Abstract
In this study, we investigated the use of three-dimensional electrospun poly(lactic-co-glycolic acid)/poly(ε-caprolactone) (PLGA/PCL) scaffolds seeded and cultured with postnatal dental cells, for improved dental tissue regeneration. Wet-electrospinning combined with ultrasonic treatment was studied as a method to enhance scaffold porosity and to promote cell-cell interactions. We also investigated whether nano-hydroxyapatite (nHA) incorporation could enhance dental cell differentiation. All scaffolds were seeded with human tooth pulp-derived dental mesenchymal (hDM) cells, or a combination of hDM and pig dental epithelial (pDE) cells, cultured for up to 28 days. Developmentally staged samples were assessed using scanning electron microscopy, histological, immunohistochemical, DNA and alkaline phosphatase activity assays, and quantitative-PCR for ameloblastic, odontoblastic, and osteogenic related gene expression. Results showed that electrospun scaffolds exhibited sufficient porosity to support robust cell ingrowth. Additional ultrasonic treatment led to a less homogeneous scaffold porosity, resulting in evident cell clustering and enhanced hDM-pDE cell-cell interactions. Finally, nHA incorporation was found to enhance dental cell differentiation. However, it also resulted in smaller fibre diameter and reduced scaffold porosity, and inhibited cell ingrowth and proliferation. In conclusion, ultrasonically treated wet-electrospun PLGA/PCL scaffolds are a suitable material for dental tissue engineering, and support future in vivo evaluations of this model.
Keywords: Wet-electrospinning, Ultrasonic, Nano-hydroxyapatite, Post-natal tooth bud cells, dental epithelial-mesenchymal cell interactions
1. Introduction
Due to the limited regenerative capacity of human deciduous and adult tooth tissues, tooth loss caused by caries, trauma, periodontal disease, and genetically inherited disease, is considered to be a major health issue. Current clinical treatment, including dental implant placement or fixed and removable dentures, can partially restore the lost function. However, the ability to replace lost teeth with a vital, bioengineered tooth would be highly preferred. The creation of fully functionalized bioengineered teeth, which contain dentin, enamel, dental pulp, blood vessels, nerves and periodontal ligament, is the ultimate goal in dental regenerative therapy.1-3
Natural teeth develop from ectodermal dental epithelial (DE) cells (produce dental enamel) and neural crest-derived dental mesenchymal (DM) cells (differentiate into pulp, dentin, cementum, periodontal ligament and alveolar bone), whose interactions have been shown to be essential for dental cell differentiation and tooth development.4,5 DE-DM cell interactions are also essential for bioengineered tooth regeneration. Previous studies illustrated that single tooth regeneration could be achieved by using a re-aggregation system, where a construct of DE cells harvested from embryonic tooth buds was combined with embryonic DM cells, and then transplanted and grown in the omentum or alveolar bone of host animals.6 However, the use of cells harvested from early stage embryonic tooth buds will be difficult, if not impossible, to translate into clinical application. Thus, the use of post-natal dental epithelial-mesenchymal cells for tooth regeneration has been extensively investigated. To date, published reports using this approach have shown the formation of small tooth crowns present throughout the scaffold rather than one full-sized tooth, and that tooth root development was only rudimentary.7-9 Even using three dimensional (3D) pre-fabricated DE-DM cell constructs, the formation of mineralized dental tissue was not satisfactory.10
These results emphasize the importance of appropriate scaffold materials and design, to establish proper DE-DM cell interactions for tooth regeneration. Electrospinning, as a simple and versatile method to produce nano- and micro-scale fibrous polymeric meshes, holds promise in this application.11 Since electrospun fibres are morphologically similar to natural extracellular matrix (ECM), they can provide appropriate cues to direct dental cell proliferation and differentiation. Moreover, bioactive particles, such as nano-hydroxyapatite (nHA) which showed positive effect on osteogenic differentiation of bone marrow-derived mesenchymal cells, can easily be integrated into tooth scaffolds during electrospinning.12,13
A major concern, especially with electrospinning, is the creation of scaffolds with sufficient porosity.14 Most current electrospinning techniques create scaffolds with insufficient inter-fibre space to simulate the physiological three dimensional tissue microenvironment and proper cell infiltration.15,16 To solve this problem, wet electrospinning techniques have been developed. During this process, electrospun fibres are collected in a liquid bath (e.g. ethanol) to form a random three dimensional structure.17 Previous reports have also shown that the spatial density of electrospun fibres could be further improved by ultrasonic separation. During ultrasonic treatment, water can infiltrate into the gaps between the fibres and mechanically agitate them.18
To address all of these aforementioned issues, we have taken the following approach. First, 3D fibrous scaffolds were fabricated using wet electrospinning; Secondly, ultrasonic treatment and nHA were incorporated into the scaffolds preparation to improve the scaffold porosity and cell differentiation, respectively; and third, scaffolds seeded with DM cells alone, or a mixture of DE-DM cells, were investigated using in vitro cell culture. Replicate samples were examined for cell infiltration, proliferation, ameloblastic, odontoblastic and osteogenic differentiation, and DE-DM cell-cell interactions. We hypothesized that (1) wet electrospinning and additional ultrasonic treatment can improve scaffold porosity; (2) incorporation of nHA improves DM cell differentiation; (3) the highly porous scaffold with or without nHA will benefit DE-DM cell-cell interaction.
2. Materials and methods
2.1. Materials
Poly(lactic-co-glycolic acid) (PLGA; Purasorb® PDLG8515, Mw 150 kDa) and poly(ε-caprolactone) (PCL; LACTEL® Absorbable Polymers, inherent viscosity range: 1.0 - 1.3 dl/g, Mw 80 kDa) were purchased from Purac Biomaterials BV (Gorinchem, The Netherlands) and DURECT Corporation (Pelham, AL), respectively. Nano-hydroxyapatite (nHA; Budenheim, Tri-Cafos P/c53-80) was kindly provided by Dr. Marc Bohner (RMS foundation, Bettlach, Switzerland). Dextran sodium sulfate (DSS) was purchased from Sigma-Aldrich (St. Louis, MO). Organic solvents 2,2,2-trifluorethanol (TFE; purity ≥ 99.8%) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP; purity ≥ 99.0%) were obtained from Acros (Geel, Belgium) and Sigma-Aldrich, respectively.
2.2. Scaffold preparation
Five different groups of scaffolds were prepared: 1) conventional electrospun scaffolds (2D); 2) wet electrospun scaffolds (3D); 3) wet electrospun scaffolds with ultrasonic treatment (3Du); 4) wet electrospun scaffolds supplemented with nHA (3DH); and 5) wet electrospun scaffolds with ultrasonic treatment and nHA supplement (3DHu). The preparation procedures were as follows.
To prepare electrospinning solution, PLGA/PCL (w/w = 3:1) was dissolved in TFE at a concentration of 0.12 g/ml. For the electrospinning solution containing nHA, a defined amount of nHA and DSS (w/v = 0.5%) was suspended in HFIP/TFE/phosphate buffered saline (PBS) (v/v = 10:9:1) solution by ultrasonic and vigorous stirring (UP50H Ultrasonic Processor, Hielscher Ultrasound Technology, Teltow, Germany) for 30 minutes. Then PLGA/PCL (w/w = 3:1) was dissolved in the solvent at a concentration of 0.2 g/ml. The weight ratio of polymer:nHA was 4:1. After magnetic stirring overnight, the prepared solution was fed into a plastic syringe with a blunt-end nozzle (18G), and fixed in the syringe holder of electrospinning machine (Esprayer ES-2000S, Fuence Co., Ltd, Tokyo, Japan).
For conventional electrospun scaffolds, a flat aluminium foil was used to collect the fibres, positioned 20 cm under the nozzle. The feeding rate of electrospinning solution was 20 μl/min, and a high voltage of 18.0 kV was applied to generate a stable polymer jet. The collection time was about 4 hours. For wet electrospun scaffolds, a grounded bath filled with 100% ethanol was used as collector. The other parameters were similar to those in the preparation of conventional scaffolds. To obtain the desired thickness, the process was stopped every 10 minutes for fibre mesh collection.
Subsequently, all the scaffolds were washed with Milli-Q water and lyophilized for 72 hours, then punched into disk-shaped forms (6 mm) using a biopsy punch (Kai medical, Gifu, Japan). 3Du and 3DHu scaffolds were further treated by UP50H Ultrasonic Processor (cycle 1, amplitude 100%) in a 50 ml centrifuge tube filled with 50% ethanol solution for 75 seconds and 120 seconds, respectively. Thereafter, the scaffolds were lyophilized again and stored at −80°C.
2.3. Porosity measurement
Porosity of the scaffolds was evaluated by a gravimetric measurement.17 The volume of the electrospun scaffold (n = 4) was calculated by measuring the dimensions of the scaffold. The weight of the scaffold was also measured to determine the apparent density of the scaffolds (ρap). Porosity was then calculated by using the following formula:
where ρm is the density of the blended PLGA/PCL (1.216 g/cm3) or PLGA/PCL/nHA (1.604 g/cm3), which was calculated based on the weight ratio and respective densities of each ingredient (ρPLGA= 1.24 g/cm3, ρPCL= 1.145 g/cm3, ρnHA= 3.156 g/cm3, from manufactures' databases).
2.4. Cell culture and scaffold seeding
Pig dental epithelial (pDE) cells and human tooth pulp derived dental mesenchymal (hDM) cells were isolated as described previously.19 The pDE cells were recovered from cryopreservation, seeded in a T175 cm2 flask (Corning Inc., Corning, NY), and expanded using epithelial medium [LHC-8, (GIBCO, Invitrogen, Carlsbad, CA), supplemented with 10% FBS, 1% penicillin/streptomycin/amphotericin, and 0.5 μg/ml epinephrine]. The hDM cells were expanded in mesenchymal medium [Advanced DMEM/F12 (GIBCO, Invitrogen, Carlsbad, CA), supplemented with 10% FBS, 25 μg/ml ascorbic acid, 1% penicillin/streptomycin/amphotericin, 1% Glutamax] after recovery from cryopreservation. Cells were cultured at 37°C in a humidified 5% CO2 atmosphere. The media for both cell types was refreshed every 2 - 3 days.
To prepare cells for seeding scaffolds, the cells were trypsinized, centrifuged at 1500 rpm for 5 minutes, then re-suspended in media. Scaffolds were sterilized in 70% ethanol for 2 h and socked in medium overnight at 37°C. Then a 15 μl cell suspension containing either hDM cells alone, or a mixture of pDE and hDM cells (1:1) was seeded to each scaffold, to achieve a final concentration of 2 × 105 cells/scaffold. Cell-seeded scaffolds were incubated at 37°C for 20 minutes in low-adhesive 24-well plates before adding medium. For further culture, osteogenic factors (100 nM dexamethasone, 10 mM β-glycerolphosphate and 50 μg/ml ascorbic acid) were added to the medium. For samples containing both pDE and hDM cells, a mixture of epithelial cell medium and mesenchymal cell medium was used (v/v = 1:1). Scaffolds without cells were used as controls, receiving identical pDE/hDM culture medium with osteogenic factors. Samples were collected after 1, 4, 9, 14 and 28 days in vitro culture.
2.5. SEM analysis
Scaffold morphology of acellular control samples (days 1 and 28) was observed by scanning electron microscopy (SEM; Zeiss, EVO MA series, Göttingen, Germany) after being sputter-coated with gold-platinum. Fibre diameters were measured from SEM micrographs that were obtained at random locations (n = 25) using Image J software (National Institutes of Health, Bethesda, MD).
Cell morphology on each type of scaffold (day 1 and 28) was also assessed by SEM. Samples were fixed in 2.5% (v/v) glutaraldehyde for 2 hours, washed with PBS, then additionally fixed with 1% (v/v) osmiumtetraoxyde for 2 hours. After been dehydrated in a graded ethanol, and dried in tetramethyl silane, samples were sputter-coated with gold-platinum, and imaged using SEM.
2.6. Histological analysis
To visualize cell distribution throughout each sample and obtain the cross-section view of these scaffolds, haematoxylin and eosin (H&E) and immunofluorescence (IF) staining was performed. Samples cultured in vitro for 1, 14 and 28 days were washed in PBS, fixed in 3.7% (v/v) formalin for 5 hours, dehydrated in a graded ethanol, and embedded in paraffin. Following de-paraffinization in xylene and rehydration through graded ethanol, 7-μm-thick sections were cut with a microtome (Leica RM2165, Nuss-loch, Germany).
For H&E staining, sections were stained with Haematoxylin for 1 minute, then washed with tap water, diluted hydrochloric acid (0.3%) and ammonia water. Sections were next stained with eosin for 20 seconds, dehydrated with ethanol (95%, 100%) and xylene, and then mounted with coverslips using Permount (Fisher Chemicals, Pittsburg, PA). Micrographs were taken using a Zeiss Axiophot microscope equipped with digital Zeiss Axiocam camera (Zeiss, Stuttgart, Germany).
For IF staining, pDE cells were stained with primary antibody rabbit-anti-human keratin (1:1 000, Euro-diagnostica, 2203PKE, Malmö, Sweden) and secondary antibody Alexa Fluor 594 Red antibody (1:50; Invitrogen, Life technologies Europe BV, A11012, Bleiswijk, The Nederland). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) according to standard protocol. Immunofluorescence images were obtained using a fluorescent microscope (Axio Imager Z1, Zeiss, Göttingen, Germany). Porcine skin control was used to confirm epithelial staining of the primary antibody. To control for non-specific staining, samples stained without primary antibody were included.
2.7. DNA content and alkaline phosphatase (ALP) activity assays
Samples (n = 4) from day 1, 4, 9, 14 and 28 were washed in PBS, then 1 mL of Milli-Q water was added to each well. After that, two freeze-thaw cycles were performed to lyse the cells. For the total DNA content assay, a Quan-iT™ Picogreen Kit (Invitrogen, Grand Island, NY) was used according to manufacturer's instruction. Briefly, 100 μL supernatant was added to 100 μL Picogreen working solution. After 5 minutes incubation at RT, the amount of DNA was measured using an ELISA microplate reader (VersaMax, Sunnyvale, CA) with a 485 nm excitation filter and a 530 nm emission filter. DNA content was determined from a standard curve with known amounts of DNA.
For the ALP activity assay, 80 μL supernatant was added to 120 μL working solution (Alkaline phosphatase Liquicolor no. 2900, Stanbio, Boerne, TX), and incubated at 37°C for 1 hour. Absorbance value at 405 nm was measured by an ELISA microplate reader (VersaMax). The standard curve was made by serial dilutions of 4-nitrophenol at final concentrations of 0 – 25 nM. ALP activity was normalized to the DNA content.
2.8. Quantitative polymerase chain reaction (qPCR)
After 14 and 28 days of culture, 3 scaffolds per group were pooled as one sample (n = 2), and RNA of the seeded cells was extracted using RNeasy Mini Kit (Qiagen Sciences, 74104, Valencia, CA) according to the manufacturer's instructions. RNA concentration was measured with spectrophotometer (NanoDrop 1000, Thermal scientific, Wilmington, DE). Subsequently, first strand cDNA was reverse transcribed from RNA using Rt2 First strand kit (Qiagen Sciences, 330411), according to the manufacturer's protocol.
cDNA was then amplified and specific gene expression was quantified using real-time PCR. Real-time PCR was performed using RT2 qPCR primers from Qiagen as specified in Table 2. Quantitative amplification detection was achieved using SABiosciences qPCR SYBR Green Master Mix (SABiosciences, Qiagen, 330521) in a real-time PCR (MxPro Mx3000P, Agilent technologies/genomics, Santa Clara, CA). Ameloblastic, odontoblastic and osteogenic-related gene markers were evaluated, including pig ameloblastin (pAMBN), human dentin sialophosphoprotein (hDSPP) and human bone gamma-carboxyglutamic acid-containing protein (hBGLAP), respectively. Housekeeping genes including pig glyceraldehyde 3-phosphate dehydrogenase (pGAPDH) and human Glyceraldehyde 3-phosphate dehydrogenase (hGAPDH) were used as reference genes. The cycling conditions were as follows: an initial 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 s and 60 °C for 1 minute. Then, a melting curve was constructed by heating at 95°C for 1 minute, 55 °C for 30 s and 95°C for 30 s. All qPCR data differentiation markers were analysed using the 2−ΔΔCt method and normalized against a reference gene. Relative expression was measured by comparing different scaffolds at different time-point with the 2D scaffold.
Table 2.
Real-time PCR primers in this study.
| Gene target | RefSeq Accession no.a | Reference positionb | Qiagen catalog no. |
|---|---|---|---|
| hDSPP | NM_014208.3 | 1318 | 330001 PPH57747E |
| hBGLAP | NM_199173.4 | 357 | 330001 PPH01898A |
| pAMBN | NM_214037.1 | 1610 | 330001 PPS00441A |
| hGAPDH | NM_002046.4 | 828 | 330001 PPH00150F |
| pGAPDH | NM_001206359.1 | 601 | 330001 PPS00192A |
Sequence used for primer design.
Position of amplicon within gene.
2.9. Statistical analysis
Data were expressed as mean standard ± deviation. Statistical analysis was carried out using Graphpad (GraphPad Inc, San Diego, CA) by one-way ANOVA and post hoc Tukey testing, for which differences were considered significant at p < 0.05.
3. Results
3.1. Scaffold characterization
By visual inspection, 2D scaffolds exhibited a compressed membrane-like structure, with thickness up to100 μm. In contrast, wet electrospun scaffolds exhibited a cotton-like appearance. Ultrasonic treatment led to an even more loose scaffold structure, although scaffold diameter remained the same (∼ 6 mm). Addition of nHA into wet electrospun scaffolds made the scaffolds denser. Even after ultrasonic treatment, 3DHu scaffolds still appeared less porous than 3Du scaffolds (Figure 1).
Figure 1.

Optical micrographs of 2D, 3D, 3Du, 3DH and 3DHu scaffolds; SEM images and H&E staining of each type of scaffold after 1 day and 28 days of culture without cells. Wet electrospun scaffolds exhibited a cotton-like appearance; ultrasonic treatment led to an even more loose scaffolds structure, and more gaps among fibres; Addition of nHA into wet electrospun scaffolds made the scaffolds denser, and led to a decreased fibre diameter. Arrows indicate the HA particles, some HA particles aggregated, and showed lager size than others; Scale bar = 2 mm in optical micrographs (1×); Scale bar = 10 μm in SEM images (2000×); Scale bar = 100 μm in H&E staining (40×).
SEM micrographs of 2D and 3D scaffolds showed that ultrasonic treatment resulted in a more diverse fibre arrangement, and larger pores among fibres. For the scaffolds containing nHA, fibre diameters were visibly smaller than those without nHA, and some fibres were stuck together to form a dense structure. Also, aggregated HA particles could occasionally be observed (Figure 1). Fibre diameter measurement confirmed that the addition of nHA led to a decreased fibre diameter. None of the groups showed degradation of electrospun fibres or decreased fibre diameter after 4 weeks of in vitro culture (p > 0.05) (Table 1).
Table 1.
Fibre diameter and porosity of the scaffolds. Note that the addition of nHA led to a decrease in fiber diameter. No changes in fiber diameter could be detected after 4 weeks of culture (p > 0.05, n = 25).
| Scaffold | Fiber diameter (day 1) | Fiber diameter (day 28) | Porosity (v/v) % |
|---|---|---|---|
| 2D | 2.239 μm ± 0.543 | 2.277 μm ± 0.665 | 93.972 ± 1.170 |
| 3D | 1.918 μm ± 0.546 | 2.150 μm ± 0.247 | 99.068 ± 0.266 |
| 3Du | 2.193 μm ± 0.783 | 2.198 μm ± 0.396 | 99.476 ± 0.151 |
| 3DH | 0.767 μm ± 0.311 | 0.675 μm ± 0.397 | 97.801 ± 0.609 |
| 3DHu | 0.983 μm ± 0.371 | 0.810 μm ± 0.435 | 99.000 ± 0.298 |
The H&E staining of the cross-section views of the different scaffolds corroborated the SEM results (Figure 1). The 2D scaffolds showed evenly distributed fibres, whereas the 3D scaffolds were generally less organized, and showed more gaps among the fibres. Ultrasonic treatment induced a looser structure where larger gaps could be found. Incorporation of nHA was confirmed by the purple-stained particles found aggregated along the fibres. Finally, 3DHu showed elongated separations in the scaffold architecture. No differences in morphological appearance were noticed over the time of the experiment.
The porosity measurements (Table 1) showed the highest porosity in 3Du scaffolds (99.476 ± 0.151%), while the lowest porosity was found in 2D scaffolds (93.972 ± 1.170%). Statistical analysis revealed that all wet electrospun scaffolds showed significantly higher porosities than the 2D scaffolds. Ultrasonically treated scaffolds showed significantly increased porosity compared to the non-treated ones. While nHA incorporation significantly decreased the scaffold porosity.
3.2. Cell morphology
SEM micrographs (Figure 2) revealed even cell distribution over all scaffold types at day 1. Still, there was an evident difference between the scaffold with nHA and the scaffold without nHA. On 2D, 3D, and 3Du scaffolds, cells had penetrated in between the scaffold fibres. In contrast, on 3DH and 3DHu scaffolds, cells mainly attached to the outer surface, without visible infiltration into the scaffold porosity. After 28 days of culture, a thick layer of extracellular matrix had formed on the scaffolds surface. 3D and 3Du scaffolds, which had higher porosity than the other scaffolds, also showed cell ingrowth into the deeper parts of the scaffolds. No obvious visual differences could be detected on cell morphology between hDM culture groups and hDM+pDE culture groups (Figure 2).
Figure 2.
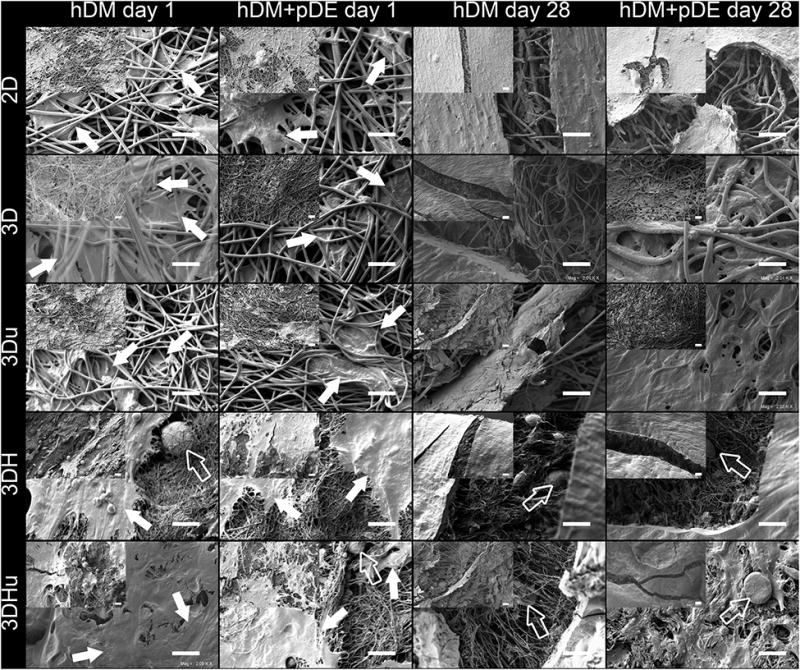
SEM images of 2D, 3D, 3Du, 3DH and 3DHu scaffolds seeded with either with hDM cells, or with hDM+pDE cells, at day 1 and at day 28. Even cell distribution over all scaffold could be found. Arrows indicate the evenly distributed cell at day 1, hollow arrows indicate the HA particles. Magnification = 2000×, scale bars = 20 μm; Lower magnification (500×) images were located in the upper-left corner of each image, scale bars = 40 μm.
3.3. H&E staining
Cell distribution throughout the scaffolds was investigated by histological analysis (Figure 3). At day 1, all scaffolds seeded with either hDM or hDM+pDE cells exhibited a cell layer on top. 3D, 3Du, 3DH and 3DHu scaffolds also showed cellular ingrowth in between the fibres after cell seeding (data not shown). After 14 and 28 days of culture, cell proliferation and migration into the scaffold became evident for all groups. 2D scaffold showed relatively poor cell infiltration at day 14, while the 3D scaffolds showed distribution of cells throughout the entire scaffold. Upon visual inspection, the hDM+pDE cell culture groups showed lower cell proliferation than hDM cell culture groups. Furthermore, evident clustering of cells in certain areas could be found in 3Du and 3DHu scaffolds seeded with hDM+pDE cells. Both scaffolds types containing nHA exhibited low cell proliferation, especially evident in hDM+pDE cell-seeded scaffolds.
Figure 3.

H&E staining of 2D, 3D, 3Du, 3DH and 3DHu scaffolds cultured with either hDM cells or hDM+pDE cells for 28 days. For each sample, low magnification (10×, scale bar = 500 μm) image provides the overview of cell distribution in the scaffold. High magnification (40×, scale bar = 100 μm) image of the framed area displays cell morphology and possible cell-cell interaction. Cell proliferation and migration into the scaffold were obvious for all group. Evident clustering of cells could be found in 3Du and 3DHu scaffolds seeded with hDM+pDE cells.
3.4. IF staining
To visualize the cell-cell interactions between hDM and pDE cells, and to identify pDE cells from hDM cells, immunofluorescent staining was performed (Figure 4). All nuclei were stained blue with DAPI. Rabbit-anti-human keratin and Alexa Fluor 594 was used to identify pDE cell staining (red). Figure 4C showed positive staining of epithelial cells in control tissue, i.e. porcine skin (keratin, red). No positive staining could be found in 3D scaffold seeded with hDM cells only (Figure 4D), or 3D scaffold seeded with hDM+pDE cells and stained without first antibody (Figure 4E). Therefore, the red stained cells were pDE cells, and the other blue stained nuclei in the hDM+pDE cell culture group represented hDM cells. In accordance with the H&E staining, 3D scaffolds seeded with either hDM or with hDM+pDE cells showed that cells could penetrate throughout the scaffold. pDE cells tended to concentrate in the centre of the scaffold, surrounded by the hDM cells (Figure 4A). In 3DHu group, pDE cells had a tendency to form denser cell clusters, where the rest of the scaffold was mainly filled by hDM cells (Figure 4B).
Figure 4.

Immunofluorescent staining with rabbit-anti-human keratin, Alexa Fluor 594 and DAPI. Cells stained in red are pDE cells, the other blue stained nuclei represent hDM cells. (A) 3D scaffold seeded with hDM+pDE cells at day 28, pDE cells tended to concentrate in the centre of the scaffold, surrounded by the hDM cells; (B) 3DHu scaffold seeded with hDM+pDE cells at day 28; (C) Positive staining of epithelial cells in porcine skin control; (D) 3D scaffold seeded with hDM cells at day 28; (E) 3D scaffold seeded with hDM+pDE cells at day 28 (stained without first antibody). 3D scaffolds seeded with either hDM or with hDM+pDE cells showed that cells could penetrate throughout the scaffold. Scale bars = 100 μm.
3.5. DNA content and ALP activity
Cell number and ALP activity in each scaffold was quantified by total DNA content and ALP activity assays at day 4, 9, 14, and 28 (Figure 5A). In general, all scaffolds seeded with hDM or hDM+pDE cells showed a significant increase in DNA content over time, although the mixed cell cultures showed weak initial proliferation in every scaffold type. For the scaffolds containing nHA, significantly lower DNA contents were detected. In the hDM culture groups, the 2D group showed the highest DNA content at day 28, with no statistical difference with 3D group at day 4, 9 and 14. The 3Du group showed equally high DNA content with 2D and 3D groups at day 4 and 9, but exhibited lower DNA content at day 14. Although 3DH and 3DHu groups showed the lowest DNA content at day 4, hDM cells continued to proliferate on these scaffolds over time. In hDM+pDE cell culture groups, similar results were obtained. In particular, the 3DHu group showed significantly lower DNA content than the 3DH group, at days 9, 14 and 28.
Figure 5.

DNA content (A) and ALP activity (B) on 2D, 3D, 3Du, 3DH and 3DHu scaffolds after cultured with either hDM cells or hDM+pDE cells for 4, 9, 14 and 28 days. All scaffolds seeded with hDM or hDM+pDE cells showed a significant increase in DNA content over time, although the mixed cell cultures showed weak initial proliferation in every scaffold type. For the scaffolds containing nHA, significant lower DNA contents were detected. The ALP activity of the 3DH group was significantly higher than the other hDM cell-seeded scaffold groups at day 4, and also the 3DHu group showed significantly higher ALP activity than the other scaffold groups seeded with hDM+pDE cells at days 4, 9 and 14. Error bars represent standard deviation (n = 4).
For the ALP activity of hDM cell only seeded scaffolds (Figure 5B), the 2D, 3D and 3Du groups showed slightly increased ALP activity over time, with significantly higher ALP activity in the 2D group at day 9. The 3DH group showed significantly higher ALP activity than 2D, 3D, 3Du and 3DHu groups at day 4, with no statistical differences among these last 4 groups. ALP activity was lower at day 9, and increased gradually at day 14 and 28. A similar trend could also be found in the 3DHu group. Both the 3DH and 3DHu groups showed significantly higher ALP activity than the 2D group at day 28. In the hDM+pDE cell-seeded scaffold groups, 2D and 3D groups showed a slightly increased ALP activity at day 9, which subsequently decreased over time. For the 3Du group, this trend was even more obvious, especially at day 9. Both the 3DH and 3DHu groups showed high ALP activity at day 4 and 9. In particular, 3DHu groups showed significantly higher ALP activity than the other groups from days 4 to 14. At day 28, ALP activity in hDM cell culture groups was significantly higher than that in hDM+pDE cell culture groups.
3.6. qPCR
The mRNA expression of odontoblastic (hDSPP), osteogenic (hBGLAP) and ameloblastic (pAMBN) related genes markers was quantitatively analysed by qPCR. In hDM cell-seeded scaffold groups, an 18-fold higher level of hDSPP expression was observed in the 3DH group, and an 8-fold higher level of hDSPP expression was observed in the 3DHu group at day 28, as compared to 2D, 3D and 3Du groups (Figure 6A). In hDM+pDE cell culture groups, scaffolds containing nHA also exhibited higher levels of hDSPP expression at days 14 and 28. Meanwhile, the 3DHu group showed at least 4-fold higher level of hDSPP expression than the 3DH group (Figure 6B). Compared to the hDM cell alone seeded scaffolds, the 2D scaffold seeded with hDM+pDE cells presented higher levels of hDSPP expression at both days 14 and 28.
Figure 6.
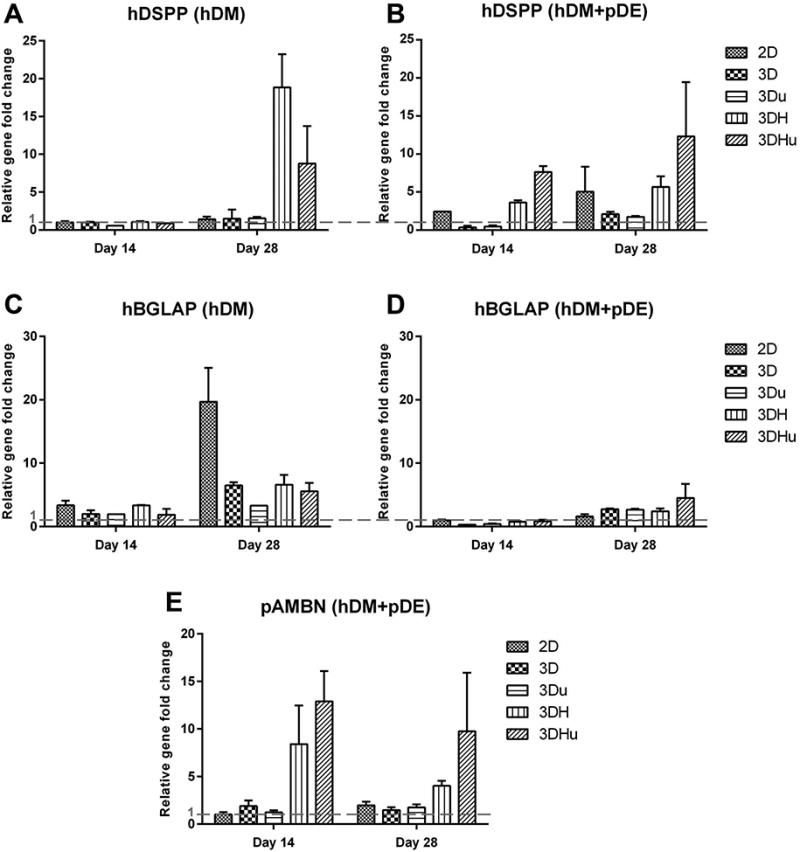
Human DSPP(A, B, to evaluate the odontoblastic differentiation of DM cells) and human BGLAP (C, D, to evaluate the osteogenic differentiation of DM cells) gene expression in hDM cells after 14 days and 28 days of culture on different scaffolds. Pig AMBN (E, to evaluate the ameloblastic differentiation of DE cells) gene expression in pDE cells after 14 days and 28 days of cultured with different scaffolds. The gene expression level of either hDM cells or hDM+pDE cells seeded on 2D scaffold at day 14 was used for normalization. The red dash line indicates the gene expression level of the reference group. 3DHu group exhibited at least a 4-fold higher level of hDSPP and pAMBN expression than 3DH group at day 14 and 28; hDM+pDE cell culture groups showed comparative higher expression of hDSPP in most of the groups, but lower hDSPP expression in hDM cell alone groups; hBGLAP expression in hDM cell-seeded scaffold groups was much higher than that in hDM+pDE cell-seeded scaffold groups.
As shown in Figure 6C and 6D, hBGLAP expression in hDM cell-seeded scaffold groups was much higher than that in hDM+pDE cell-seeded scaffold groups, which was in accordance with the ALP activity results obtained at day 28. In the hDM cell-seeded scaffold groups at day 28, no less than a 13-fold higher level of hBGLAP expression was observed in the 2D group as compared to the other groups.
Similar to the hDSPP gene expression results, scaffolds containing nHA exhibited higher levels of pAMBN expression as compared to the other hDM+pDE cell-seeded scaffold groups. Also, 3DHu group showed 4-fold and 6-fold higher level of pAMBN expression than 3DH group at day 14 and 28, respectively. No pAMBN expression could be detected in the hDM cell-seeded scaffold groups.
4. Discussion
Although human incisor, canine, premolar and molar teeth exhibit distinct size, shape and morphology, their development basically begins in the same manner: the reciprocal interactions between the dental epithelium and the neural crest cell-derived ectomesenchyme.2,20 By establishing proper methods to achieve accurate DE-DM cell-cell interactions, the in vitro formation of bioengineered tooth formation might be facilitated.10,21 To date, the most successful tooth organ regeneration was obtained by seeding DE and DM cells harvested from E14.5 tooth buds into adjacent regions within a collagen gel drop.6 However, such methods do not allow for translation to clinically achievable procedures, due to lack of autologous embryonic tooth cells to use for these applications, and that collagen gels do not provide appropriate stiffness to form teeth of pre-determined size and shape. No such results have yet been reported using post-natal dental cells, likely due to inappropriate cell-cell or cell-ECM interactions in a variety of scaffold materials used in these efforts. In the current study, wet electrospun PLGA/PCL scaffolds combined with or without ultrasonic treatment and nHA supplementation were fabricated and seeded with post-natal DE and DM tooth bud cells, in an attempt to establish 3D bioengineered tooth bud constructs in which more appropriate DE-DM cell interactions exist, to facilitate tooth regeneration.
Although electrospun scaffolds have the potential to resemble natural ECM, a common problem encountered with these scaffolds is an inherently low porosity, which inhibits adequate cellular infiltration.15,16,22 Therefore, in the present study, wet electrospinning technique and ultrasonic treatment were employed to achieve highly porous PLGA/PCL scaffolds. Furthermore, nHA was incorporated into the scaffolds to enhance dental cell differentiation. Overall, our results showed that wet electrospun PLGA/PCL scaffolds were highly porous and permitted ingrowth of hDM and pDE cells. Further ultrasonic treatment induced diverse fibre arrangement and large pores among the fibres, which led to evident cell clustering and thus to a more enhanced DE-DM cell interactions. Finally, the addition of nHA indeed had a positive effect on dental cell differentiation. However, such addition at the same time inhibited scaffold porosity and cell proliferation.
Regarding the experimental design, several remarks can be made. Firstly, the cell seeding was performed in a static way. Earlier studies, using other cells and scaffold types, often applied dynamic seeding methods. For instance, the scaffolds were rotated in cell suspensions, or slight vacuum was used to enhance cell infiltration throughout the scaffold.18,22 In a pilot study used to test this approach, a dynamic seeding method was found to create a rotational turbulence that over time proved to be too harsh for the DE cells (data not shown). Therefore, a static seeding method was used in the current study. Also, earlier investigations demonstrated that the cell seeding efficiency did not differ significantly between cell seeding methods.23,24 Secondly, only hDM and hDM+pDE cell populations were tested in this study. This is due to the fact that prior published reports from our group showed that scaffolds containing nHA inhibited DE cell proliferation, and that DE cells seeded on their own would not survive during cell culture.15,25
In the present study, five different scaffolds were fabricated. The 2D scaffolds were prepared according to conventional electrospinning protocol.26 Higher porosity was obtained by applying an ethanol collection bath (wet electrospinning) and further ultrasonic treatment. Moreover, the wet electrospun samples were prepared with or without the addition of nHA. Although we used “porosity” in scaffold characterization, of course the samples were not porous but rather were fibrous in nature. The porosity in this sense was regarded as an average inter-fibre space. As to the high porosity in the wet electrospun scaffolds, the effect of ethanol bath collection on the porosity was evident in SEM, porosity measurement and H&E staining results. Previous study demonstrated that ultrasonic treatment could loosen the densely packed electrospun poly(l-lactic) scaffolds.18 By mechanically separating them through vibration for 5 minutes, a 6-fold higher of fibre distance could be obtained. Also it was assumed that such an effect would increase the porosity of wet electrospun scaffolds, and a larger porosity would lead to better nutritional support, oxygen exchange and optimal cell infiltration. However, the results showed that the ultrasonic treatment mostly changed the homogeneity of the scaffold, with only a slight increase in porosity. This treatment did not lead to an uniform increase of inter-fibre distance, but rather to the creation of large gaps among fibres. Although unforeseen, this alteration in scaffolds architecture proved very beneficial in the cell culture. This special architecture namely lead to an apparent clustering of cells in hDM+pDE cell culture group, which subsequently optimally facilitated the interactions between hDM and pDE cells.
In IF staining, pDE cells tended to concentrate in the centre of the scaffold, surrounded by the hDM cells (Figure 4A). This cell distribution is similar with other study that co-culture DE-DM cells in vitro: DE cells formed rosette and tubular-like structures, DM cells located around DE cells. Moreover, DM cells proliferate faster than DE cells in vitro, then DE cells were trapped by the fast growing DM cells.10 It is also similar with that found in vivo: at the early stage of tooth development, tooth bud forms as a proliferation of the dental epithelium into the underlying dental mesenchyme. Further in vivo evaluation and long-term observation will be needed to investigate the DE-DM cell distribution.
As to the cell-cell interaction between hDM and pDE cells, both H&E and IF staining confirmed that ultrasonic treatment could induced intense clustering of hDM and pDE cells, while the distribution of these cells was relatively more separate in 3D and 3DH scaffolds. The 3Du and 3DHu scaffolds also supported the proliferation of hDM and pDE cells. The 3Du group showed equally high DNA content with 2D and 3D scaffolds at day 28, while cell proliferation in 3DHu group was inhibited by nHA. Both 3Du and 3DHu group showed significantly higher ALP activity than 2D and 3D groups at day 9. Similarly, the qPCR results exhibited that 3DHu group exhibited at least a 4-fold higher level of hDSPP and pAMBN expression than 3DH group at day 14 and 28. Together, these results confirmed that ultrasonic treatment could induce cell clustering in the 3D scaffold, which could result in more intense hDM-pDE cell-cell interactions resulting in higher ameloblastic and odontoblastic differentiation. Interestingly, the fact that hDM+pDE cell culture groups showed comparative higher expression of hDSPP in most of the groups, but lower hDSPP expression in hDM cell alone groups suggest DE cells promote the differentiation of DM cells to odontoblastic direction rather than osteogenic direction. For DE-DM co-culture group, it is favourable to guide DM cells to odontoblastic direction for dentin formation, and guide DE cells to ameloblastic direction for enamel formation, then it will ultimately regenerate the whole tooth. As to DM cells cultured alone, it is favourable to guide DM cells to osteogenic direction for alveolar bone formation, then it will support the regenerated tooth.
Regarding the incorporation of nHA into the scaffolds, the beneficial effect seems to be controversy. According to literature, nHA can enhance the attachment, proliferation and differentiation of bone forming cells, as well as in vivo bone generation.27,28 Such nHA incorporation was shown to enhance the expression of osteogenic related genes in human mesenchymal stem cells, such as ALP, osteocalcin and bone sialoprotein, and promote ECM mineralization. In dental regeneration, a previous study also indicated that nHA had an effect on cell proliferation, differentiation and ECM production of porcine tooth bud cells.15 Such results were partially similar to our current findings. In 3DH and 3DHu groups, DNA content did not increase much over time, and they were significantly lower than 2D, 3D and 3Du groups. The ALP activity of the 3DH group was significantly higher than the other hDM cell-seeded scaffold groups at day 4, and also the 3DHu group showed significantly higher ALP activity than the other scaffold groups seeded with hDM+pDE cells at days 4, 9 and 14. This could be interpreted as the result of DE-DM cells differentiating immediately after seeding under the influence of nHA, and thus the actual cell proliferation was inhibited. The inhibited cell proliferation may lead to delayed tooth regeneration, or even fail to regenerate a whole tooth. Likewise, our qPCR results also indicated that incorporation of nHA benefited odontoblastic differentiation of hDM cells, and ameloblastic differentiation of pDE cells when co-cultured with hDM. Low expression of pAMBN in 3Du group suggest the cell clustering may not benefit the ameloblastic differentiation necessarily. The present of nHA is essential in this case.
Still, in the current study, we also found that the nano-scale apatite crystals were of great influence to the spinning process. Scaffolds exhibited lower fibre diameter and porosity than the scaffolds without nHA, and exhibited a lesser uniform appearance, especially at the surface. This may be because the addition of nHA and dextran sodium sulfate can reduce the viscosity, and increase the conductivity of the PLGA/PCL solution. Such undesirable side-effects of nHA on scaffold morphology have also been noticed by other researchers.29 For future studies, higher fibre diameter and porosity of these scaffolds may be created using higher polymer concentrations and lower nHA content, or with a higher spinning flow rate. Other methods to incorporate or release nHA from the scaffold should also be investigated, and additional DE-DM cell stimulation by other means such as the addition of growth factors.
5. Conclusion
Based on our findings, and within the limitations of this study, it can be concluded that ultrasonic treated wet electrospun PLGA/PCL scaffolds are a suitable scaffold material for dental tissue engineering. In this study, wet electrospun scaffolds exhibited sufficient porosity to support dental cell ingrowth. Additional ultrasonic treatment led to a less homogeneous scaffold porosity, resulting in evident cell clustering, thus benefiting DM-DE cell-cell interactions. Finally, the addition of nHA had a positive effect on dental cell differentiation. However, the addition of nHA at the same time induced lower fibre diameter and porosity, as well as inhibited cell ingrowth and proliferation. Further in vivo evaluation of this cell-scaffold construct is warranted for the future.
Acknowledgments
The authors appreciate the Medical Honours Programme (Radboud University Nijmegen) and Ms. Natasja van Dijk for immunofluorescent staining. This study was financially supported by Royal Netherlands Academy of Arts and Sciences (KNAW; project number CEP-10CDP025), and NIH/NIDCR R01 DE016132 (PCY).
Footnotes
No benefit of any kind will be received either directly or indirectly by the authors.
References
- 1.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 2.Duailibi SE, Duailibi MT, Vacanti JP, Yelick PC. Prospects for tooth regeneration. Periodontol. 2006;200041:177–187. doi: 10.1111/j.1600-0757.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 3.Chai Y, Slavkin HC. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003;60:469–479. doi: 10.1002/jemt.10287. [DOI] [PubMed] [Google Scholar]
- 4.Peters H, Balling R. Teeth - where and how to make them. Trends in Genetics. 1999;15:59–65. doi: 10.1016/s0168-9525(98)01662-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. Making a tooth: growth factors, transcription factors, and stem cells. Cell Research. 2005;15:301–316. doi: 10.1038/sj.cr.7290299. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 8.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 9.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WB, Ahluwalia IP, Yelick PC. Three dimensional dental epithelial-mesenchymal constructs of predetermined size and shape for tooth regeneration. Biomaterials. 2010;31:7995–8003. doi: 10.1016/j.biomaterials.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji W, Sun Y, Yang F, van den Beucken JJJP, Fan MW, Chen Z, Jansen JA. Bioactive Electrospun Scaffolds Delivering Growth Factors and Genes for Tissue Engineering Applications. Pharm Res. 2011;28:1259–1272. doi: 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomaterialia. 2011;7:2769–2781. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Both SK, Yang XC, Walboomers XF, Jansen JA. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomaterialia. 2009;5:3295–3304. doi: 10.1016/j.actbio.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2010;93:247–257. doi: 10.1002/jbm.a.32535. [DOI] [PubMed] [Google Scholar]
- 15.van Manen EH, Zhang W, Walboomers XF, Vazquez B, Yang F, Ji W, Yu N, Spear DJ, Jansen JA, Yelick PC. The influence of electrospun fibre scaffold orientation and nano-hydroxyapatite content on the development of tooth bud stem cells in vitro. Odontology. 2014;102:14–21. doi: 10.1007/s10266-012-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rampichova M, Chvojka J, Buzgo M, Prosecka E, Mikes P, Vyslouzilova L, Tvrdik D, Kochova P, Gregor T, Lukas D, et al. Elastic three-dimensional poly (epsilon-caprolactone) nanofibre scaffold enhances migration, proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2013;46:23–37. doi: 10.1111/cpr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Yang F, Wang Y, Both SK, Jansen JA. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater. 2013;9:4505–4512. doi: 10.1016/j.actbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Lee JB, Jeong SI, Bae MS, Yang DH, Heo DN, Kim CH, Alsberg E, Kwon IK. Highly porous electrospun nanofibers enhanced by ultrasonication for improved cellular infiltration. Tissue Eng Part A. 2011;17:2695–2702. doi: 10.1089/ten.TEA.2010.0709. [DOI] [PubMed] [Google Scholar]
- 19.Abukawa H, Zhang W, Young CS, Asrican R, Vacanti JP, Kaban LB, Troulis MJ, Yelick PC. Reconstructing Mandibular Defects Using Autologous Tissue-Engineered Tooth and Bone Constructs. Journal of Oral and Maxillofacial Surgery. 2009;67:335–347. doi: 10.1016/j.joms.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morotomi T, Kawano S, Toyono T, Kitamura C, Terashita M, Uchida T, Toyoshima K, Harada H. In vitro differentiation of dental epithelial, progenitor cells through epithelial-mesenchymal interactions. Arch Oral Biol. 2005;50:695–705. doi: 10.1016/j.archoralbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Tan LJ, Ren YJ, Kuijer R. A 1-min Method for Homogenous Cell Seeding in Porous Scaffolds. Journal of Biomaterials Applications. 2012;26:877–889. doi: 10.1177/0885328210389504. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YF, Sae-Lim V, Chou AM, Hutmacher DW, Lim TM. Does seeding density affect in vitro mineral nodules formation in novel composite scaffolds? Journal of Biomedical Materials Research Part A. 2006;78A:183–193. doi: 10.1002/jbm.a.30685. [DOI] [PubMed] [Google Scholar]
- 24.Buizer AT, Veldhuizen AG, Bulstra SK, Kuijer R. Static versus vacuum cell seeding on high and low porosity ceramic scaffolds. Journal of Biomaterials Applications. 2014;29:3–13. doi: 10.1177/0885328213512171. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Lee DS, Choung HW, Shon WJ, Seo BM, Lee EH, Cho JY, Park JC. Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials. 2011;32:9696–9706. doi: 10.1016/j.biomaterials.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Ji W, Yang F, Seyednejad H, Chen Z, Hennink WE, Anderson JM, van den Beucken JJ, Jansen JA. Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation. Biomaterials. 2012;33:6604–6614. doi: 10.1016/j.biomaterials.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Qian JM, Xu WJ, Yong XQ, Jin XX, Zhang W. Fabrication and in vitro biocompatibility of biomorphic PLGA/nHA composite scaffolds for bone tissue engineering. Materials Science & Engineering C-Materials for Biological Applications. 2014;36:95–101. doi: 10.1016/j.msec.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesan J, Kim SK. Nano-hydroxyapatite composite biomaterials for bone tissue engineering--a review. J Biomed Nanotechnol. 2014;10:3124–3140. doi: 10.1166/jbn.2014.1893. [DOI] [PubMed] [Google Scholar]
- 29.You Y, Lee SJ, Min BM, Park WH. Effect of solution properties on nanofibrous structure of electrospun poly(lactic-co-glycolic acid) Journal of Applied Polymer Science. 2006;99:1214–1221. [Google Scholar]


