analysis to the overall cohort. In addition, we were unable to perform consistent multivariate adjustments across studies by combining models with the same set of potential confounders owing to our reliance on published data with variable levels of adjustments. Despite our efforts to provide results in a consistent manner, there remained heterogeneity among the available studies that require further investigation. Importantly, the observational data may be impacted by selection bias and confounding with may impact the results. Lastly, there were no uniform doses of ACE‐I and ARBs in all of the studies included that may have affected efficacy of the drugs.
These findings indicate that it may be important to further investigate ACE‐I and ARB in patients with HFpEF in prospective randomized clinical trials with longer follow‐ups, more defined population (ideally with higher event rates), and possibly stratified by phenotype or stage of disease progression. Also, such trials need to be large in size and avoid significant crossover all in an effort to be adequately powered.
Conflict of interest
G.C.F. has received research support from National Heart Lung Blood Institute and consults for Amgen, Bayer, Janssen, Novartis, and Medtronic. S.D.A. is a consultant for Amgen. M.G. has been a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, Corthera, Cytokinetics, Debiopharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, PeriCor Therapeutics, Protein Design Laboratories, Sanofi‐Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical, and Trevena Therapeutics. J.B. has received research support from the National Institutes of Health, European Union, and PCORI and served as a consultant for Amgen, Bayer, Boehringer Ingelheim, BMS, CVRx, Gilead, Medtronic, Novartis, Relypsa, and Stealth Peptide.
Khan, M. S. , Fonarow, G. C. , Khan, H. , Greene, S. J. , Anker, S. D. , Gheorghiade, M. , and Butler, J. (2017) Renin–angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Failure, 4: 402–408. doi: 10.1002/ehf2.12204.
References
- 1. Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 2005; 47: 320–332. [DOI] [PubMed] [Google Scholar]
- 2. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013; 10: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owan TE, Do H, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 4. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 6. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, PEP‐CHF Investigators . The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J 2006; 27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 7. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I‐PRESERVE Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 8. McMurray J. Renin angiotensin blockade in heart failure with preserved ejection fraction: the signal gets stronger. Eur Heart J 2006; 27: 2257–2259. [DOI] [PubMed] [Google Scholar]
- 9. Mujib M, Patel K, Fonarow GC, Kitzman DW, Zhang Y, Aban IB, Ekundayo OJ, Love TE, Kilgore ML, Allman RM, Gheorghiade M, Ahmed A. Angiotensin‐converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med 2013; 126: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lund LH, Benson L, Dahlström U, Edner M. Association between use of renin‐angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012; 308: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Q, Chen Y, Liu Q, Shan Q. Effects of renin‐angiotensin‐aldosterone system inhibitors on mortality, hospitalization, and diastolic function in patients with HFpEF: a meta‐analysis of 13 randomized controlled trials. Herz 2016; 41: 76–86. [DOI] [PubMed] [Google Scholar]
- 12. Fu M, Zhou J, Sun A, Zhang S, Zhang C, Zou Y, Fu M, Ge J. Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction—a meta analysis of 7 prospective clinical studies. Int J Cardiol 2012; 155: 33–38. [DOI] [PubMed] [Google Scholar]
- 13. Rain C, Rada G. Are angiotensin‐converting enzyme inhibitors or angiotensin 2 receptor antagonists effective in heart failure with preserved ejection fraction? Medwave 2015; 15: e6101. [DOI] [PubMed] [Google Scholar]
- 14. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 15. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 2008; 94: 573–580. [DOI] [PubMed] [Google Scholar]
- 17. Philbin EF, Rocco TA. Use of angiotensin‐converting enzyme inhibitors in heart failure with preserved left ventricular systolic function. Am Heart J 1997; 134: 188–195. [DOI] [PubMed] [Google Scholar]
- 18. Philbin EF, Rocco TA, Lindenmuth NW, Ulrich K, Jenkins PL. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin converting enzyme inhibitors. Am J Med 2000; 109: 605–613. [DOI] [PubMed] [Google Scholar]
- 19. Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther 2003; 17: 133–139. [DOI] [PubMed] [Google Scholar]
- 20. Grigorian LG, Roman AV, Ramos PM, Veloso PR, Bandin Dieguez MA, Gonzalez‐Juanatey JR. Angiotensin‐converting enzyme inhibitors prescription is associated with longer survival among patients hospitalized for congestive heart failure who have preserved systolic function: a long‐term follow‐up study. J Card Fail 2006; 12: 128–133. [DOI] [PubMed] [Google Scholar]
- 21. Tribouilloy C, Rusinaru D, Leborgne L, Peltier M, Massy Z, Slama M. Prognostic impact of angiotensinconverting enzyme inhibitor therapy in diastolic heart failure. Am J Cardiol 2008; 101: 639–644. [DOI] [PubMed] [Google Scholar]
- 22. Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM. A randomized, double‐blind, placebo‐controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail 2009; 11: 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, Little WC. Randomized double‐blind trial of enalapril in older patients with heart failure and preserved ejection fraction. Circ Heart Fail 2010; 3: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel K, Fonarow GC, Kitzman DW, Aban IB, Love TE, Allman RM, Gheorghiade M, Ahmed A. Angiotensin receptor blockers and outcomes in real‐world older patients with heart failure and preserved ejection fraction: a propensity‐matched inception cohort clinical effectiveness study. Eur J Heart Fail 2012; 14: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J 2014; 35: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 26. Persson H, Lonn E, Edner M, Investigators of the CHARM Echocardiographic Substudy—CHARMES . Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy—CHARMES. J Am Coll Cardiol 2007; 49: 687–694. [DOI] [PubMed] [Google Scholar]
- 27. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino‐terminal pro‐brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I‐PRESERVE trial. Circ Heart Fail 2011; 4: 569–577. [DOI] [PubMed] [Google Scholar]


