Abstract
Aims
Intermittent levosimendan administration has been suggested to improve survival in patients with advanced heart failure (AdHF). Quality of life is a key issue for AdHF patients and is negatively affected by frequent hospitalizations.
Methods and results
CENTRAL, Google Scholar, MEDLINE/PubMed, Scopus, and the Cochrane Central Register of clinical trials (updated 15/1/2017) were searched for randomized controlled trials investigating the effect of intermittent levosimendan administration in patients with AdHF. The primary outcome was the number of patients requiring rehospitalization 3 months after the end of treatment. A total of 319 patients from six trials were included. Overall pooled analysis showed that the use of levosimendan was associated with a significant reduction in the number of rehospitalizations at 3 months: 33/207 (16%) vs. 39/113 (35%), risk ratio 0.40, 95% confidence interval 0.27–0.59, P < 0.001, I 2 = 0%. This result was confirmed by sensitivity analyses.
Conclusions
Within the limitations of this meta‐analysis including also studies in which endpoints were not independently adjudicated and not clearly specified, repetitive or intermittent administration of levosimendan for patients with AdHF was associated with a reduction in the rehospitalization rate at 3 months. Large, high‐quality randomized controlled trials are needed to confirm this finding.
Keywords: Advanced heart failure, Intermittent, Repetitive, Levosimendan, Meta‐analysis, Rehospitalization
Introduction
Use of the calcium‐sensitizer and inodilator levosimendan in intermittent cycles has been shown to benefit patients with advanced heart failure (AdHF). The largest single trial of this intervention so far completed is the Levo‐REP trial,1 in which administration of four cycles of levosimendan therapy (0.2 μg/kg/min for 6 h) at 2‐week intervals substantially improved event‐free survival, defined as freedom from death from all causes, heart transplantation/implantation of a ventricular assist device, or acute heart failure. Previous studies with intermittent levosimendan in a range of patient populations, including those with AdHF, documented a range of haemodynamic and neuro‐hormonal effects considered likely to be beneficial. In several instances, these were accompanied by an indication of improved survival that was amplified in two separate meta‐analyses.2, 3
These indications of a survival benefit from intermittent levosimendan in patients with AdHF offer a distinct contrast to experiences with traditional adrenergic inotropes such as dobutamine. Taken in conjunction with the proven utility of levosimendan in patients treated with beta‐blockers,4 plus its ability to confer positive inotropic, vasodilatory, and cardioprotective effects without significant increases in myocardial oxygen requirements,5, 6 this identifies levosimendan as a qualitatively distinct addition to the treatment options for late‐stage heart failure (HF).
For many patients with AdHF, however, quality of life (QoL) is as much a priority as duration of life. One of the larger negative influences on QoL is the need for repeated hospitalizations to stabilize a condition that is prone to clinical exacerbations and deterioration despite maximal use of first‐line therapies such as diuretics, beta‐blockers, and drugs that target the renin–angiotensin system. With this in mind, we undertook, as part of an ongoing evaluation of intermittent levosimendan therapy, an analysis of the impact of this intervention on rehospitalization events during a total treatment period of 3 months.
Methods
Search strategy
Pertinent studies were independently searched for in CENTRAL, Google Scholar, MEDLINE/PubMed, Scopus, and the Cochrane Central Register of clinical trials (updated 15/1/2017) by two of the authors. Our search strategy aimed to include any randomized controlled trials (RCTs) ever performed in which levosimendan was intermittently administered in patients with AdHF. In addition, we contacted international experts and employed backward snowballing (i.e. scanning of references from the retrieved articles and other pertinent reviews) to obtain further studies. The full PubMed search strategy7 is available in the Supporting Information. No language restriction was enforced.
Study selection
References obtained from database and literature searches were first independently examined at a title/abstract level by the two authors, with divergences being resolved by consensus. Then, if potentially pertinent, they were retrieved as complete articles. The following inclusion criteria were used for potentially relevant studies: random allocation to treatment; comparison of levosimendan vs. any control; studies performed in AdHF patients; use of intravenous repetitive administration; and no restrictions on dose or time of administration. The exclusion criteria were as follows: duplicate publications either acknowledged or not (in this case we referred to the article with the longest follow‐up period available); non‐RCT; non‐adult studies; and oral administration of levosimendan. Two investigators independently assessed compliance with selection criteria and selected studies for the final analysis, with divergences being resolved by consensus.
Data abstraction and study
Baseline and outcome data were independently abstracted by the two authors, with divergences being resolved by consensus. Specifically, we extracted potential sources of significant clinical heterogeneity, such as study design, sample size, clinical setting/indication, bolus and infusion doses of levosimendan, and duration of treatment, control treatment, and follow‐up duration, as well as rehospitalization data. At least two separate attempts to contact the original authors were made in cases of missing data. The primary endpoint of our meta‐analysis was the number of rehospitalizations 3 months after the end of treatment.
Internal validity and risk of bias assessment
The internal validity of and risk of bias in the included trials was appraised by two independent investigators according to the latest version of the ‘Risk of bias assessment tool’ developed by The Cochrane Collaboration,8 with divergences being resolved by consensus. Visual inspection of a funnel plot was performed to assess the presence of publication bias.
Data analysis and synthesis
Dichotomous data were extrapolated to compute the individual and pooled risk ratio (RR) with pertinent 95% confidence interval (CI) using the Mantel–Haenszel method. For continuous outcomes, the mean difference (MD) was computed using the inverse variance method. We used a fixed‐effects model in cases of low statistical inconsistency (I 2 ≤ 25%) and a random‐effects model (which better accommodates clinical and statistical variations) in cases of high statistical inconsistency (I 2 > 25%). Statistical significance was set at the two‐tailed 0.05 level for hypothesis testing. The hypothesis of statistical heterogeneity was tested by means of the Cochran Q test, with statistical significance set at the two‐tailed 0.10 level, whereas the extent of statistical consistency was measured with I 2, defined as 100% × (Q − df )/Q, where ‘Q’ is Cochran's heterogeneity statistic and ‘df ’ is the degrees of freedom. In addition to the principal analysis in which all the studies that fulfilled the inclusion criteria were considered, we performed secondary analyses to assess the effect of different control treatment and levosimendan infusion schemes on the primary outcome. The effect of intermittent levosimendan administration on left ventricular ejection fraction (LVEF) and New York Heart Association (NYHA) class was also assessed. Sensitivity analyses were performed by sequentially removing each study and reanalysing the remaining dataset (performing a new analysis after removal of each study) and by changing the summary statistics (from RR to odds ratio and risk difference) and analysis method (from Mantel–Haenszel to inverse variance and Peto). Unadjusted P‐values are reported throughout. All data were analysed according to the intention‐to‐treat principle whenever possible. Data were analysed using Review Manager version 5.3 (Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark, 2014).
This study was performed in compliance with appendix S1 of The Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines.9, 10, 11, 12
Results
Study characteristics
Our search strategy yielded a total of 362 articles. After exclusion of 344 non‐pertinent titles or abstracts, 18 papers were retrieved in complete form and assessed according to the selection criteria (Figure 1 ). Of these, a total of 12 papers were excluded because of non‐compliance with the pre‐specified selection criteria, leaving six studies13, 14, 15, 16, 17, 18 for the final analysis. A complete list of the excluded studies, together with the reasons for exclusion, is provided in Table S1 .
Figure 1.
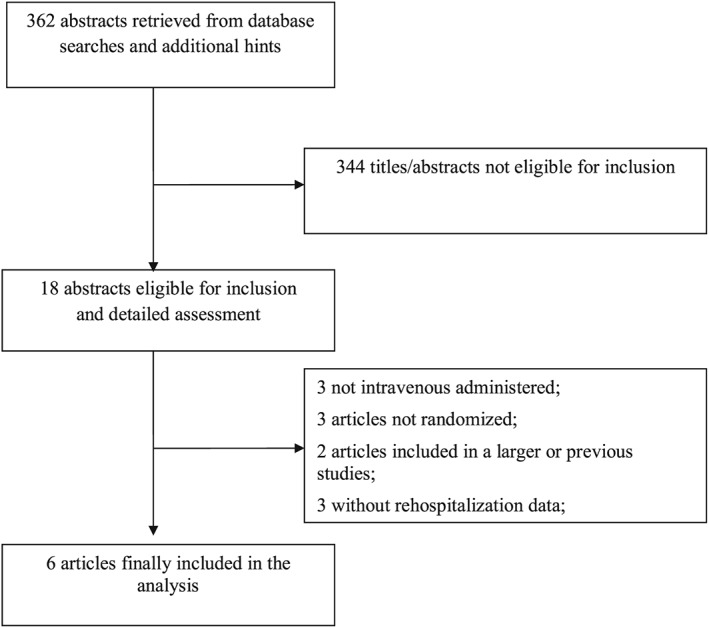
Flow diagram for selection of articles.
The six included studies randomized a total of 319 enrolled patients: 207 to levosimendan and 113 to control (Table 1). Different comparators were used: placebo in three studies,14, 17, 18 standard treatment in one study,13 dobutamine in one study,15 and furosemide in one study.16 Three studies were multicentre.14, 15, 16
Table 1.
Characteristics of the studies
| Reference | Year | Multicentre? | Control | No. of patients | Loading dose? | Continuous infusion dose (μg/kg/min) | Duration of administration (h) | Dosing interval | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | ||||||||
| Mavrogeni et al. 13 | 2007 | No | Best available treatment | 30 | 30 | 6 μg/kg | 0.1–0.2 | 24 | Every month |
| Kleber et al. 14 | 2009 | Yes | Placebo | 18 | 9 | 12 μg/kg over 10 min | 0.1 for 50 min followed by 0.2 for 23 h | 24 (first infusion); 6 (subsequent infusions) | Every 2 weeks |
| Bonios et al. 15 | 2012 | No | Dobutamine | 19 | 15 | No | Levosimendan: 0.3; dobutamine: 10 | 6 | Every week |
| Malfatto et al.16 | 2012 | No | Furosemide | 22 | 11 | No | 0.1–0.4 | 24 | Every month |
| Comin‐Colet et al. 17 | 2015 | Yes | Placebo | 48 | 21 | No | 0.2 | 6 | Every 2 weeks |
| García‐González et al. 18 | 2016 | Yes | Placebo | 70 | 27 | No | 0.1 | 24 | Every 30 days |
Data on 3‐month rehospitalization rates were available for three studies. We obtained further data from the authors in three cases.13, 14, 16 Quality appraisal of the studies indicated that three of them were judged to have an unclear risk of bias14, 17, 18 and three to have a high risk of bias13, 15, 16 ( Table S2 ).
Patient characteristics and levosimendan regimen
The cardiac conditions of the enrolled patients are reported in Table 2, and some available not cardiologic variables are reported in Table S3 . Most of the trials enrolled chronic heart failure (CHF) patients with a severely depressed LVEF, NYHA class III or IV, and who were experiencing frequent decompensation.
Table 2.
Cardiac conditions of the enrolled patients
| Reference | Year | Setting | LVEF (%) | NYHA class | Proportion of patients with HF of ischaemic aetiology (%) |
|---|---|---|---|---|---|
| Mavrogeni et al. 13 | 2007 | CHF (LVEF <30%) | 22 ± 6 (levosimendan group); 22 ± 5 (control group) | III–IV | 50 |
| Kleber et al. 14 | 2009 | Pulmonary hypertension with signs of right HF in the previous 6 months | Not reported | III–IV | Not available |
| Bonios et al. 15 | 2012 | Chronic systolic HF with recent decompensation | 23 ± 7 (levosimendan group); 21 ± 5 (control group) | IV | 50 |
| Malfatto et al. 16 | 2012 | CHF (LVEF <35%) requiring at least two hospitalizations in the previous 6 months | 26 ± 5 (levosimendan group); 28 ± 8 (control group) | 3 ± 0.4 (levosimendan group); 3 ± 0.4 (control group) | 72 |
| Comin‐Colet et al. 17 | 2015 | CHF (LVEF <35%) with decompensation in the previous 12 months | 25 ± 7 (levosimendan group); 25 ± 6 (control group) | III–IV | 61 |
| García‐González et al . 18 | 2016 | CHF with decompensation in the previous 6 months | 25 ± 8 (levosimendan group); 26 ± 10 (control group) | III–IV | Not available |
CHF, chronic heart failure; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The dose regimen and schedule for levosimendan administration varied between studies and are detailed in Table 1. A loading dose of 6 or 12 μg/kg was administered in two trials, while a continuous infusion (dose range 0.1–0.4 μg/kg/min) was administered in all trials. The infusion was continued for 6 h in two trials and for 24 h in three trials. In one trial, levosimendan was initially infused for 24 h and for 6 h in the subsequent administrations. The time lag between infusions varied from 1 week (one trial) to 2 weeks (two trials) and 1 month (three trials). Infusions were repeated for at least 2 months and for up to 12 months.
Quantitative data synthesis
The overall pooled analysis showed that the use of levosimendan was associated with a significant reduction in the number of rehospitalizations at 3 months: 33/207 (16%) vs. 39/113 (35%), RR 0.40, 95% CI 0.27–0.59, P < 0.001, I 2 = 0% (Table 3, Figure 2 ). These results were confirmed when analysing the only three multicentre studies14, 17, 18 in which placebo was used as comparator [25/136 (18%) vs. 27/57 (47%), RR = 0.40, 95% CI = 0.26 to 0.62, P < 0.001, I 2 = 0% (Table 3, Figure 2 ).
Table 3.
Secondary and sensitivity analyses
| Analysis | No. of trials included | No. of events | RR | 95% CI | P for effect | P for heterogeneity | I 2 (%) | |
|---|---|---|---|---|---|---|---|---|
| Levosimendan group | Control group | |||||||
| Overall | 6 | 33/207 | 39/113 | 0.40 | 0.27–0.59 | <0.001 | 0.79 | 0 |
| Placebo‐controlled trials | 3 | 25/136 | 27/57 | 0.40 | 0.26–0.62 | <0.001 | 0.62 | 0 |
| Multicentre trials | 3 | 25/136 | 27/57 | 0.40 | 0.26–0.62 | <0.001 | 0.62 | 0 |
| Lapse ≤2 weeks | 3 | 17/85 | 21/45 | 0.39 | 0.23–0.65 | <0.001 | 0.58 | 0 |
| Lapse >2 weeks | 3 | 16/122 | 18/68 | 0.42 | 0.23–0.74 | 0.003 | 0.55 | 0 |
| Study period <6 months | 3 | 23/88 | 24/41 | 0.45 | 0.29–0.69 | <0.001 | 0.46 | 0 |
| Study period ≥6 months | 3 | 10/119 | 15/72 | 0.32 | 0.15–0.68 | 0.003 | 0.77 | 0 |
| Loading dose used | 2 | 5/48 | 7/39 | 0.43 | 0.16–1.19 | 0.10 | 0.31 | 2 |
| Loading dose not used | 4 | 28/159 | 32/74 | 0.39 | 0.26–0.59 | <0.001 | 0.75 | 0 |
| Excluding studies providing data via personal communication | 3 | 21/137 | 26/63 | 0.35 | 0.22–0.57 | <0.001 | 0.94 | 0 |
| Analysis using inverse variance | 6 | 33/207 | 39/113 | 0.42 | 0.29–0.61 | <0.001 | 0.80 | 0 |
| Analysis using Peto | 6 | 33/207 | 39/113 | 0.24a | 0.14–0.44a | <0.001 | 0.83 | 0 |
| Analysis using OR | 6 | 33/207 | 39/113 | 0.25b | 0.14–0.46b | <0.001 | 0.85 | 0 |
| Analysis using RD | 6 | 33/207 | 39/113 | −0.20c | −0.31 to −0.08c | <0.001 | 0.17 | 36d |
| Analysis removing each trial and reanalysing the dataset | P < 0.001, 95% CI <1, and I 2 = 0% for all | |||||||
CI, confidence interval; OR, odds ratio; RD, risk difference; RR, risk ratio.
Peto OR and 95% CI for Peto OR are shown.
OR and 95% CI for OR are shown.
RD and 95% CI for RD are shown.
Data analysed with random‐effects model.
Figure 2.
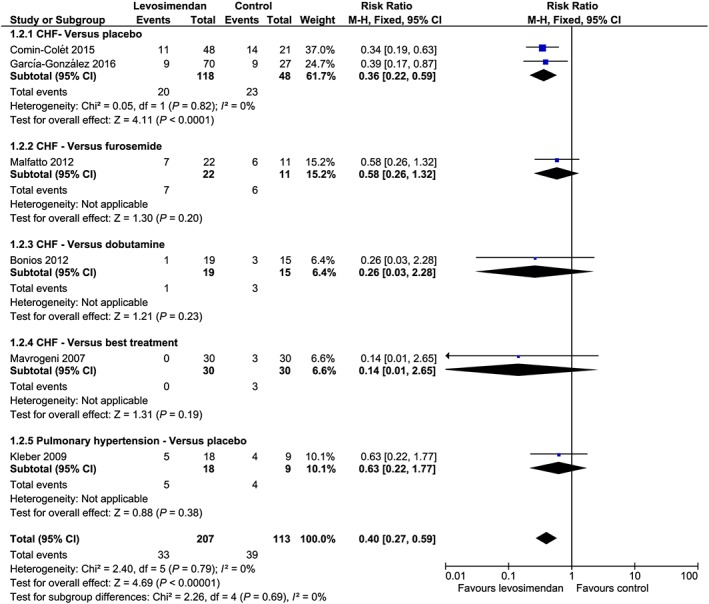
Forest plot. CI, confidence interval; CHF, chronic heart failure; M‐H, Mantel–Haenszel.
In addition, the results were confirmed when analysing only studies in which levosimendan was administered every 2 weeks or less, every month, for less than 6 months, or for at least 6 months.
Statistical significance was lost when analysing studies in which a loading dose was administered but was evident when analysis was restricted to studies in which only continuous infusion was administered (Table 3).
Removing each trial and reanalysing the remaining dataset, and changing the analysis method or summary statistics did not change either the significance or magnitude of the results (Table 3).
Visual inspection of the funnel plot did not suggest the presence of publication bias (Figure S1 ).
LVEF was significantly different between the two groups after treatment with levosimendan (MD 3.87, 95% CI 0.15–7.60, P = 0.04, I 2 = 62%; three studies included; Figure S2 ), but no significant difference in NYHA class was observed (MD −0.30, 95% CI −1.03 to 0.42, P = 0.41, I 2 = 80%; two studies included; Figure S3 ).
Discussion
The intermittent use of levosimendan infusions as a bridge to heart transplantation/implantation of a ventricular assist device, as well as its use as a destination therapy in palliative care for advanced/end‐stage HF patients, has increased in importance over the years since the first attempts to evaluate the efficacy of such therapy for improving the outcome and QoL of patients. Among other properties of levosimendan, the persistence of its haemodynamic effects for ≈2 weeks is obtained in particular with the active metabolite OR‐1896,19 which is mainly responsible for the efficacy of the intermittent repetitive infusion scheme of treatment.2
The papers regarding the repetitive intermittent administration of levosimendan selected for this meta‐analysis exhibited a heterogeneous methodology and variable results. In particular, there were significant differences between study designs, levosimendan comparators, levosimendan dosages, the duration of the infusions, and the frequency of repetitive treatment, not to mention endpoints were independently adjudicated and not clearly specified in several cases. This notwithstanding, all of the studies showed some beneficial effects of levosimendan in these clinical settings.
Mavrogeni et al. 13 assessed the effects of monthly levosimendan infusions on LVEF, end‐diastolic and end‐systolic volumes, and diameters. In addition to the good echocardiographic results obtained, the authors also demonstrated an improvement in symptoms and a reduction in mortality.
Echocardiographic indices and QoL were also the outcomes of the study by Malfatto et al. 16: compared with furosemide, they obtained significant reductions in NYHA class, ventricular volumes, and N terminal pro brain natriuretic peptide (NT‐proBNP) levels, and an increase in LVEF, with monthly levosimendan infusions. One‐year mortality did not reach statistical significance, although a positive trend towards a reduction with levosimendan was noted.
The study by Kleber et al. 14 evaluated both the short‐term and long‐term (8 weeks) effects of levosimendan on pulmonary vascular resistance and other haemodynamic parameters in patients with various forms of pulmonary hypertension. The decrease in pulmonary vascular resistance observed differed significantly between the levosimendan and placebo groups (P = 0.009), with the best results obtained 6 h after the start of the infusion. The authors also reported that repeated infusions maintained the clinical effects without the development of tolerance to the drug and concluded that shorter dosing intervals might render even better results.
Bonios et al. 15 randomized 63 patients to levosimendan, dobutamine, or the combination of both. The investigators found a significant benefit on event‐free survival with levosimendan, with 6‐month mortality lowered to 19%, as opposed to 38% with dobutamine (P = 0.037 vs. levosimendan) and 48% in the combination group (P = 0.009 vs. levosimendan).
Two new studies in this clinical setting were presented during the 2015 and 2016 European Society of Cardiology–Heart Failure Association Congresses.
The first is the LION‐Heart study,17 a multicentre, randomized, double‐blind, parallel group, placebo‐controlled trial. It aimed to evaluate the efficacy and safety of intermittent 6‐h administrations of levosimendan every 2 weeks in 69 outpatients with CHF. The primary endpoint of the study was changes in NT‐proBNP levels assessed bi‐weekly, and the results were significantly in favour of levosimendan (P < 0.005). This is consistent with the results of levosimendan trials in acute HF,4, 19 and has not been reported with any other inodilator.
The LAICA study18, 20 aimed to test the effects of monthly 24‐h infusions of levosimendan on the incidence of in‐hospital admissions for acute decompensated HF in 97 patients in the advanced stages of the disease. This was a multicentre, prospective, randomized, double‐blind, placebo‐controlled, parallel‐group trial, with several additional secondary endpoints. Although statistical significance for the primary endpoint was missed, fewer admissions for acute decompensated HF, as well as lower mortality rates, were recorded among the levosimendan‐treated patients.
Within the limitations of this meta‐analysis on rehospitalization including few studies, in which endpoints were not clearly specified, at least three reasons exist to support the repetitive levosimendan treatment of AdHF patients, at least three reasons exist to support this repetitive treatment in selected patients. Firstly, it has been demonstrated that repeated levosimendan administrations improve the haemodynamic stability of patients; secondly, this treatment improves symptoms; and thirdly, it reduces episodes of acute decompensation.
We noticed that in the studies in which a loading dose of levosimendan was administered,13, 14 the statistical significance in the advantages of levosimendan vs. comparators is lost. This observation matches well with the analysis by Landoni et al. 21 Indeed, in that previous meta‐analysis of 45 randomized levosimendan clinical trials in all settings, a trend towards a lesser reduction in mortality in the bolus subset (P = 0.2) was observed. One may speculate that the pronounced haemodynamic effects/adverse effects (i.e. reduction of blood pressure) induced by a loading dose of levosimendan in hypotense or hypovolemic patients could be the cause of the observed reduction of benefits.
Rehospitalizations and quality of life
Advanced heart failure is characterized by potentially and partially reversible symptoms and cardiac dysfunction,2 together with frequent episodes of acute decompensation requiring prolonged hospitalization,22 and thus, a severely compromised QoL. Each exacerbation of the condition is characterized by worsening of symptoms, greater limitation of functional capacity, and deterioration of QoL.23 Moreover, the repeated hospitalizations also contribute to a further decrease in cardiac function in a circular fashion towards progressive decline of the patient's clinical condition, which ends with death.24
Rehospitalization is an important marker of the QoL of AdHF patients, although it is rarely evaluated when QoL is measured. Registries and administrative data have shown that early HF‐related readmission after discharge from hospital is associated with worse long‐term outcomes and a decline in QoL, with ≈25% of preventable readmission cases happening within 1 month of the initial phase.25, 26 For this reason, the possibility of including rehospitalization in future HF studies as a composite endpoint, along with death and other indices of clinical stability (such as NT‐proBNP), has evoked some interest. Recently, such an attempt was made by Margulies et al. 27 In their study, the primary endpoint was a global rank score that assigned a numerical value to each patient on the basis of their clinical condition, considering clinical events first (death and/or acute decompensation) and changes in NT‐proBNP levels thereafter. This endpoint was then analysed in hierarchical categories, with the first being time to death, the second time to HF hospitalization, and the third time‐averaged proportional change in NT‐proBNP.
A surrogate to measure QoL can be the assessment of functional capacity by NYHA classes, although QoL encompasses much more than functional capacity alone. Indeed, it is commonly perceived that in the advanced NYHA classes III or IV, Qol is very low. As it regards the effects of levosimendan on QoL, a recent review22 listed and discussed encouraging data present in the literature. In parallel, some recent papers show promising effects by levosimendan on NYHA in acute heart failure patients.28 We hypothesize that the lack of improvement of NYHA class with the repetitive treatment with levosimendan observed in some of the studies utilized for the present meta‐analysis may be due to the subjectivity of assessing such parameter, as compared with the more objective improvements in ejection fraction (EF) and NT‐proBNP.
Limitations
This study has several limitations. First of all, the relatively small number of studies and patients included in the meta‐analysis and the heterogeneous population involved should be taken into consideration when interpreting the results. This is a common and intrinsic limitation of meta‐analyses.9 Data on rehospitalizations were available in the original publications only for three trials, while data from the remaining three were provided by trials' authors. However, a sensitivity analysis including only studies with published data confirmed magnitude and directions of results. A further limitation is the heterogeneous selection of comparators in the six studies. A clear definition of a strategy for readmission in the individual studies was lacking, and in several cases, there was an uncertain adjudication of reason for readmissions. A time‐dependent analysis for readmission was lacking in the majority of included trials. Moreover, the heterogeneous dosing and interval of administration of levosimendan can also be seen as a severe limitation. Last but not least, the assessment of the patients was non‐blinded in some studies: both Bonios et al. 15 and Malfatto et al. 16 adopted randomized assignment but open‐label protocols, while Mavrogeni et al. 13 run an open labeled study. The remaining three studies by Comin‐Colet et al., 17 Garcia‐Gonzalez et al.,18 and Kleber et al. 14 (accounting for 72% of the weight in this meta‐analysis) were double‐blind trials. However, because the performance of a meta‐analysis is meant to shed light on the overall safety and efficacy of a drug and to help in powering future clinical trials, we consider our results useful as providing a strong rationale for a properly powered study on the effect of levosimendan on rehospitalization.
Future steps
In the light of our results, and taking into due account all the limitations of our study, it appears that a repeated administration of levosimendan to patients with AdHF brings advantages as less frequent rehospitalization. The present meta‐analysis is based on data from clinical trials where levosimendan was compared with other treatments mainly on top of standard of care (e.g. diuretics). Often the rationale of those studies was the hypothesis that levosimendan would bring additional advantages as sustained improvements of haemodynamics and symptoms due to its prolonged pharmacokinetics. Still open is the question if levosimendan would be more beneficial in patients with HF with reduced EF or HF with preserved EF, and future studies should tackle also this aspect. Certainly, our analysis supports the use of rehospitalization as one of the endpoints.
The fact that recent studies of new drugs developed as treatments of acute and AdHF failed to show benefits (see the fate of intravenous omecamtiv mecarbil,29 ularitide,30 serelaxin,31 and liraglutide28) brings this field in focus again. In fact, despite effective new drugs contributing to prolong the life of CHF patients, the rate of hospitalization for acute events is not decreasing at all in the developed countries,32, 33 making AdHF an even greater burden. A properly powered clinical trial on repetitive levosimendan use in these patients is thus strongly advocated.
Conclusions
This meta‐analysis supports the hypothesis that repetitive or intermittent administration of levosimendan for patients with AdHF is associated with a reduction in rehospitalization rate at 3 months. There is therefore a strong rationale for an RCT with levosimendan in these settings to be designed and carried out that has adequate power to investigate patient rehospitalization within its endpoints.
Conflict of interest
A.F. and P.P. are employed by the company that discovered and developed levosimendan. None of the other authors have any conflict of interest.
Author contributions
A.B. and S.S. independently performed the preliminary searches for the relevant publications. P.P. and A.F. contributed substantially to the discussions of the existing literature and to the final text. All authors reviewed the manuscript before submission.
Funding
None.
Supporting information
Table S1. List of the 12 excluded studies, together with reason for exclusion.
Table S2. Assessment of the risk of bias of included studies.
Table S3. Analysis of the serum electrolytes.
Figure S1. Funnel Plot.
Figure S2. Forest plot for LVEF after study drug administration.
Figure S3. Forest plot for NYHA after study drug administration.
Silvetti, S. , Belletti, A. , Fontana, A. , and Pollesello, P. (2017) Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: a meta‐analysis of randomized trials. ESC Heart Failure, 4: 595–604. doi: 10.1002/ehf2.12177.
References
- 1. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 2. Nieminen MS, Altenberger J, Ben‐Gal T, Böhmer A, Comin‐Colet J, Dickstein K, Edes I, Fedele F, Fonseca C, García‐González MJ, Giannakoulas G, Iakobishvili Z, Jääskeläinen P, Karavidas A, Kettner J, Kivikko M, Lund LH, Matskeplishvili ST, Metra M, Morandi F, Oliva F, Parkhomenko A, Parissis J, Pollesello P, Pölzl G, Schwinger RH, Segovia J, Seidel M, Vrtovec B, Wikström G. Repetitive use of levosimendan for treatment of chronic advanced heart failure: clinical evidence, practical considerations, and perspectives: an expert panel consensus. Int J Cardiol 2014; 15: 360–367. [DOI] [PubMed] [Google Scholar]
- 3. Silvetti S, Greco T, Di Prima AL. Intermittent levosimendan improves mid‐term survival in chronic heart failure patients: meta‐analysis of randomised trials. Clin Res Cardiol 2014; 103: 505–513. [DOI] [PubMed] [Google Scholar]
- 4. Mebazaa A, Nieminen MS, Packer M, Cohen‐Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Põder P, Kivikko M, SURVIVE Investigators . Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 2007; 297: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 5. Ukkonen H, Saraste M, Akkila J, Knuuti MJ, Lehikoinen P, Någren K, Lehtonen L, Voipio‐Pulkki LM. Myocardial efficiency during calcium sensitization with levosimendan: a noninvasive study with positron emission tomography and echocardiography in healthy volunteers. J Clin Pharmacol Ther 1997; 61: 596–607. [DOI] [PubMed] [Google Scholar]
- 6. Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, Lehikoinen P, Någren K, Lehtonen L, Voipio‐Pulkki LM. Myocardial efficiency during levosimendan infusion in congestive heart failure. J Clin Pharmacol Ther 2000; 68: 522–531. [DOI] [PubMed] [Google Scholar]
- 7. Biondi‐Zoccai GG, Agostoni P, Abbate A, Testa L, Burzotta F. A simple hint to improve Robinson and Dickersin's highly sensitive PubMed search strategy for controlled clinical trials. Int J Epidemiol 2005; 34: 224–225. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greco T, Zangrillo A, Biondi‐Zoccai G, Landoni G. Meta‐analysis: pitfalls and hints. Heart Lung Vessel 2013; 5: 219–225. [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. http://handbook.cochrane.org/ (2 February 2017).
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6 e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mavrogeni S, Giamouzis G, Papadopoulou E, Thomopoulou S, Dritsas A, Athanasopoulos G, Adreanides E, Vassiliadis I, Spargias K, Panagiotakos D, Cokkinos DV. A 6‐month follow‐up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire, and arrhythmia in advanced heart failure. J Card Fail 2007; 13: 556–559. [DOI] [PubMed] [Google Scholar]
- 14. Kleber FX, Bollmann T, Borst MM, Costard‐Jäckle A, Ewert R, Kivikko M, Petterson T, Pohjanjousi P, Sonntag S, Wikström G. Repetitive dosing of intravenous levosimendan improves pulmonary hemodynamics in patients with pulmonary hypertension: results of a pilot study. J Clin Pharmacol 2009; 49: 109–115. [DOI] [PubMed] [Google Scholar]
- 15. Bonios MJ, Terrovitis JV, Drakos SG, Katsaros F, Pantsios C, Nanas SN, Kanakakis J, Alexopoulos G, Toumanidis S, Anastasiou‐Nana M, Nanas JN. Comparison of three different regimens of intermittent inotrope infusions for end stage heart failure. Int J Cardiol 2012; 159: 225–229. [DOI] [PubMed] [Google Scholar]
- 16. Malfatto G, Della Rosa F, Villani A, Rella V, Branzi G, Facchini M, Parati G. Intermittent levosimendan infusions in advanced heart failure: favourable effects on left ventricular function, neurohormonal balance and one‐year survival. J Cardiovasc Pharmacol 2012; 60: 450–455. [DOI] [PubMed] [Google Scholar]
- 17. Comin‐Colet J, on behalf of the LION Heart Study Investigators . Multicenter, double‐blind, randomized, placebo‐controlled trial evaluating the efficacy and safety of intermittent levosimendan in outpatients with advanced chronic heart failure: the LION Heart study. Paper presented at the European Society of Cardiology–Heart Failure Association Congress, Seville, Spain, 24 May, 2015. https://spo.escardio.org/SessionDetails.aspx?eevtid=1077&sessId=14858&subSessId=0&searchQuery=%2fdefault.aspx%3feevtid%3d1077%26days%3d%26topics%3d%26types%3d%26rooms%3d%26freetext%3dcomin-colet%26sort%3d1%26page%3d1%26showResults%3dTrue%26nbPerPage%3d20%26WithWebcast%3d%26WithSlides%3d%26WithAbstract%3d%26WithReport%3d%26scroll%3D456#.WT4tn7h_S3o (15 May 2017).
- 18. García‐González MJ, on behalf of the LAICA Study Investigators . Efficacy and security of intermittent repeated levosimendan administration in patients with advanced heart failure: a randomized, double‐blind, placebo controlled multicenter trial: LAICA study. Paper presented at the European Society of Cardiology–Heart Failure Association Congress, Florence, Italy, 21 May, 2016. https://spo.escardio.org//SessionDetails.aspx?eevtid=1126&presId=136186&doc=Webcast#.WT4sdrh_S3o (15 May 2017).
- 19. Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation 2003; 107: 81–86. [DOI] [PubMed] [Google Scholar]
- 20. García‐González MJ, de Mora‐Martín M, López‐Fernández S, López‐Díaz J, Martínez‐Sellés M, Romero‐García J, Cordero M, Lara‐Padrón A, Marrero‐Rodríguez F, del Mar García‐Saiz M, Aldea‐Perona A, LAICA study investigators . Rationale and design of a randomized, double‐blind, placebo controlled multicenter trial to study efficacy, security, and long term effects of intermittent repeated levosimendan administration in patients with advanced heart failure: LAICA study. Cardiovasc Drugs Ther 2013; 27: 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landoni G, Biondi‐Zoccai G, Greco M, Greco T, Bignami E, Morelli A, Guarracino F, Zangrillo A. Effects of levosimendan on mortality and hospitalization. A meta‐analysis of randomized controlled studies. Crit Care Med. 2012. Feb; 40: 634–646. [DOI] [PubMed] [Google Scholar]
- 22. Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, Wikström G, Agostoni P, Atar S, Baholli L, Brito D, Colet JC, Édes I, Gómez Mesa JE, Gorjup V, Garza EH, González Juanatey JR, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira‐Barretto A, Pollesello P, Rudiger A, Schwinger RH, Wieser M, Yavelov I, Zymliński R. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015; 191: 256–264. [DOI] [PubMed] [Google Scholar]
- 23. Fruhwald S, Pollesello P, Fruhwald F. Advanced heart failure: an appraisal of the potential of levosimendan in this end‐stage scenario and some related ethical considerations. Expert Rev Cardiovasc Ther 2016; 14: 1335–1347. [DOI] [PubMed] [Google Scholar]
- 24. Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 96: 11G–17G. [DOI] [PubMed] [Google Scholar]
- 25. Mills RM. The heart failure frequent flyer: an urban legend. Clin Cardiol 2009; 32: 67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation 2012; 126: 501–506. [DOI] [PubMed] [Google Scholar]
- 27. Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP, Heart Failure Clinical Research Network NHLBI. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016; 316: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mushtaq S, Andreini D, Farina S, Salvioni E, Pontone G, Sciomer S, Volpato V, Agostoni P. Levosimendan improves exercise performance in patients with advanced chronic heart failure. ESC Heart Fail. 2015; 2: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teerlink JR, Felker GM, McMurray JJ, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JG, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM, ATOMIC‐AHF Investigators . Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC‐AHF study. J Am Coll Cardiol. 2016; 67: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 30. Packer M, O'Connor C, McMurray JJ, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J, TRUE‐AHF Investigators . N Engl J Med. 2017; 376: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 31. Teerlink JR, on behalf of the RELAX‐AHF‐2 Study Investigators . RELAX‐AHF‐2: Serelaxin in acute heart failure. Presentation at the ESC Heart Failure congress 2017, Paris. http://spo.escardio.org/SessionDetails.aspx?eevtid=1226&sessId=20859&subSessId=0&searchQuery=%2fdefault.aspx%3feevtid%3d1226%26days%3d%26topics%3d%26types%3d%26rooms%3d%26freetext%3dmetra%26sort%3d1%26page%3d1%26showResults%3dTrue%26nbPerPage%3d20%26WithWebcast%3d%26WithSlides%3d%26WithAbstract%3d%26WithReport%3d%26scroll%3D228#WRv5CTHkW3E (May 15 2017).
- 32. Blecker S, Ladapo JA, Doran KM, Goldfeld KS, Katz S. Emergency department visits for heart failure and subsequent hospitalization or observation unit admission. Am Heart J. 2014; 168: 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayodele L, Rudnika‐Noulin D. Heart Failure Epidemiology. LLC: Decision Resources Group publisher; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of the 12 excluded studies, together with reason for exclusion.
Table S2. Assessment of the risk of bias of included studies.
Table S3. Analysis of the serum electrolytes.
Figure S1. Funnel Plot.
Figure S2. Forest plot for LVEF after study drug administration.
Figure S3. Forest plot for NYHA after study drug administration.


