Abstract
Aims
We aimed to assess determinants of anorexia, that is loss of appetite in patients with heart failure (HF) and aimed to further elucidate the association between anorexia, functional capacity, and outcomes in affected patients.
Methods and results
We assessed anorexia status among 166 patients with HF (25 female, 66 ± 12 years) who participated in the Studies Investigating Co‐morbidities Aggravating HF. Anorexia was assessed by a 6‐point Likert scale (ranging from 0 to 5), wherein values ≥1 indicate anorexia. Functional capacity was assessed as peak oxygen uptake (peak VO2), 6 min walk test, and short physical performance battery test. A total of 57 patients (34%) reported any anorexia, and these patients showed lower values of peak VO2, 6 min walk distance, and short physical performance battery score (all P < 0.05). Using multivariate analysis adjusting for clinically important factors, only high‐sensitivity C‐reactive protein [odds ratio (OR) 1.24, P = 0.04], use of loop diuretics (OR 5.76, P = 0.03), and the presence of cachexia (OR 2.53, P = 0.04) remained independent predictors of anorexia. A total of 22 patients (13%) died during a mean follow‐up of 22.5 ± 5.1 months. Kaplan‐Meier curves for cumulative survival showed that those patients with anorexia presented higher mortality (Log‐rank test P = 0.03).
Conclusions
Inflammation, use of loop diuretics, and cachexia are associated with an increased likelihood of anorexia in patients with HF, and patients with anorexia showed impaired functional capacity and poor outcomes.
Keywords: Heart failure, Anorexia, Cachexia, Functional capacity
Introduction
Heart failure (HF) is a complex clinical syndrome and carries a poor prognosis, and this syndrome is progressive and characterized by worsening quality of life. Importantly, the prevalence of HF increases dramatically with age, and therefore, elderly people represent over 80% of all HF patients.1 The majority of elderly HF patients presents with co‐morbidities. Co‐morbidities are generally considered to have strong impact on physical function, quality of life, and survival of patients with HF in late life.
Muscle wasting is not only a problem in the elderly but also a co‐morbidity of chronic diseases such as HF.2 Our group has recently demonstrated that muscle wasting, as defined using the criteria for sarcopenia, is present in nearly 20% of ambulatory HF patients and that affected HF patients have an impaired physical function.3, 4 In addition, cachexia, associated with various pathophysiological mechanisms including neuroendocrine abnormalities, inflammatory system activation, increased lipolysis, and muscle wasting, affects about 10‐15% of patients with HF, and its presence is also an independent predictor of poor overall prognosis.5, 6 Overall, a wasting continuum from sarcopenia to cachexia has been proposed to occur in patients with advanced HF.7, 8
Currently, it is acknowledged that the term malnutrition suggests a nutritional problem or failure, according to the combination of loss of food intake with age‐related changes and other HF‐related co‐morbidities. Anorexia, defined as the loss of desire to eat, is a multifactorial process, despite caloric deprivation is frequently seen in HF patients.9, 10 Anorexia is also reported as an independent and strong predictor of morbidity and mortality among patients in various clinical settings.11 In oncology patients, the anorexia–cachexia syndrome is diagnosed as weight loss accompanied by anorexia, associated with impaired functional capacity and worse outcomes.12 Despite the growing importance of anorexia, cachexia, and functional capacity for quality of life, there is still a lack of information explaining whether and how they relate to each other in patients with HF.13 However, the reasons for anorexia are multifactorial; drug therapy, hepatic or gastrointestinal congestion and gastrointestinal dysfunction, and metabolic disturbance could affect appetite. Therefore, the determinants of anorexia and influence of anorexia on functional capacity and outcome are of outstanding interests in patients with HF.
Using data from the Studies Investigating Co‐morbidities Aggravating HF (SICA‐HF), a prospective, observational study into the co‐morbidities of chronic HF,14 we aimed to investigate predictors of anorexia and to assess the association with functional capacity and outcomes in ambulatory patients with HF.
Methods
Study design and patients
Between February 2010 and March 2014, we enrolled clinically stable 328 ambulatory patients into SICA‐HF at Charité‐Universitätsmedizin Berlin, Campus Virchow‐Klinikum, Berlin, Germany, 166 of whom answered a 6‐point Likert scale to assess the presence of anorexia. The study was funded by the European Commission's 7th Framework programme (FP7/2007–2013) under grant agreement number 241558 and fulfils all principles of the Declaration of Helsinki. SICA‐HF is registered under the ClinicalTrials.gov unique identifier: NCT01872299. Clinically stable ambulatory patients with chronic HF were eligible if they satisfied the following criteria: age > 18 years old and clinical signs and symptoms of chronic HF with (i) a left ventricular ejection fraction (LVEF) ≤ 40%, (ii) a left atrial dimension > 2.5 cm/m height, (iii) a left atrial dimension > 4.0 cm, or (iv) N terminal pro brain natriuretic peptide > 400 pg/mL or BNP > 150 pg/mL. Patients with previous heart transplantation or a history of unstable angina, myocardial infarction, stroke, cardiovascular revascularization, or open abdominal surgery within 6 weeks prior to enrolment were excluded.14 In the SICA‐HF study protocol, patients were divided according to LVEF into HF with reduced ejection fraction with LVEF ≤ 40% and HF with LVEF > 40% and a left atrial dimension > 4.0 cm (HF with preserved ejection fraction).3, 14 In patients with HF, medications consisted of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, digoxin, diuretics including loop diuretics and thiazides, aspirin, statins, and amiodarone in varying combinations.
Evaluation of anorexia
A single‐item measure was used to assess the presence of anorexia in all participants.15, 16 Subjects were asked to answer the question ‘Do you have appetite loss?’ (rated as 6‐point Likert scale 0 = not at all, 1 = very rarely, 2 = rarely, 3 = occasionally, 4 = frequently, and 5 = very frequently with higher number indicating increased frequency of anorexia). A cut‐off value ≥ 1 on the anorexia scale was used to define anorexia, because the response option ‘very rarely’ indicates that patients experience their appetite to be different from normal (‘not at all’), which was described by Blauwhoff‐Buskermolen.16
Nutritional assessment
The Nutritional Risk Index (NRI), an easily calculated measure that incorporates albumin and body size, predicts mortality in ambulatory and hospitalized HF patients.17, 18, 19 NRI was calculated as NRI = (1.519 × serum albumin, g/dL) + {41.7 × present weight (kg)/usual weight (kg)} as previously described. The ideal body weight (IBW) was used as usual weight, and calculated with the Devine formula for men [IBW (kg) = 50 kg + 2.3 kg for each inch of 110 height >5 ft] and the Robinson formula for women [IBW (kg) = 48.67 kg + 1.65 kg for each inch of height >5 ft].20 As in other previous studies, in patients with present weight greater than IBW, we set this ratio =1.21
Body composition and assessment of muscle wasting (sarcopenia)
Dual‐energy X‐ray absorptiometry was used to evaluate body composition such as total and appendicular lean and fat mass. Appendicular lean mass was defined as the lean mass of both arms and legs combined.22, 23 A scanner model ‘Lunar Prodigy’ and ‘Lunar en core 2002’ software was used to analyse the data (GE Medical Systems, Madison, WI, USA). Muscle wasting was defined according to previously published criteria for sarcopenia,24 that is appendicular skeletal muscle mass 2 standard deviations below the mean of a healthy young reference group aged 18–40 years old. The appendicular muscle mass index was calculated as the lean mass of both arms and legs combined divided by the height in metre squared.22
Evaluation of cachexia
Body weight was measured with light clothes without shoes. Body weight change history up to 24 months prior to enrolment was documented based on medical reports or patients' personal statements. Cachexia was defined according to the current consensus‐based diagnostic criteria.25 Hence, the diagnosis included the presence of non‐oedematous, non‐intentional weight loss of ≥5% over a period of at least 6 months.
Functional capacity assessment
The maximal muscle strength was assessed by handgrip strength measured by a handgrip dynamometer. The best of three measurements was used. The Short Physical Performance Battery (SPPB) test was performed according to standard protocol.26 SPPB, a highly standardized geriatric test, consists of tests for balance, gait, strength, and endurance and evaluates the ability to stand with both feet together side‐by‐side, semi‐tandem, and tandem positions, time to walk 4 m, and time to rise from a chair and return to the seated position 5 times. SPPB scores range from 0 to 12 points, and lower SPPB scores indicate impaired mobility. In addition, all patients underwent treadmill cardiopulmonary exercise testing (spiroergometry) according to the modified Bruce protocol.27 The modified Naughton protocol was used in selected patients.28 A 6 min walk test was performed according to standard protocol29 as previously described in the SICA‐HF study.3
Statistical analysis
Data are presented as percentages, mean ± standard deviation. For all statistical analyses, IBM Statistical Package for the Social Sciences (spss) version 24.0 (IBM Co., Armonk, NY, USA) was used. Categorical data were analysed using Pearson's χ2 test or Fisher's exact test as appropriate, and parametric variables were analysed by Student's unpaired t‐test. Predictors of anorexia were evaluated by binary logistic regression analysis. To estimate the influence of anorexia on survival, Kaplan–Meier cumulative survival curves were constructed for illustrative purposes and compared by the Mantel–Haenzel log‐rank test. Moreover, the relationship of anorexia and cachexia with survival was assessed by Cox proportional hazard analysis (single predictor and multivariable analysis). Hazard ratio (HR) and 95% confidence interval (CI) for risk factors and significance level were used. A value of P < 0.05 was considered to indicate statistical significance in all analyses.
Results
Patient characteristics
Baseline characteristics of the study population are displayed in Table 1. We enrolled 166 patients with clinically stable HF patients (25 female patients, 66 ± 12 years, LVEF 33.4 ± 9.9%, New York Heart Association class 2.3 ± 0.7, and 87.3% of whom presented with HF with reduced ejection fraction and the remaining with HF with preserved ejection fraction). Out of 166 total patients with HF, 57 patients (34%) reported any anorexia (Figure 1). Patients with anorexia were those who had more advanced HF according to their functional class assessment (P = 0.008), higher impairment of their LVEF (P = 0.011), increased levels of high sensitive C‐reactive protein (P = 0.004) and serum creatinine (P = 0.009), were more likely to present with concomitant chronic kidney disease (P = 0.011) and cachexia (P = 0.013). However, no significant difference was found with regard to albumin level (37.0 ± 3.2 vs. 37.1 ± 4.2; P = 0.91) and NRI score (89.7 ± 9.0 vs. 92.4 ± 9.7; P = 0.64) in patients with vs. without anorexia. Moreover, body composition analysis also showed no significant differences in fat mass and fat‐free mass between the two groups. In addition, compared with patients without anorexia, anorexic patients were more likely to be on diuretic medication (75.5% vs. 96.5%, P = 0.001) and loop diuretics usage (56.9% vs. 78.9%, P = 0.006). The presence of muscle wasting without weight loss (sarcopenia) was not significantly different between anorexic and non‐anorexic patients. However, the patients suffering from both anorexia and cachexia showed higher prevalence of muscle wasting (patients with both anorexia and cachexia 40%, anorexia 13.9%, cachexia 10.0%, and neither anorexia nor cachexia 11.2%).
Table 1.
Clinical characteristics
| No anorexia (n = 109) | Anorexia (n = 57) | P‐value | |
|---|---|---|---|
| Age (years) | 65.8 ± 10.5 | 68.9 ± 10.3 | 0.067 |
| Female (n, %) | 17.3 | 14.0 | 0.663 |
| NYHA class I/II/III/IV (n) | 17/55/37/1 | 1/23/32/1 | 0.008 |
| BMI (kg/m2) | 29.2 ± 5.0 | 27.8 ± 5.4 | 0.084 |
| Obesity (%) | 38.2 | 30.4 | 0.077 |
| Ischaemic HF (%) | 60.0 | 61.4 | 1.000 |
| LVEF (%) | 34.8 ± 9.7 | 30.6 ± 10.1 | 0.011 |
| Co‐morbidity | |||
| Hypertention (%) | 68.2 | 63.2 | 0.293 |
| Hyperlipidaemia (%) | 71.1 | 67.3 | 0.701 |
| Diabetes (%) | 40.0 | 29.8 | 0.236 |
| CKD (%) | 30.9 | 52.6 | 0.011 |
| Anaemia (%) | 27.3 | 40.4 | 0.115 |
| Laboratory findings | |||
| High sensitive C‐reactive protein (mg/dL) | 2.2 ± 1.6 | 3.1 ± 2.2 | 0.004 |
| Creatinin (mg/dL) | 1.2 ± 0.4 | 1.4 ± 0.5 | 0.009 |
| Haemoglobin (g/dL) | 13.5 ± 1.5 | 13.2 ± 1.7 | 0.362 |
| Albumin (mg/dL) | 37.1 ± 4.2 | 37.0 ± 3.2 | 0.911 |
| Na (mEq/L) | 141.1 ± 3.6 | 140.5 ± 4.6 | 0.384 |
| K (mEq/L) | 4.5 ± 0.6 | 4.4 ± 0.6 | 0.683 |
| Cl (mEq/L) | 103.0 ± 3.7 | 101.5 ± 4.3 | 0.024 |
| NRI (points) | 92.4 ± 9.7 | 89.7 ± 9.0 | 0.635 |
| Medication | |||
| ACE inhibitor (%) | 68.8 | 61.4 | 0.394 |
| ARB (%) | 49.5 | 54.4 | 0.625 |
| β‐blocker (%) | 94.5 | 91.2 | 0.546 |
| Digoxin (%) | 10.1 | 14.0 | 0.450 |
| Diuretics (%) | 76.1 | 96.5 | 0.001 |
| Loop diuretics (%) | 56.9 | 78.9 | 0.006 |
| Thiazides (%) | 30.3 | 31.6 | 0.861 |
| Aspirin (%) | 74.3 | 61.4 | 0.114 |
| Statin (%) | 72.5 | 68.4 | 0.721 |
| Amiodarone (%) | 11.9 | 15.8 | 0.478 |
| Functional capacity | |||
| Handgrip strength (kg) | 37.9 ± 12.0 | 35.8 ± 11.8 | 0.063 |
| SPPB (point) | 10.8 ± 2.0 | 10.4 ± 1.6 | 0.047 |
| 6 min walk test (m) | 439.0 ± 144.4 | 403.2 ± 162.9 | 0.010 |
| Peak VO2 (mL/min/kg) | 18.3 ± 5.1 | 15.2 ± 4.1 | 0.001 |
| Sarcopenia (%) | 10.9 | 21.1 | 0.064 |
| Cachexia (%) | 18.2 | 36.8 | 0.013 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, Body mass index; Cl, chloride; CKD, Chronic kidney disease; K, potassium; LVEF, left ventricular ejection fraction; Na, sodium; NYHA, New York Heart Association; NRI, Nutritional Risk Index; peak VO2, peak oxygen uptake; SPPB, short physical performance battery; β‐blocker, beta‐adrenergic blocking agents.
Figure 1.
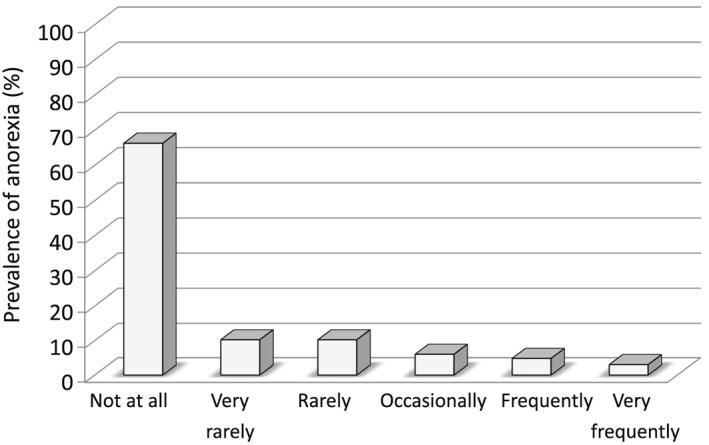
The prevalence of anorexia.
Association between anorexia and functional capacity
Functional capacity measurements are presented in Table 1. Regarding physical performance and functional capacity, patients who reported anorexia showed lower SPPB score (10.4 ± 1.6 vs. 10.8 ± 2.0 points; P = 0.047), 6 min walk distance (403.2 ± 162.9 vs. 439.0 ± 144.4 m; P = 0.010), and peak VO2 (15.2 ± 4.1 vs. 18.3 ± 5.1 mL/min/kg; P = 0.001).
Finally, we investigated the effects of cachexia among patients with or without anorexia. Figure 2 demonstrates the association between functional capacity and the presence of anorexia in the respective groups. The SPPB score, 6 min walk distance, and peak VO2 showed worse results among patients suffering from both anorexia and cachexia.
Figure 2.
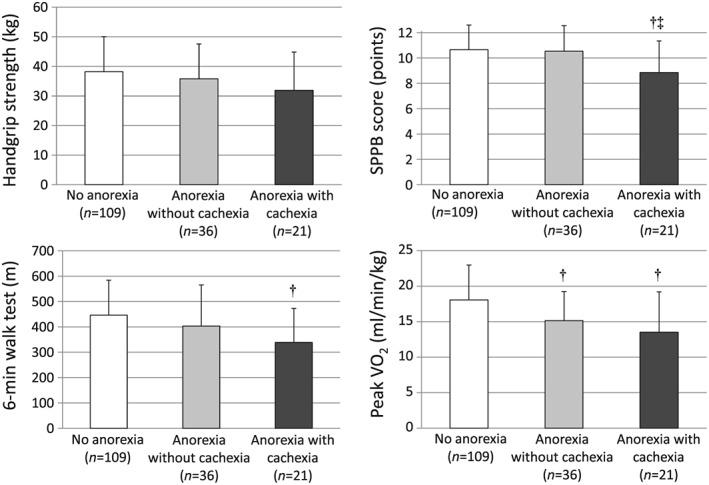
Impact of anorexia and cachexia on functional capacity in patients with heart failure. †P < 0.05 vs. no anorexia, ‡P < 0.05 vs. anorexia without cachexia. Peak VO2, peak oxygen consumption; SPPB, short physical performance battery.
Determinants of anorexia in patients with heart failure
Using logistic regression analysis with the presence of anorexia serving as the dependent variable, we found that NYHA class, LVEF, presence of chronic kidney disease, high sensitive C‐reactive protein, cachexia, and loop diuretics usage predicted the presence of anorexia in patients with HF (Table 2). Multivariate regression analysis showed that high sensitive C‐reactive protein [odds ratio (OR) 1.24, P = 0.04], loop diuretic use (OR 5.76, P = 0.03), and cachexia (OR 2.53, P = 0.04) remained independent predictors of anorexia in patients with HF (Table 2).
Table 2.
Logistic regression model with anorexia serving as the dependent variable
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Exp (Β) | P‐value | 95% CI | Exp (Β) | P‐value | 95% CI | |
| NYHA class [1 class increase] | 2.442 | 0.001 | 1.435–4.155 | 1.660 | 0.133 | 0.858–3.212 |
| LVEF [1% increase] | 0.955 | 0.013 | 0.920–0.990 | 0.997 | 0.905 | 0.956–1.041 |
| CKD [presence] | 2.418 | 0.009 | 1.250–4.678 | 1.803 | 0.172 | 0.774–4.201 |
| High sensitive C‐reactive protein [1 μg/L increase] | 1.275 | 0.012 | 1.056–1.539 | 1.240 | 0.043 | 1.007–1.527 |
| Cachexia [presence] | 2.596 | 0.010 | 1.258–5.357 | 2.532 | 0.042 | 1.036–6.190 |
| Loop diuretics [use] | 8.614 | 0.004 | 1.965–37.769 | 5.759 | 0.028 | 1.207–27.466 |
CI, confidence interval; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Impact of anorexia on cumulative survival rate in patients with heart failure
In the total population, 2‐year all‐cause mortality was 22 (13.3%) among clinically stable ambulatory HF patients. Our findings showed that all‐cause mortality was significantly higher in patients with any anorexia. The all‐cause mortality rate was 22.8% after a mean follow‐up of 22.5 ± 5.1 months (95% CI 2.336–6.634) in patients with anorexia when compared with 8.2% (95% CI 4.532–19.690) in patients without anorexia (P = 0.014). Kaplan–Meier curves for cumulative survival showed that those patients with anorexia presented higher mortality rate within 24 months (Log‐rank test P = 0.029, Figure 3) than those without. Similarly, the all‐cause mortality rate was 24.4% (95% CI 0.08–0.20) in patients with cachexia when compared with 9.6% (95% CI 0.11–0.38) in patients without cachexia (P = 0.03). Using univariate Cox analysis, it was shown that anorexia is associated with 2‐year all‐cause mortality (HR 2.466, 95% CI 1.065–5.709, P = 0.035). In multivariate Cox analysis, anorexia was not a significant independent predictor of 2‐year all‐cause mortality (adjusted HR 2.124, 95% CI 0.903–4.995, P = 0.054) after adjusting for potential confounders such as age, gender, LVEF, and NYHA class, unlike cachexia (adjusted HR 2.395, 95% CI 1.018–5.631, P = 0.045). We investigated the effects of cachexia among patients with or without anorexia. Multivariate Cox analysis showed that cachexia has additive effect on increasing mortality among HF patients with anorexia. Anorexic patients with cachexia showed increased mortality rates (adjusted HR: 2.856, 95% CI 1.032–7.902, P = 0.043) after adjusting for potential confounders such as age, gender, LVEF, and NYHA class (Table 3).
Figure 3.
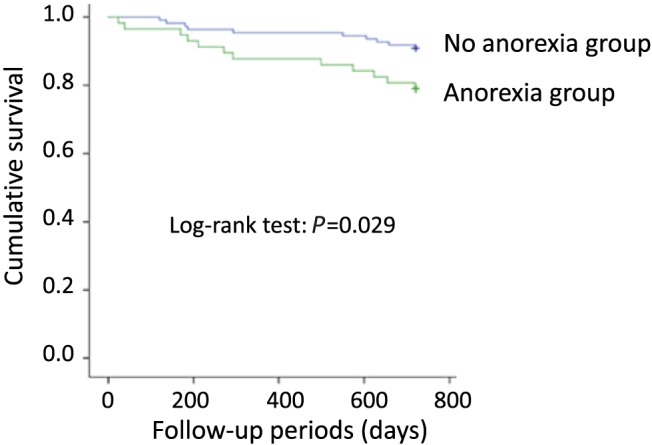
Kaplan–Meier survival curve.
Table 3.
Mortality risk according to the presence of anorexia and cachexia in heart failure patients
| Crude model | Adjusted modela | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| No anorexia | 1 (reference) | 1 (reference) | ||||
| Anorexia without cachexia | 1.903 | 0.492–7.360 | 0.351 | 1.986 | 0.501–7.872 | 0.329 |
| Anorexia with cachexia | 5.018 | 1.758–14.320 | 0.003 | 2.993 | 0.995–9.000 | 0.051 |
CI, confidence interval; HR, hazard ratio.
Adjusted model: adjusted for age, gender, left ventricular ejection fraction, and New York Heart Association class.
Discussion
In the present study, more than one‐third of clinically stable ambulatory patients with HF reported any anorexia. Using multivariable regression analysis, we identified an increased level of inflammation, loop diuretic use, and the presence of cachexia as major determinants of anorexia in patients with HF. Our findings demonstrate that patients with anorexia are more likely to present with impaired functional capacity and higher all‐cause mortality within 2 years. Moreover, cachexia showed additive effects of worsening functional capacity and poor outcomes in patients with HF and anorexia. Previous studies have shown that anorexia is not an unavoidable consequence of chronic disease, and it often promotes its development through various mechanisms.30
In the present study, cachexia was more frequently detected in patients with anorexia (36.8%) compared with those without (18.2%), and cachexia remained an independent predictor of anorexia in our cohort. The complex clinical nature of HF‐induced anorexia or HF‐induced cachexia suggested a multifactorial aetiology. In addition to cachexia, inflammation, medication side effects, depression, anxiety, sodium restricted diets, and chewing problem may also be found as cause of anorexia.31 Some patients therefore appear anorexia symptom before sign and symptoms of cachexia even though patients were not severe HF patients. In these cases, it may be suspected that anorexia inevitably leads to cachexia. On the other hand, some patients have cachexia despite maintaining a relatively normal appetite and nutritional intake.32 Therefore, elucidating the underlying mechanism for anorexia–cachexia syndrome was challenging in patients with HF. Currently, the anorexia–cachexia syndrome has been recognized as an adverse consequence of disease, and its treatment remains a clinical challenge in oncology patients.33 Anorexia–cachexia syndrome is often associated with the underlying disease process and related to both peripheral and central neurohormonal signals regulating both appetite and energy expenditure. Moreover, cachexia was related to intestinal congestion and can further worsen anorexia and impaired food intake in patients with HF.15, 34 An increase in circulating inflammatory cytokines including C‐reactive protein, which is the most frequently measured inflammatory marker is one of the pathogenic mechanism of anorexia and food intake in chronic disease.35 One of the targets of inflammatory mediators is the central nervous system, particularly on regulatory feeding centers in the hypothalamus sited in the ventral diencephalon. Recently, several studies explained the mechanisms by which inflammation reaches the hypothalamus and the neural substrates underlying inflammatory anorexia.36 Furthermore, pro‐inflammatory cytokines persistently activate pro‐opiomelanocortin neurons and inhibit neuropeptide Y neurons, and both are involved in the alteration of satiety and hunger signals (the clinical signs of anorexia and cachexia).37
Conventional HF treatment includes angiotensin‐converting enzyme inhibitors and beta‐blockers and reduces the risk of weight loss.38, 39 In this context, one of the most important issues for managing anorexia is to maintain optimal HF treatment. Moreover, energy‐protein nutritional supplement and essential amino acids were efficacious in body weight, muscle metabolism, and inflammatory system in patients with HF.40, 41 Recently, several agents such as ghrelin, megestrol acetate, medroxyprogesterone acetate, and synthetic cannabinoids are used clinically to stimulate appetite in patients with disease‐related wasting; however, these agents are very limited in patients with HF.42 However, another possible cause of anorexia could be a side effect of treatment of HF or HF‐induced wasting.43 Several HF treatments could contribute to inadequate food intake, especially overzealous diuretic use, which can cause anorexia in animal experiments.44 For instance, the use of loop diuretics is associated with up‐regulation of the activity of the renin–angiotensin–aldosterone system,45, 46 and higher doses of diuretics are associated with poor outcomes in patients with HF.47 Angiotensin II, the primary contributor of renin–angiotensin–aldosterone system, causes anorexia and reduces food intake in rodents.48 Long‐term use of diuretics in HF is also linked to the deficiency of micronutrients such as zinc.30, 49 Diuretics could stimulate the urinary excretion of zinc decreasing perception of taste, anorexia, and inadequate food intake.50 In addition, appetite stimulants, megestrol acetate and medroxyprogesterone acetate, were generally not recommended for patients with HF, because these appetite stimulants increase in weight gain and peripheral oedema caused by fluid retention.51 Similarly, synthetic cannabinoids have adverse side effects such as postural hypotension owing to reduced vascular resistance. For this reason, it is recommended to avoid usage of these appetite stimulants in patients with HF.
Several studies conducted in elderly patients with cancer and other chronic diseases showed that anorexia was associated with impaired muscle mass, functional capacity, increased risk of frailty, disability, and impaired quality of life.13, 52, 53 Landi and collaborators showed significantly lower SPPB score in anorexic patients compared with those without (−1.0 point, P = 0.03).52 Moreover, they clearly showed that these patients have remarkable decline in functional capacity and activities of daily living. Comparably, our findings also show declined functional capacity including SPPB score, distance of 6 min walk test, and peak VO2 in patients with HF and anorexia. The frequency of anorexia symptoms is associated with the presence of sarcopenia in our patients. In addition, cachexia showed an additive effect in worsening functional capacity in patients with HF and anorexia. Therefore, it is crucial to detect the presence of anorexia and cachexia to reduce co‐morbidities and to preserve functional capacity in patients with HF.
Another important finding of our study is the association between anorexia and increased mortality. Older nursing home residents with anorexia had a higher risk of death for all‐causes compared with non‐anorexic participants (HR 2.26, 95% CI 2.14–2.38).54 In chronic renal failure, mortality risk in anorexic patients was 4 to 5 times that of those without anorexia.55 Thus, several studies mentioned that anorexia is one of the predictors of mortality in elderly subjects and in patients with chronic disease.35, 56 However, there are few reports pointing out the association between anorexia and clinical outcome in patients with HF. In the unadjusted model of our findings, we observed that anorexia potentially correlated with poor clinical outcome in patients with HF. Moreover, patients with anorexia and cachexia were strongly associated with mortality risk after adjusting for potential confounders. Our findings have an important clinical implication, because our results may help to better understand the relationship between anorexia, cachexia and impaired functional capacity and poor outcomes in patients with HF.
There are limitations associated with our study. In order to diagnose anorexia, it is important to have valid and reliable instruments. Recently, two instruments with high face validity were recommended to diagnose anorexia in patients with cancer anorexia–cachexia syndrome.53 The first instrument is a yes/no question ‘Did you have a decrease appetite during the last month?’. The second instrument is anorexia symptom scale, which consists of one item of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐C30.57 On the other hand, there is no standard instrument to diagnose anorexia in patients with HF. Moreover, we did not collect the information of dietary intake, and therefore, we do not know whether the patients with anorexia have deficiency of energy or protein intake. Future research should focus on the definitions and instruments of anorexia and dietary intake in patients with HF.
Conclusions
The present study shows that inflammation, loop diuretic use, and cachexia are independent predictors of anorexia in patients with HF. Moreover, anorexia is associated with impaired functional capacity and increased mortality risk. Nutritional habits and status are frequently disregarded, and its evaluation should be included as a fundamental part in the overall assessment of ambulatory patients with HF.
Conflict of interest
S.v.H. has received consulting honoraria from Thermo Fisher Scientific, Roche, Novartis, Vifor Pharma, Respicardia, Pfizer, Chugai, Professional Dietetics, and Solartium Dietetics as well as lecture fees from Amgen and Novartis. S.D.A. reports receiving consulting fees from Alere, BRAHMS GmbH, Abbott laboratories, and Vifor Pharma, honoraria from Alere, BRAHMS GmbH, and Vifor Pharma, and research support from BRAHMS GmbH. All other authors do not have a conflict of interest to report.
Funding
The project was supported by the European Commission's 7th Framework programme (FP7/2003‐2013) under grant agreement number 241558; the Russian Ministry of Science and Education within the FTP ‘R&D in priority fields of the S&T complex of Russia 2007–2012’ under state contract number 02.527.11.0007; the Innovative Medicines Initiative – Joint Undertaking (IMI‐JU 115621); and The German Centre for Cardiovascular Disease (DZHK).
Saitoh, M. , dos Santos, M. R. , Emami, A. , Ishida, J. , Ebner, N. , Valentova, M. , Bekfani, T. , Sandek, A. , Lainscak, M. , Doehner, W. , Anker, S. D. , and von Haehling, S. (2017) Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). ESC Heart Failure, 4: 448–457. doi: 10.1002/ehf2.12209.
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013; 127: e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sente T, Van Berendoncks AM, Hoymans VY, Vrints CJ. Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle 2016; 7: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 4. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD, von Haehling S. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016; 222: 41–46. [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 6. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016; 7: 507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Haehling S. The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc 2015; 74: 367–377. [DOI] [PubMed] [Google Scholar]
- 8. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017; 14: 323–341. [DOI] [PubMed] [Google Scholar]
- 9. Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 2000; 8: 175–179. [DOI] [PubMed] [Google Scholar]
- 10. Invernizzi M, Carda S, Cisari C. Società Italiana per lo Studio della Sarcopenia e della Disabilità Muscolo‐Scheletrica (SISDIM). Possible synergism of physical exercise and ghrelin‐agonists in patients with cachexia associated with chronic heart failure. Aging Clin Exp Res 2014; 26: 341–351. [DOI] [PubMed] [Google Scholar]
- 11. Landi F, Laviano A, Cruz‐Jentoft A. The anorexia of aging: is it a geriatric syndrome? J Am Med Dir Assoc 2010; 11: 153–156. [DOI] [PubMed] [Google Scholar]
- 12. Tarricone R, Ricca G, Nyanzi‐Wakholi B, Medina‐Lara A. Impact of cancer anorexia‐cachexia syndrome on health‐related quality of life and resource utilisation: a systematic review. Crit Rev Oncol Hematol 2016; 99: 49–62. [DOI] [PubMed] [Google Scholar]
- 13. Morley JE. Anorexia, sarcopenia, and aging. Nutrition 2001; 17: 660–663. [DOI] [PubMed] [Google Scholar]
- 14. von Haehling S, Lainscak M, Doehner W, Ponikowski P, Rosano G, Jordan J, Rozentryt P, Rauchhaus M, Karpov R, Tkachuk V, Parfyonova Y, Zaritskey AY, Shlyakhto EV, Cleland JG, Anker SD. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). J Cachexia Sarcopenia Muscle 2010; 1: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murín J, Anker SD, Sandek A. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J 2016; 37: 1684–1691. [DOI] [PubMed] [Google Scholar]
- 16. Blauwhoff‐Buskermolen S, Ruijgrok C, Ostelo RW, de Vet HCW, Verheul HMW, de van der Schueren MAE, Langius JAE. The assessment of anorexia in patients with cancer: cut‐off values for the FAACT‐A/CS and the VAS for appetite. Support Care Cancer 2016; 24: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adejumo OL, Koelling TM, Hummel SL. Nutritional risk index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant 2015; 34: 1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aziz EF, Javed F, Pratap B, Musat D, Nader A, Pulimi S, Alivar CL, Herzog E, Kukin ML. Malnutrition as assessed by nutritional risk index is associated with worse outcome in patients admitted with acute decompensated heart failure: an ACAP‐HF data analysis. Heart Int 2011; 6: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Najjar Y, Clark AL. Predicting outcome in patients with left ventricular systolic chronic heart failure using a nutritional risk index. Am J Cardiol 2012; 109: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 20. Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother 2000; 34: 1066–1069. [DOI] [PubMed] [Google Scholar]
- 21. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 22. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 23. Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN Jr. Appendicular skeletal muscle mass: measurement by dual‐photon absorptiometry. Am J Clin Nutr 1990; 52: 214–218. [DOI] [PubMed] [Google Scholar]
- 24. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001; 137: 231–243. [DOI] [PubMed] [Google Scholar]
- 25. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr Edinb Scotl 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 26. Guralnik JM, Simonsick EM, Ferrucci L. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–M94. [DOI] [PubMed] [Google Scholar]
- 27. Bruce RA, Blackmon JR, Jones JW, Strait G. Exercise testing in adult normal subjects and cardiac patients. Pediatrics 1963; 32(Suppl): 742–756. [PubMed] [Google Scholar]
- 28. Naughton J, Sevellus G, Balke B. Physiologic responses of normal and pathologic subjects to a modified work capacity test. J Sports Med 1963; 31: 201. [PubMed] [Google Scholar]
- 29. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 30. Sandek A, Doehner W, Anker SD, von Haehling S. Nutrition in heart failure: an update. Curr Opin Clin Nutr Metab Care. 2009; 12: 384–391. [DOI] [PubMed] [Google Scholar]
- 31. Landi F, Lattanzio F, Dell'Aquila G, Eusebi P, Gasperini B, Liperoti R, Belluigi A, Bernabei R, Cherubini A. Prevalence and potentially reversible factors associated with anorexia among older nursing home residents: results from the ULISSE project. J Am Med Dir Assoc 2013; 14: 119–124. [DOI] [PubMed] [Google Scholar]
- 32. Blum D, Omlin A, Fearon K, Baracos V, Radbruch L, Kaasa S, Strasser F, European Palliative Care Research Collaborative . Evolving classification systems for cancer cachexia: ready for clinical practice? Support Care Cancer 2010; 18: 273–279. [DOI] [PubMed] [Google Scholar]
- 33. Larkin M. Thwarting the dwindling progression of cachexia. Lancet 1998; 351: 1336. [DOI] [PubMed] [Google Scholar]
- 34. Hughes CM, Woodside JV, McGartland C, Roberts MJ, Nicholls DP, McKeown PP. Nutritional intake and oxidative stress in chronic heart failure. Nutr Metab Cardiovasc Dis 2012; 22: 376–382. [DOI] [PubMed] [Google Scholar]
- 35. Braun TP, Marks DL. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J Cachexia Sarcopenia Muscle 2010; 1: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Molfino A, Iannace A, Colaiacomo MC, Farcomeni A, Emiliani A, Gualdi G, Laviano A, Rossi Fanelli F. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite‐regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle 2017; 8: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martone AM, Onder G, Vetrano DL, Ortolani E, Tosato M, Marzetti E, Landi F. Anorexia of aging: a modifiable risk factor for frailty. Nutrients 2013; 5: 4126–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003; 361: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 39. Lainscak M, Laviano A. ACT‐ONE – ACTION at last on cancer cachexia by adapting a novel action beta‐blocker. J Cachexia Sarcopenia Muscle 2016; 7: 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar‐Zadeh K, Polonski L, Anker SD. The effects of a high‐caloric protein‐rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double‐blind pilot study. J Cachexia Sarcopenia Muscle 2010; 1: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aquilani R, Opasich C, Gualco A, Verri M, Testa A, Pasini E, Viglio S, Iadarola P, Pastoris O, Dossena M, Boschi F. Adequate energyprotein intake is not enough to improve nutritional and metabolic status inmuscle‐depleted patients with chronic heart failure. Eur J Heart Fail 2008; 10: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 42. von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther 2009; 121: 227–252. [DOI] [PubMed] [Google Scholar]
- 43. Dunn SP, Bleske B, Dorsch M, Macaulay T, Van Tassell B, Vardeny O. Nutrition and heart failure: impact of drug therapies and management strategies. Nutr Clin Pract 2009; 24: 60–75. [DOI] [PubMed] [Google Scholar]
- 44. Lundy RF Jr, Caloiero V, Bradley C, Liang NC, Norgren R. Furosemide‐induced food avoidance: evidence for a conditioned response. Physiol Behav 2004; 81: 397–408. [DOI] [PubMed] [Google Scholar]
- 45. He XR, Greenberg SG, Briggs JP, Schnermann J. Effects of furosemide and verapamil on the NaCl dependency of macula densa‐mediated renin secretion. Hypertension 1995; 26: 137–142. [DOI] [PubMed] [Google Scholar]
- 46. Bayliss J, Norell M, Canepa‐Anson R, Sutton G, Poole‐Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J 1987; 57: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007; 9: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin‐like growth factor I in rats through a pressor‐independent mechanism. J Clin Invest 1996; 97: 2509–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Witte KK, Clark AL. Micronutrients and their supplementation in chronic cardiac failure. an update beyond theoretical perspectives. Heart Fail Rev 2006; 11: 65–74. [DOI] [PubMed] [Google Scholar]
- 50. Pinho CPS, da Silveira AC. Nutritional aspects in heart failure. J Nutr Health Sci 2014; 1: 305. [Google Scholar]
- 51. Nelson KA. The cancer anorexia–cachexia syndrome. Semin Oncol 2000; 27: 64–68. [PubMed] [Google Scholar]
- 52. Landi F, Russo A, Liperoti R, Tosato M, Barillaro C, Pahor M, Bernabei R, Onder G. Anorexia, physical function, and incident disability among the frail elderly population: results from the ilSIRENTE study. J Am Med Dir Assoc 2010; 11: 268–274. [DOI] [PubMed] [Google Scholar]
- 53. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Group [SIG] “cachiexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010; 29: 154–159. [DOI] [PubMed] [Google Scholar]
- 54. Landi F, Lattanzio F, Dell’Aquila G, Eusebi P, Gasperini B, Liperoti R, Belluigi A, Bernabei R, Cherubini A. Prevalence and potentially reversible factors associated with anorexia among older nursing home residents: results from the ULISSE project. J Am Med Dir Assoc 2013; 14: 119–124. [DOI] [PubMed] [Google Scholar]
- 55. Kalantar‐Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 2004; 80: 299–307. [DOI] [PubMed] [Google Scholar]
- 56. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, Sisto A, Marzetti E. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients 2016; 8: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]


