Abstract
Aims
Cardiac involvement in sarcoidosis is reported in up to 30% of patients. Left ventricular involvement demonstrated by contrast‐enhanced cardiac magnetic resonance has been well validated. We sought to determine the prevalence and distribution of right ventricular late gadolinium enhancement in patients diagnosed with pulmonary sarcoidosis.
Methods and results
We prospectively evaluated 87 patients diagnosed with pulmonary sarcoidosis with contrast‐enhanced cardiac magnetic resonance for right ventricular involvement. Pulmonary artery pressures were non‐invasively evaluated with Doppler echocardiography. Patient characteristics were compared between the groups with and without right ventricular involvement, and right ventricular enhancement was correlated with pulmonary hypertension, ventricular mass, volume, and systolic function. Left ventricular late gadolinium enhancement was demonstrated in 30 patients (34%). Fourteen patients (16%) had right ventricular late gadolinium enhancement, with sole right ventricular enhancement in only two patients. The pattern of right ventricular enhancement consisted of right ventricular outflow tract enhancement in 1 patient, free wall enhancement in 8 patients, ventricular insertion point enhancement in 10 patients, and enhancement of the right side of the interventricular septum in 11 patients. Pulmonary arterial hypertension correlated with the presence of right ventricular enhancement (P < 0.001). Right ventricular enhancement correlated with systolic ventricular dysfunction (P < 0.001), hypertrophy (P = 0.001), and dilation (P < 0.001).
Conclusions
Right ventricular enhancement was present in 16% of patients diagnosed with pulmonary sarcoidosis and in 48% of patients with left ventricular enhancement. The presence of right ventricular enhancement correlated with pulmonary arterial hypertension, right ventricular systolic dysfunction, hypertrophy, and dilation.
Keywords: Cardiomyopathy, Magnetic resonance imaging, Pulmonary hypertension, Right ventricle, Sarcoidosis
Introduction
Sarcoidosis is a rare, inflammatory condition, resulting from an uncontrolled cellular inflammatory response in genetically predisposed individuals, which affects the heart in approximately a third of patients.1 Left ventricular involvement demonstrated by contrast‐enhanced cardiac magnetic resonance has been well validated.2 Until recently, limited attention has been given to right ventricular involvement in cardiac sarcoidosis, its prevalence, relevance, and prognostic value.3 Cardiac magnetic resonance imaging is the preferred imaging tool to evaluate the healthy and diseased right ventricle.4, 5 Right ventricular volumes, mass, and function can be quantified without geometric assumptions and excellent intra‐observer and inter‐observer agreement and inter‐study reproducibility.4, 5, 6 Delayed contrast‐enhanced magnetic resonance allows for the detection and quantification of focal scar and interstitial fibrosis. Although there are numerous reports on delayed contrast‐enhanced cardiac magnetic resonance delineating left ventricular sarcoidosis, relatively few studies have reported on right ventricular involvement.3, 7, 8, 9, 10 We sought to determine the prevalence and distribution of right ventricular late gadolinium enhancement in patients diagnosed with pulmonary sarcoidosis and determine the relationship with pulmonary hypertension, ventricular volume, mass, and systolic function.
Methods
Patient selection
Between July 2001 and March 2014, we enrolled 87 consecutive patients with histologically proven pulmonary sarcoidosis. Cardiac evaluation was performed because of symptoms or routine screening to exclude cardiac involvement. Patients were excluded when the standard contra‐indications for contrast‐enhanced cardiac magnetic resonance existed. Institutional Review Board approval was obtained for this study.
Baseline investigations
Baseline investigations included 12‐lead electrocardiography, Doppler echocardiography, and contrast‐enhanced cardiac magnetic resonance. Pulmonary artery systolic pressure was estimated from the tricuspid regurgitant velocity plus an estimate of right atrial pressure derived from the inferior vena cava.11 Right‐sided heart studies12 were performed in patients with pulmonary hypertension and congestive heart failure and in patients in whom automated cardioverter defibrillators were implanted. Coronary angiography was performed to exclude underlying coronary artery disease in patients with documented ventricular tachy‐arrhythmias, pathological Q‐waves, impaired systolic function, regional wall motion abnormalities, and/or late gadolinium enhancement.
Cardiac magnetic resonance protocol and analysis
Studies were performed using a commercial 1.5 T scanner with a cardiac‐dedicated phased‐array coil. The cardiac magnetic resonance studies were electrocardiographically triggered by standard software. Studies consisted of multi‐phase multi‐slice steady‐state‐free precession and fat‐saturated T2‐weighted (69) breath‐hold sequences of the short axis, vertical long axis, and horizontal long axis views. Outflow tract views were generated in patients with right ventricular abnormalities. The short‐axis images covered the left ventricle from base to apex. The steady‐state‐free precession sequences (typical repetition time 3.5 ms; echo time 1.4 ms; flip angle 55°; temporal resolution 50 ms; voxel size 1.6 × 1.6 × 10 mm, no gap) were performed to assess regional wall‐motion abnormalities, left and right ventricular masses, volumes, and ejection fractions. Papillary muscles were included when determining right ventricular mass and excluded when determining volumes. Contrast‐enhanced and T2‐weighted images were obtained in diastole to minimize artefact due to cardiac motion. Ten minutes after the additional administration of 0.1 mmol/kg gadolinium‐diethylenetriaminepenta‐acetic acid (Schering, Berlin, Germany), a two‐dimensional segmented inversion recovery‐gradient echo breath‐hold sequence (short axis, vertical long axis, horizontal long axis, and right ventricular outflow tract in selected patients, voxel size 1.6 × 1.6 × 10 mm, without gap) was used to assess for late gadolinium enhancement. The inversion time (250 to 400 ms) was determined on an individual basis to obtain optimal nulling of the unenhanced myocardial signal. Two experienced, blinded, and independent observers used commercially available software (CAAS MRV 3.4, Pie Medical Imaging, Maastricht, the Netherlands) to determine the standard parameters delineated in Table 1. The distribution of right ventricular late gadolinium enhancement was determined by consensus and characterized as free wall, apical, outflow tract, right‐sided interventricular septal, and/or including the ventricular insertion points. Late ventricular gadolinium enhancement was considered present only if confirmed on both short‐axis and matching long‐axis myocardial locations. Late left ventricular gadolinium enhancement was quantified by a semiautomatic detection method using the signal intensity threshold of ≥2 SD above a remote reference region. The intra‐observer and inter‐observer variabilities were determined by calculating the variability coefficients and intra‐class correlations for each parameter in 18 randomly selected studies.
Table 1.
Characteristics of patients with and without right ventricle late gadolinium enhancement
|
Patients without RV LGE n = 73 |
Patients with RV LGE n = 14 |
P value | |
|---|---|---|---|
| Male | 48 (66) | 9 (64) | 0.779 |
| Caucasian | 58 (79) | 8 (57) | 0.074 |
| Age (years) | 52.8 ± 10.2 | 55.7 ± 9.1 | 0.460 |
| Cardiac presentation | 18 (25) | 10 (71) | <0.001 |
| Syncope | 4 (5) | 1 (8) | 0.564 |
| Palpitations | 7 (10) | 3 (21) | 0.167 |
| Clinical congestive heart failure | 4 (5) | 6 (43) | 0.001 |
| Sustained ventricular Tachycardia | 6 (8) | 4 (29) | 0.039 |
| Chest discomfort | 2 (3) | 1 (8) | 0.388 |
| Dyspnoea | |||
| NYHA 0–2 | 72 (98) | 12 (86) | 0.388 |
| NYHA 3–4 | 2 (3) | 1 (7) | 0.388 |
| Diabetes mellitus | 3 (4) | 0 | 1.000 |
| Hypertension | 7 (10) | 0 | 0.588 |
| Medication at any time | 51 (70) | 12 (86) | 0.102 |
| Steroids | 5 (7) | 2 (14) | 0.280 |
| Methotrexate | 5 (7) | 5 (36) | 0.006 |
| Loop diuretics | 5 (7) | 6 (43) | 0.001 |
| Spironolactone | 5 (7) | 7 (50) | <0.001 |
| Ace inhibitors/ATIIRB | 7 (9) | 7 (50) | 0.001 |
| Beta blockers | 9 (12) | 6 (43) | 0.008 |
| Amiodarone | |||
| Abnormal ECG | 18 (25) | 10 (71) | <0.001 |
| Pulmonary hypertension | 5 (7) | 9 (64) | <0.001 |
| CMR imaging parameters | |||
| LVEF, % | 60 [54–66] | 50 [42–58] | 0.015 |
| LVEF ≤ 50% | 8 (11) | 5 (36) | 0.039 |
| LVEDV, mL | 113 [90–136] | 134 [81–187] | 0.261 |
| LVEDV index, mL/m2 | 58 [47–69] | 75 [70–100] | 0.142 |
| LV mass | 112 [72–152] | 122 [83–161] | 0.550 |
| LV mass index, g/m2 | 64 [44–84] | 65 [38–92] | 0.780 |
| LVH | 20 (27) | 3 (23) | 0.747 |
| LV dilation | 5 (8) | 4 (29) | 0.035 |
| LV LGE | 18 (25) | 12 (86) | <0.001 |
| LV LGE, % | 12 [4–20] | 28 [18–38] | 0.002 |
| RVEF, % | 49 [43–55] | 33 [24–42] | 0.001 |
| RVEDV, mL | 148 [108–188] | 188 [141–235] | 0.034 |
| RVEDV index, mL/m2 | 78 [58–98] | 96 [68–124] | 0.018 |
| RVESV | 72 [47–97] | 102 [70–134] | 0.05 |
| RVESV index, mL/m2 | 37 [26–48] | 58 [38–78] | 0.046 |
| RVH | 5 (7) | 6 (43) | 0.001 |
| RV mass, g | 42 [34–50] | 53 [35–71] | 0.068 |
| RV mass index, g/m2 | 21 [17–25] | 28 [22–34] | 0.075 |
| RV dilation | 3 (4) | 6 (43) | <0.001 |
| RVEF ≤ 45% | 6 (8) | 10 (71) | <0.001 |
| T2 positive | 7/60 (12) | 3/9 (33) | 0.112 |
CI, confidence interval; CMR, cardiac magnetic resonance; EDV, end‐diastolic volume; LGE, late adolinium enhancement; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; RV, right ventricle; RVEDV, right ventricular end‐diastolic volume; RVEDVI, right ventricular end‐diastolic volume index; RVEF, right ventricular ejection fraction; RVH, right ventricular hypertrophy.
Bold signifies significance i.e. P < 0.05.
Values are n (%), median [IQR], or mean ± SD.
Variables and definitions
Peak systolic right ventricular pressures over 35 mmHg were considered to represent pulmonary hypertension. Right ventricular hypertrophy was defined as right ventricular weight exceeding normal values as published by Maceira et al. and/or right ventricular end‐diastolic wall thickness over 5 mm.6 Right ventricular systolic dysfunction was defined as an ejection fraction below 45%.13
Statistical analysis
All statistical analyses were performed using statistical software (Version 21.0, SPSS; Chicago, IL, USA). Continuous normal distributed variables were expressed as mean ± SD and, between group comparisons, were made using the parametric t‐test for independent samples or the Mann–Whitney test when appropriate. In the non‐normally distributed continuous data, the median and interquartile range were determined and, between group correlations, were made with the Wilcoxon test. Categorical variables were assessed using the χ2 or Fisher's exact test when appropriate. Statistical significance was defined at P < 0.05.
Results
Patient characteristics
Table 1 demonstrates the baseline characteristics in the included 87 patients. Twenty‐seven (31%) patients presented with cardiac symptoms, while the remaining 60 either suffered from non‐specific symptoms or were routinely screened for cardiac sarcoidosis. According to the ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities, an implantable cardioverter defibrillator or pacemaker was implanted in respectively 14 and 3 patients after the baseline cardiac magnetic resonance study.14 All had late gadolinium enhancement and suffered from cardiac symptoms. When applying the recently published Heart Rhythm Society expert consensus criteria, we diagnosed 31 patients (36%) with cardiac involvement.15
Cardiac magnetic resonance analyses
Thirty patients (34%) had left ventricular and 14 patients (16%) had right ventricular enhancement, with enhancement limited to the right ventricle in only two patients (2%). The group of patients with right ventricular enhancement had significantly more extensive left ventricular enhancement compared with those without (Table 1, P = 0.007). Table 2 describes the distribution of right ventricular enhancement. Right ventricular enhancement correlated with early diastolic left‐sided septal displacement (P = 0.002), systolic right ventricular dysfunction (P < 0.001), hypertrophy (P < 0.001), and dilation (P = 0.009). Fifteen patients had pulmonary hypertension, eight of which had right ventricular enhancement. The presence of ventricular insertion point enhancement (P = 0.007), right‐sided septal (P < 0.001), and right ventricular free wall enhancement (P = 0.016) correlated with the presence of pulmonary hypertension. Right ventricular hypertrophy correlated with the presence of ventricular insertion point enhancement (P = 0.004) and right‐sided septal (P = 0.001), but not with free wall enhancement (P = 0.076). In five patients, early diastolic septal displacement towards the left ventricle was observed, two of which had insertion point enhancement (P = 0.099), and three of which had right‐sided septal enhancement (P = 0.013). Neither the chronicity of pulmonary sarcoidosis, as determined by the time since diagnosis, nor the extent of lung disease as determined by high‐resolution computed tomography correlated with pulmonary hypertension, right ventricular systolic function, or myocardial enhancement. The right ventricular end‐diastolic volume index in patients with pulmonary Stage 4 (fibro‐cystic disease) was significantly larger compared with the earlier disease Stage 1 (hilar nodes only) (P = 0.027). The T2‐weighted studies suggested active, granulomatous disease in only 10/69 (14%) of patients. The intra‐observer variability for right ventricular end‐diastolic volume was 2%, end‐systolic volume 3.4%, ejection fraction 3.4%, and mass 4%. The interobserver variability for right ventricular end‐diastolic volume was 1.6%, end‐systolic volume 5%, ejection fraction 3.2%, and mass 8%.
Table 2.
Characteristics of patients with right ventricle late gadolinium enhancement
| Enhanced segments | Patients (n = 14) | Combination of enhanced segments | Patients (n = 14) | Patients with pulmonary hypertension | Patients with end‐systolic septal shift (n = 5) |
|---|---|---|---|---|---|
| RV septal | 11 | RV septal | 1 | 1 | 0 |
| VIP | |||||
| RV free wall | |||||
| RVOT | |||||
| VIP | 10 | RV septal | 3 | 2 | 1 |
| VIP | |||||
| RV free wall | |||||
| RV free wall | 8 | RV septal | 4 | 1 | 0 |
| VIP | |||||
| RVOT | 1 | RV septal | 2 | 2 | 1 |
| RV free wall | |||||
| RV septal | 1 | 1 | 1 | ||
| RV free wall | 1 | 0 | |||
| VIP | 1 | 1 | 1 | ||
| RV free wall | 1 | 0 | 0 | ||
| VIP |
RV, right ventricular; RVOT, right ventricular outflow tract; VIP, ventricular insertion points.
Discussion
This is the first prospective cardiac magnetic resonance study to specifically report on the prevalence and distribution of right ventricular involvement in cardiac sarcoidosis. Predominant or isolated right ventricular involvement is rare, with nearly all patients suffering from left ventricular disease.16 With Murtagh et al., Crawford et al., and Cheong et al., we found more extensive left ventricular enhancement to be associated with right ventricular involvement.8, 10, 17 Previous studies reported a correlation between the presence and extent of left ventricular enhancement, impaired left and right ventricular systolic function, and higher adverse event rates. The direct relationship between right ventricular enhancement, its size and systolic function, as previously demonstrated in left ventricular sarcoidosis, has not been reported before.
Right ventricular enhancement, inflammation, and impaired systolic function have been associated with adverse outcome, particularly ventricular tachy‐arrhythmias.10, 17, 18, 19, 20 However, the localizations of the arrhythmogenic foci were not reported on. Because right ventricular sarcoidosis occurs in patients with more extensive left ventricular disease, the reported prognostic relevance of right ventricular disease may at least partly reflect the extent of left ventricular arrhythmogenic substrate.
Several post‐transplant and post‐mortem studies in sarcoidosis patients have reported right ventricular involvement to range from 6%, in patients dying from alternate causes, to as high as 65% in those dying from sudden cardiac deaths.21, 22, 23, 24, 25 Generally, patients with congestive failure were found to have extensive biventricular sarcoid (Figure 1), and in those who had died suddenly, active granulomatous infiltration and patchy scar were present. Right ventricular outflow tract involvement was rare (Figure 2).
Figure 1.
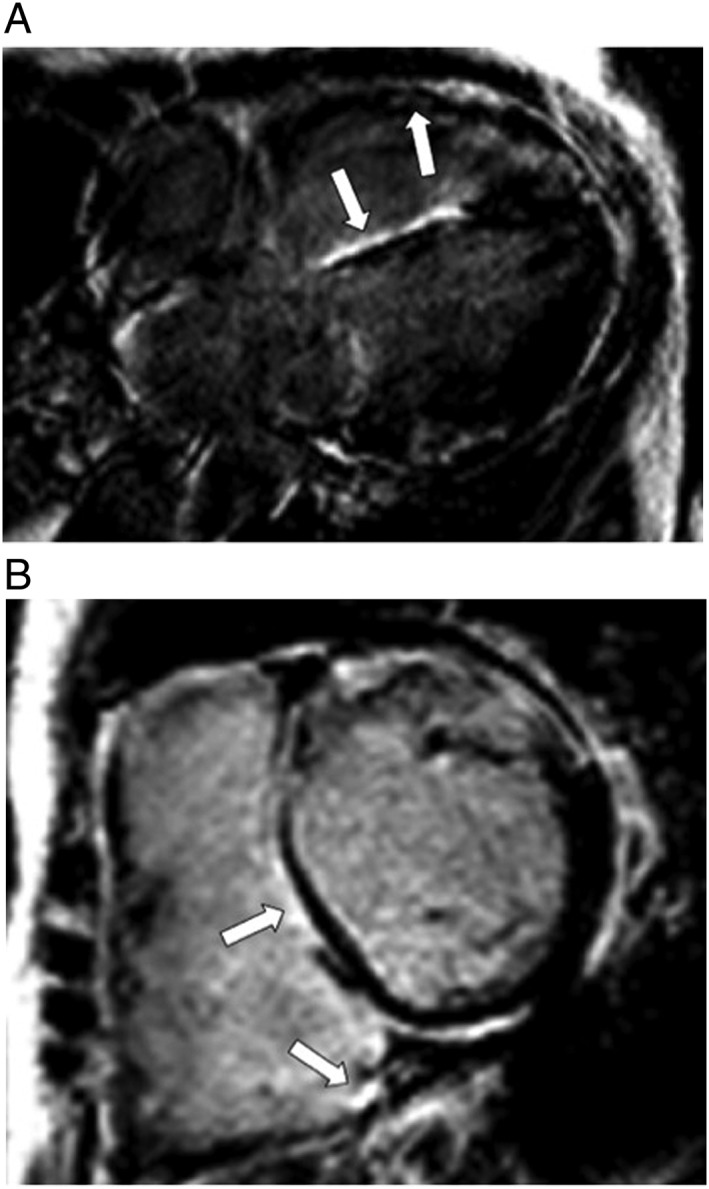
(A) Contrast‐enhanced magnetic resonance study in a patient with biventricular congestive heart failure (inversion‐recovery gradient echo sequence and horizontal long axis view) demonstrates enhancement of the right ventricular free wall and right‐sided interventricular septum. (B) Short axis view in the same patient demonstrates enhancement of the right‐sided interventricular septum and inferior right ventricular insertion point.
Ten recent studies employing contrast‐enhanced magnetic resonance in the assessment of cardiac sarcoidosis reported on right ventricular disease in sarcoidosis (Table 3).3, 7, 8, 9, 10, 17, 18, 19, 20, 26 Our findings in an unselected population with pulmonary sarcoidosis compare. Right ventricular enhancement was reported in five of these studies and ranged from 2% in unselected to 48% in high‐risk populations, which compares with our 47% of patients with left ventricular sarcoidosis.3, 7, 8, 9, 10 Similar to our findings, nearly all patients with right ventricular enhancement had left ventricular enhancement. Samar et al., Patel et al., and Crawford et al. were the only researchers to report on right ventricular enhancement in more detail.3, 7, 10 Contrary to our and other studies, Samar et al. reported that right ventricular enhancement did not correlate with a difference in left ventricular ejection fraction. His data were included in a poster presentation, which precluded detail, while relevant data were not available in 19% of patients.3 Crawford reported multi‐focal right ventricular enhancement in nearly half of those with left ventricular enhancement, basal, mid, or apical right ventricular segments being equally involved.10 Similar to Crawford et al., the majority of our patients (70%) had multi‐focal right ventricular enhancement. However, most of our patients had right‐sided septal enhancement, similar to Patel's findings.7 Right‐sided septal and insertion point enhancement were related to pulmonary hypertension in eight of our patients. Crawford et al. and Patel et al. did not include data on right ventricular or pulmonary pressures. Compared with Crawford's population, our patients with right ventricular enhancement had on average poorer systolic function and more extensive left ventricular enhancement.
Table 3.
Cardiac magnetic resonance studies reporting on right ventricular involvement in cardiac sarcoidosis
| Authors | Type of study | Patients | Conclusion |
|---|---|---|---|
| Cheong et al.8 | Prospective, single centre | 31 patients asymptomatic biopsy proven systemic sarcoidosis, 8 (26%) LV LGE of whom 2 (25%) with RV LGE, inferobasal RV LGE in patients with most LV LGE | Asymptomatic small amount of LGE (average 3.2% of LV) in 26%, no cardiac events after 1 year |
| Patel et al.7 | Prospective, single centre | 81 patients with extra‐cardiac sarcoidosis, 21 (26%) with LV LGE (average 6 g), 14 (67%) had right‐sided septal LGE incl 4 RV free wall/outflow tract/anterobasal segments | Patients with LGE had 9‐fold higher rate of adverse events |
| Patel et al.19 | Retrospective, single centre | 152 patients extra‐cardiac sarcoidosis, LVEF ≥ 50%, 29 (19%) LV LGE, no data on RV LGE | Patients with LV LGE had lower RVEF, because of either presumed biventricular disease or pulmonary hypertension |
| Schuller et al.18 | Retrospective, multi‐centre | 112 CS patients with ICDs for primary or secondary prevention, no data on LGE | Impaired systolic LV and RV function correlates with more ICD therapy |
| Samar et al. (poster)3 | Retrospective, single centre | 122 sarcoidosis patients, 37 (22%) LV LGE, 18 (49%) of these also RV LGE | LVEF, LVEDV, RVEDV similar in groups with/without RV LGE |
| Crawford et al.10 | Retrospective, multi‐centre | 52 CS patients, all LVEF > 35% 32 (62%) with LV LGE of which 13 (41%) also had RV LGE | Multi‐focal LGE correlated with VT/VF, patients with RV LGE had more extensive LV LGE |
| Nadel et al.9 | Retrospective, single centre | 106 sarcoidosis patients, 32 CS‐defined by CMR LGE—32 LV LGE, 2 (6%) RV LGE | LGE only independent predictor of adverse outcome |
| Muser et al.20 | Prospective, single centre | 31 CS patients with VTs pre‐ablation, 23 had CMR, 21 (68%) LV LGE, 11 (35%) RV LGE, no data on RV distribution or extent | LGE extent predicted VT‐free survival |
| Ekström et al.26 | Retrospective, single centre | 50 CS patients, 48 (96%) with LV LGE, not reported on RV LGE | LV extent of LGE and RVEF, correlated with adverse outcome |
| Murtagh et al.17 | Retrospective, single centre | 205 patients extra‐cardiac sarcoidosis, LVEF ≥ 50%, 41 (20%) LV LGE, ≥4 patients with VIP LGE, no specific data on RV LGE | For every 1% in LGE burden, the hazard for an event increased by 8%; mild impaired RV dysfunction correlated with increased event rate |
CMR, cardiac magnetic resonance; CS, cardiac sarcoidosis; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; RVEF, right ventricular ejection fraction.
The presence of right ventricular enhancement correlated with hypertrophy, dilation, and systolic dysfunction. The relationship between pulmonary hypertension, right ventricular hypertrophy, dilation, systolic dysfunction, septal displacement, and septal and insertion point enhancement has been demonstrated before12 (Figure 3). Right ventricular enhancement in sarcoidosis may be caused by direct granulomatous infiltration but also be related to pulmonary hypertension12 (Figures 1 and 3). Right ventricular hypertrophy, dilation, dysfunction, and enhancement may be secondary to pulmonary hypertension and are associated with worse prognosis.12, 27 Pulmonary hypertension is found in 6–28% of the general sarcoidosis outpatient setting and may be secondary to pulmonary fibrosis, angiitis, and/or congestive heart failure.27 The fact that pulmonary hypertension in our population did not correlate with disease extent as determined by computed tomography illustrates the variety in pathophysiology of this condition in sarcoidosis.28 Right ventricular dilation, systolic impairment, and inflammation, as demonstrated with positron emission tomography, in sarcoidosis have been demonstrated to predict adverse outcome in sarcoidosis.10, 13, 17, 18, 19, 26, 29
In patients with predominant right ventricular disease, cardiac sarcoidosis needs to be differentiated from arrhythmogenic right ventricular cardiomyopathy. Distinguishing features favouring sarcoidosis consist of an older age of onset, a non‐familiar pattern, wider QRS complexes, septal involvement with atrio‐ventricular conduction disease, multiple arrhythmogenic foci, particularly right ventricular apical tachycardia, concomitant left ventricular disease, and the presence of mediastinal lymphadenopathy.30 Electro‐anatomic mapping, contrast‐enhanced magnetic resonance, and/or positron emission tomography may guide endomyocardial biopsies needed to obtain histological confirmation of the diagnosis.
We report on ventricular insertion point enhancement, a distribution pattern not specifically mentioned in the other studies. Late enhancement of the septum and insertion points may result from delayed wash‐out of gadolinium due to altered myocardial fibre strain, fibre disarray, ischaemia, and fibrosis, secondary to right ventricular pressure or volume overload, and resulting septal shift.12, 31, 32 Ventricular insertion point enhancement has been reported in hypertrophic cardiomyopathy, atrial septal defects, severe pulmonary hypertension, tetralogy of Fallot, transposition of the great arteries, and even a proportion of veteran healthy endurance athletes31, 32, 33, 34, 35 (Figures 2, 3, 4, 5). The amount of insertion point enhancement correlates with mean pulmonary arterial pressures, right ventricular mass, volume, and ejection fraction.12 Pulmonary hypertension in sarcoidosis is associated with adverse outcome, particularly when accompanied by right ventricular dysfunction, and/or lung fibrosis.12, 27 Recently, Swift et al. reported septal extension of insertion point enhancement in pulmonary hypertension to mark more severe disease, with associated right ventricular dilation, but found it not to be an independent predictor of overall mortality.12
Figure 2.
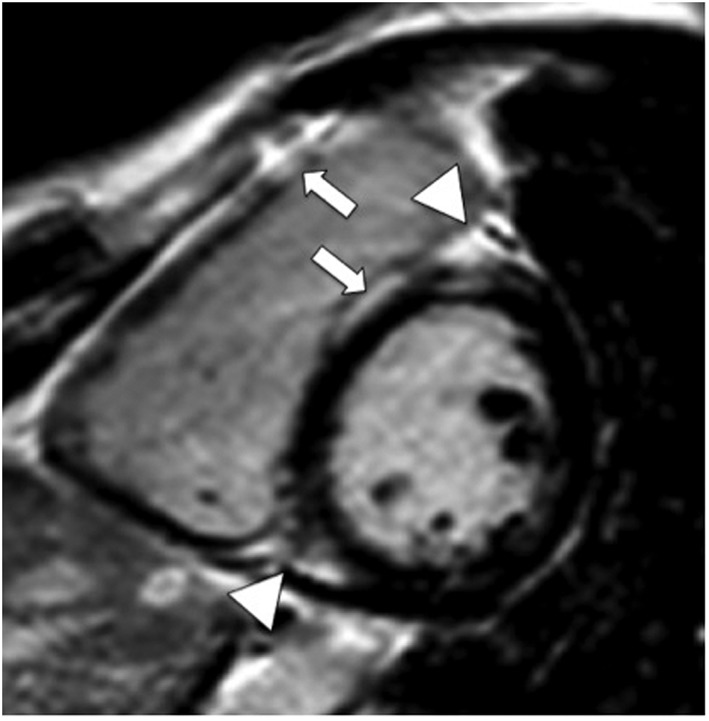
Contrast‐enhanced magnetic resonance study (inversion‐recovery gradient echo sequence, end‐diastolic frame, and short axis view) demonstrates enhancement of the right ventricular free wall (arrow), ventricular insertion points (triangles), and right‐sided septum (arrow).
Figure 3.
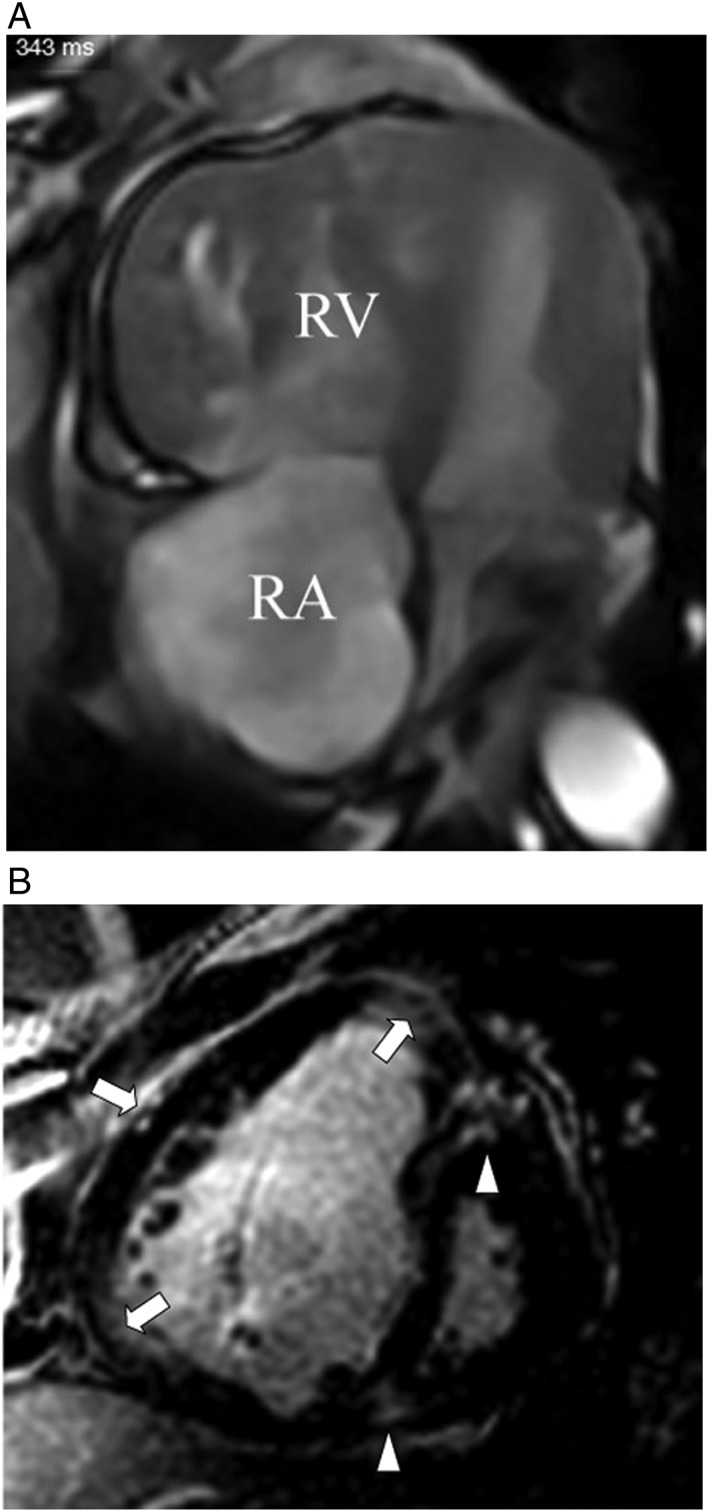
(A) Magnetic resonance study (steady‐state‐free precession sequence, horizontal long axis view, and end‐diastolic frame) demonstrates dilation of the right ventricle (RV) and right atrium (RA), marked right ventricular hypertrophy, with displacement of the interventricular septum towards the left ventricle; both left ventricle and atrium are compressed. (B) Contrast‐enhanced magnetic resonance study (inversion recovery‐gradient echo sequence, short axis view, and end‐diastolic frame) of the identical patient with pulmonary vascular sarcoidosis and resulting severe pulmonary arterial hypertension demonstrates contrast‐enhancement of the right ventricular hinge points (triangles) and free wall (arrows).
Figure 4.
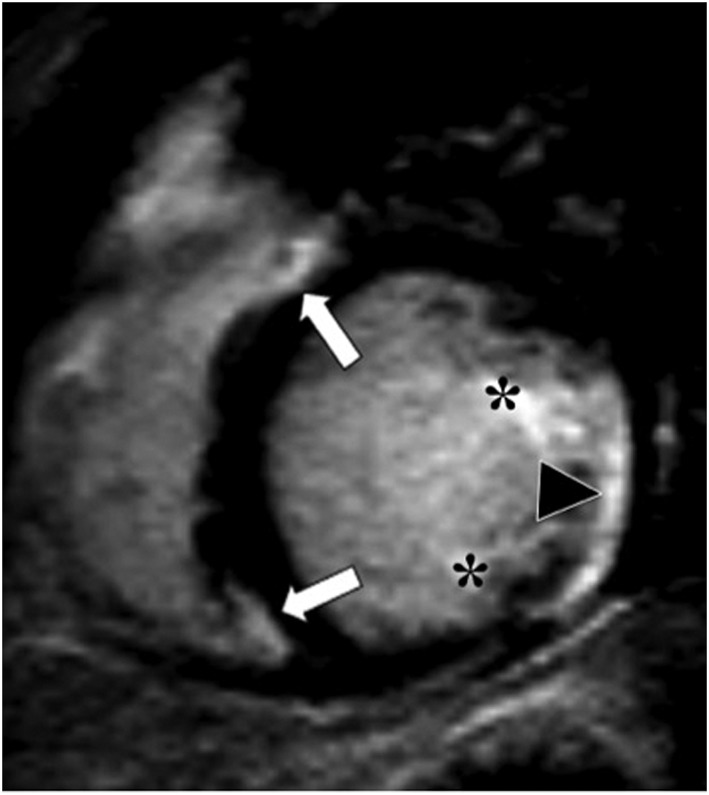
Contrast‐enhanced magnetic resonance study (inversion‐recovery gradient echo sequence and short axis view) in a patient without pulmonary hypertension demonstrates enhancement of the ventricular insertion points (arrows), papillary muscles (asterisks), and postero‐lateral left ventricular segments (triangle).
Figure 5.
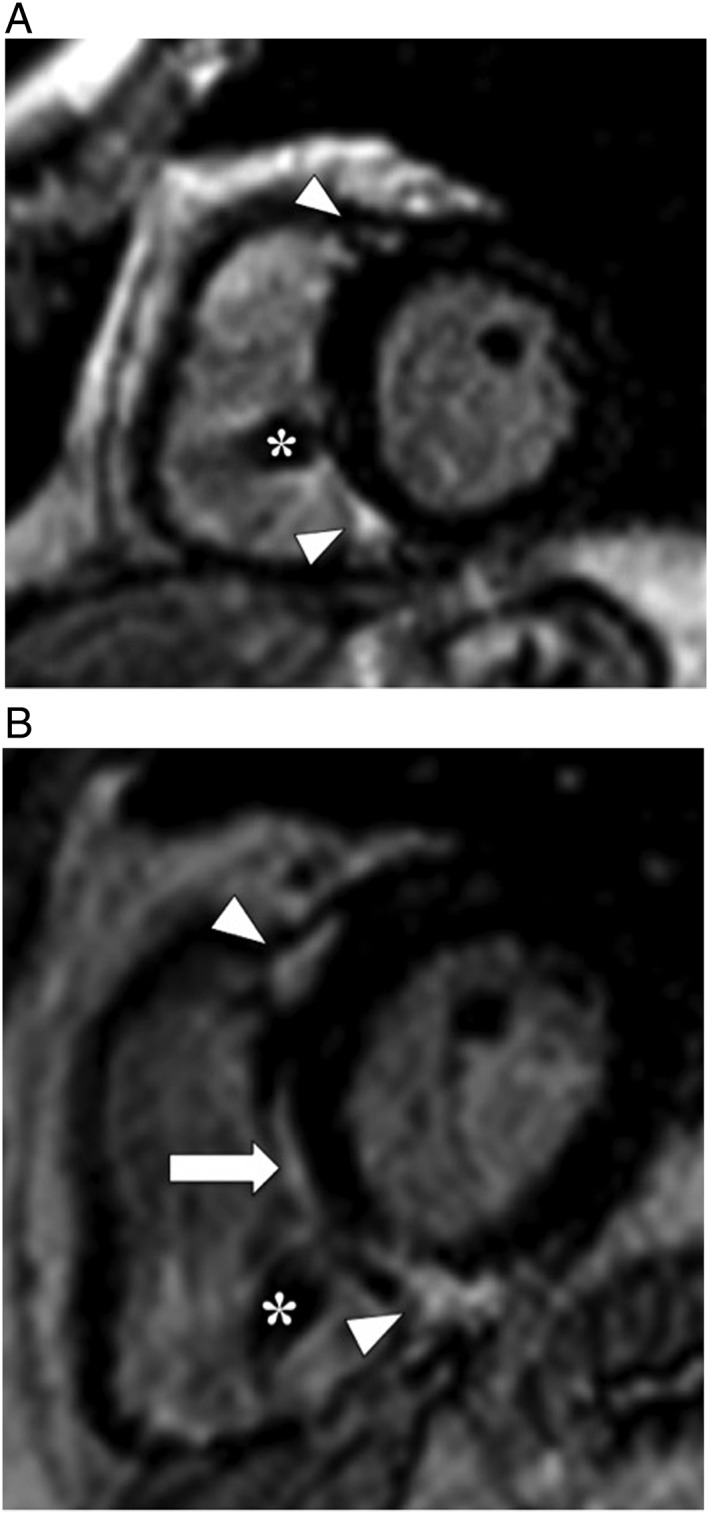
(A) Contrast‐enhanced magnetic resonance study (inversion‐recovery gradient echo sequence, end‐diastolic frame, and short axis view) in a patient diagnosed with a high‐degree atrio‐ventricular block secondary to active cardiac sarcoidosis. A dual chamber pacemaker had been inserted. Ventricular insertion point enhancement is demonstrated (triangles). Pulmonary pressures were normal (asterisk—artefact of right ventricular pace lead). (B) Contrast‐enhanced magnetic imaging study (inversion‐recovery gradient echo sequence, end‐diastolic frame, and short axis view) in the identical patient when reassessed 7 years later demonstrates substantially more enhancement of the right‐sided septum (arrow) and the insertion points (triangles). The percentage time pacing had increased from 5% to 15% of the time (asterisk—artefact produced by right ventricular pace lead).
Late gadolinium enhancement includes active, potentially reversible, granulomatous inflammation, and chronic focal scar.8, 20, 36 Immune suppressive treatment, currently a work in progress, may potentially improve systolic function and decrease arrhythmogenic substrate.10, 20, 36 The T2‐weighted spin echo‐based assessment used in our study to evaluate for active granulomatous infiltration and associated oedema is rather insensitive.8 T2 mapping and positron emission tomography have shown promise and will be included in future projects.28, 37
Conclusions
Approximately 30% of an unselected patient population with pulmonary sarcoidosis had left ventricular involvement, half of which had right ventricular involvement. More extensive left ventricular enhancement correlated with right ventricular involvement. Right ventricular enhancement may result from direct infiltration and resulting scar or pulmonary hypertension. Previous studies associated impaired systolic right ventricular function and right ventricular enhancement with ventricular tachy‐arrhythmias. We demonstrate right ventricular enhancement with cardiac magnetic resonance to be mostly multi‐focal, involve the septum, and correlate with increased right ventricular volumes, hypertrophy, and impaired systolic function.
Limitations
Pulmonary pressures were routinely determined non‐invasively in the majority of our patients, and we may have underestimated the pulmonary pressures. Our imaging protocol was not primarily adapted to evaluate the right ventricle, our slice thickness, and potentially suboptimal myocardial nulling may have resulted in an underestimation of the presence and extent of right ventricular infiltration and scarring. Because T2 mapping was not performed, we likely underestimated active granulomatous inflammation. The relatively small number of patients included limits our findings and conclusions.
Future focus of development
Customized hybrid approaches including electrocardiography, ultrasound, positron emission tomography, and contrast‐enhanced magnetic resonance will provide us with more sensitive, accurate, and comprehensive information on haemodynamic, electrical, mechanical, and inflammatory characteristics of the atriae and ventricles.
Conflict of interest
None declared.
Acknowledgement
The expert statistical advice of Dr P. Nelemans, MD, PhD, of the Department of Epidemiology at Maastricht University Medical Centre, is greatly valued.
Smedema, J.‐P. , van Geuns, R.‐J. , Ainslie, G. , Ector, J. , Heidbuchel, H. , and Crijns, H. J. G. M. (2017) Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Failure, 4: 535–544. doi: 10.1002/ehf2.12166.
References
- 1. Youssef G, Beanlands RSB, Birnie DH, Nery PB. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart 2011; 97: 2078–2087. [DOI] [PubMed] [Google Scholar]
- 2. Smedema JP, Snoep G, van Kroonenburgh MPG, van Geuns RJ, Dassen WRM, Gorgels APM, Crijns HJGM. Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 3. Samar HY, Thompson DV, Doyle M, Williams RB, Yamrozik JA, Reddy ST, Shah M, Biederman RJ. Sarcoidosis; is it confined to just the LV? An RV LGE study. From 17th Annual SCMR Scientific Sessions, New Orleans, LA, USA. 16‐19 January 2014.
- 4. Valsangiocomo Buechel ER, Mertens L. Imaging the right heart: the use of integrated multimodality imaging. Eur Heart J 2012; 33: 949–960. [DOI] [PubMed] [Google Scholar]
- 5. van de Veerdonck MC, Marcus JT, Bogaard HJ, Vonk NA. State of the art: advanced imaging of the right ventricle and pulmonary circulation in humans (2013 Grover Conference series). Pulm Circ 2014; 4: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady‐state free precession cardiovascular magnetic resonance. Eur Heart J 2006; 27: 2879–2888. [DOI] [PubMed] [Google Scholar]
- 7. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009; 120: 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheong BY, Muthupillai R, Nemeth M, Lambert B, Dees D, Huber S, Castriotta R, Flamm S. The utility of delayed‐enhancement magnetic resonance imaging for identifying non‐ischemic myocardial fibrosis in asymptomatic patients with biopsy‐proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26: 39–46. [PubMed] [Google Scholar]
- 9. Nadel J, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long‐term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging 2015; 16: 1634–1641. [Google Scholar]
- 10. Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Xioukui G, Schuller J, Kron J, Nour KA, Cheng A, Ji SY, Feinstein S, Gupta S, Ilg K, Sinno M, Abu‐Hashish S, Al‐Mallah M, Sauer WH, Ellenbogen K, Morady F, Bogun F. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014; 7: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 11. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–613. [DOI] [PubMed] [Google Scholar]
- 12. Swift AJ, Rajaram S, Capener D, Elliott C, Condliffe R, Wild JM, Kiely DJ. LGE patterns in pulmonary hypertension do not impact overall mortality. J Am Coll Cardiol Img 2014; 7: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 13. Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, Jabbour A, Alpendurada F, Guha K, Ismail NA, Raza S, Khwaja J, Brown TDH, Morarji K, Liodakis E, Roughton M, Wage R, Pakrashi PC, Sharma R, Carpenter JP, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. The prevalence and prognostic significance of right ventricular systolic dysfunction in non‐ischemic dilated cardiomyopathy. Circulation 2013; 128: 1623–1636. [DOI] [PubMed] [Google Scholar]
- 14. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) , American Association for Thoracic Surgery; Society of Thoracic Surgeons . ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities. Circulation 2008; 117: e350–e408. [DOI] [PubMed] [Google Scholar]
- 15. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Nielsen JC, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014; 11: 1305–1323. [DOI] [PubMed] [Google Scholar]
- 16. Halushka MK, Yuh DD, Russell SD. Right ventricle‐dominant cardiac sarcoidosis with sparing of the left ventricle. J Heart Lung Transplant 2006; 25: 479–482. [DOI] [PubMed] [Google Scholar]
- 17. Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Ju Z, Addetia K, Mor‐Avi V, Moss JD, Hogarth DK, Sweiss NJ, Lang RM, Patel AR. Prognosis in myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction. Risk stratification using cardiac magnetic resonance. Circ Cardiovasc Imaging 2016; 9: e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, Sweiss NJ, Nguyen DT, Aleong RG, Varosy PD, Weinberger HD, Sauer WH. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol 2012; 23: 925–929. [DOI] [PubMed] [Google Scholar]
- 19. Patel AR, Klein MR, Chandra S, Spencer KT, DeCara JM, Lang RM, Burke MC, Garrity ER, Hogarth DK, Archer SL, Sweiss NJ, Beshai AF. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail 2011; 13: 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muser D, Santangeli P, Patahk RK, Castro SA, Liang JJ, Magnani S, Hayashi T, Garcia FC, Hutchinson MD, Supple GE, Frankel DS, Riley RP, Lin D, Schaller RD, Desjardin B, Dixit S, Callans DJ, Zado ES, Marchlinski FE. Long‐term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol 2016; 9: e004333. [DOI] [PubMed] [Google Scholar]
- 21. Virmani R, Butres JC, Roberts WC. Cardiac sarcoidosis. A major cause of sudden death. Chest 1980; 77: 423–428. [DOI] [PubMed] [Google Scholar]
- 22. Roberts WC, Chung MS, Ko JM, Capehart JE, Hall SA. Morphologic features of cardiac sarcoidosis in native heart of patients having cardiac transplantation. Am J Cardiol 2014; 113: 706–712. [DOI] [PubMed] [Google Scholar]
- 23. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinico‐pathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II). Am J Med 1977; 63: 86–108. [DOI] [PubMed] [Google Scholar]
- 24. Tavora F, Creswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 2009; 104: 571–577. [DOI] [PubMed] [Google Scholar]
- 25. Bagwan IN, Hooper LVB, Shepard MN. Cardiac sarcoidosis and sudden death. The heart may look normal or mimic other cardiomyopathies. Virchows Arch 2011; 458: 671–678. [DOI] [PubMed] [Google Scholar]
- 26. Ekström K, Lehtonen J, Hänninen H, Kandolin R, Kivistö S, Kupari M. Magnetic resonance imaging as a predictor of survival free of life‐threatening arrhythmias and transplantation in cardiac sarcoidosis. J Am Heart Assoc 2016; 5: e003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baughman RP, Engel PJ, Nathan S. Pulmonary hypertension in sarcoidosis. Clin Chest Med 2015; 36: 703–714. [DOI] [PubMed] [Google Scholar]
- 28. Nunes H, Humbert M, Capron F, Brauner M, Sitbon O, Battesti JP, Simonneau G, Valeyre D. Pulmonary hypertension associated with sarcoidosis: mechanisms, hemodynamics and prognosis. Thorax 2006; 61: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blankstein R, Osborn M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014; 63: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Philips B, Madhavan S, James CA, te Riele A, Murray B, Tichnell C, Bhonsale A, Nazarian S, Judge DP, Calkins H, Tandri H, Cheng A. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis. Distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol 2014; 7: 230–236. [DOI] [PubMed] [Google Scholar]
- 31. Sato T, Tsujino I, Ohira H, Oyama‐Manabe N, Ito YM, Noguchi T. Paradoxal interventricular septal motion as a major determinant of late gadolinium enhancement in ventricular insertion points in pulmonary hypertension. PLoS One 2013; 8: e66724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol 2001; 38: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 33. Kuribayashi T, Roberts WC. Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am J Cardiol 1992; 70: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 34. McCann GP, Beek AM, Vonk‐Noordegraaf A, van Rossum AC. Delayed contrast‐enhanced magnetic resonance imaging in pulmonary arterial hypertension. Circulation 2005; 112: e268. [DOI] [PubMed] [Google Scholar]
- 35. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Non‐ischemic left ventricular scar as a substrate of life‐threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016; 9: e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ise T, Hasegawa T, Morita Y, Yamada M, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S, Shimizu W, Anzai T, Kitakaze M. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 2014; 100: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 37. Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med 2014; 189: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]


