Abstract
Background
The purpose of this study was to define and characterize the thyroid tumor-draining lymph nodes in genetically engineered mice harboring thyroid-specific expression of oncogenic BrafV600E with and without Pten insufficiency.
Methods
After intratumoral injection of methylene blue, the lymphatic drainage of the thyroid gland was visualized in real time. The thyroid gland/tumor was resected en bloc with the respiratory system for histological analysis.
Results
Although mice harboring BrafV600E mutations were smaller in body size compared with their wild-type (WT) littermates, the size of their thyroid glands and deep cervical lymph nodes were significantly larger. Additionally, the tumor-draining lymph nodes showed increased and enlarged lymphatic sinuses that were distributed throughout the cortex and medulla. Tumor-reactive lymphadenopathy and histiocytosis, but no frank metastases, were observed in all mice harboring BrafV600E mutations.
Conclusions
The tumor-draining lymph nodes undergo significant structural alterations in immunocompetent mice, and this may represent a primer for papillary thyroid carcinoma (PTC) metastasis.
Keywords: cancer, lymph node, metastasis, mouse, thyroid
1 INTRODUCTION
By the year 2019, thyroid cancer is expected to become the third most common cancer in women and will cost the United States approximately 20 billion dollars.1 Papillary thyroid carcinoma (PTC), which accounts for 85%–90% of all thyroid cancer, is generally considered a relatively indolent and highly curable disease.2 However, initial treatment is not always curative, and persistent or recurrent disease occurs in up to 30% of patients.3 The most common site for PTC metastasis and recurrent disease is the locoregional lymph nodes.3 Although PTC lymph node metastasis is associated clinically with both a higher risk for disease recurrence and disease-specific mortality, the mechanisms underlying lymphatic dissemination of tumor cells remain elusive.3–5
BRAFV600E mutation, which constitutively activates the mitogen-activated protein kinases – extracellular signal-regulated kinases pathway, is the most common genetic alteration in thyroid cancer and occurs in 44% of PTC and in 24% of PTC-derived poorly differentiated thyroid carcinoma or anaplastic thyroid carcinoma (ATC) cases.6–8 In addition to the BRAFV600E mutation, ATC also frequently harbors one or more genetic alterations in the phosphatidylinositol-4,5-bisphosphate 3-kinase – protein kinase B pathway (eg, PIK3CA, AKT1, and PTEN).9,10 ATC is one of the most lethal types of cancer, with a median survival of just 5 months.11 Although metastasis is observed in 10%–20% of cases, most patients with ATC die because of locally invasive disease, which is generally refractory to conventional treatment modalities (ie, radiation and chemotherapy).11
The lack of successful treatment options for advanced thyroid cancer underscores the need for novel therapeutic strategies, as well as in vivo models that can help predict their clinical utility. In the past, in vivo models were developed by injecting human thyroid cancer cell lines into immunodeficient mouse strains.2,7,12 Immunodeficient models, however, fail to mimic the natural tumor microenvironment, because they do not evoke protective immune responses or tumor-promoting inflammation.13 For this reason, genetically engineered strains have been developed to study tumors that arise in situ in immunocompetent hosts.2,7,12 In order to rapidly induce tumor development, orthotopic injection of tumor cell lines derived from genetically engineered mice have also been used.2,7,12 Although such models are practical for defining the underlying molecular mechanisms of tumor development, the rapid progression of disease does not allow for a step-wise analysis of later events, such as lymph node metastasis.12 Moreover, subcutaneous or orthotopic injection of tumor cells creates a wound, which may confound interpretation of the mechanisms involved in disease progression, such as, thyroid capsule invasion, extrathyroidal extension, regional lymph node metastasis.12
Mouse models have demonstrated that physiological levels of oncogenic BrafV600E expression alone is sufficient to initiate de novo thyrocyte tumorigenesis in immunocompetent mice.14 In general, the latency and extent of these tumors depends on the presence of an intact thyroid-stimulating hormone signaling axis at the time of tumor initiation.15 Despite the fact that physiological levels of oncogenic BrafV600E expression result in de novo thyrocyte tumorigenesis and invasive PTC with relatively short latency times,14,15 the tumor-draining lymph nodes have not yet been comprehensively examined in genetically engineered mouse models.10,14–17 Unfortunately, it is not always clear from the literature whether this is because lymphatic disease was not present in prior studies or whether the tumor-draining lymph nodes were not routinely examined.
In this study, we developed a technique to identify the lymphatic drainage of the murine thyroid gland in real time in order to facilitate our studies of lymph node responses to thyroid cancer. Herein, we demonstrate that the tumor-draining lymph nodes undergo significant structural alterations in mice harboring PTC, and this may represent a priming of the lymph nodes for metastasis.
2 MATERIALS AND METHODS
2.1 Experimental animals
All animal experiments were performed at the University of Arkansas for Medical Sciences and approved by the Institutional Animal Care and Use Committee. The LSL-BrafV600E, Ptenfl/fl, and thyroid peroxidase promoter (TPO)-Cre strains have previously been described.15,18 Mice were on mixed C57BL6/129SVJ (B6) genetic backgrounds. Genotypes were determined by reverse transcription-polymerase chain reaction, as previously described.18 Three male and 6 female mice: (a) wild-type (WT; 1 male and 2 female), 8–10 weeks old; (b) LSL-BrafV600ECre+ (1 male and 2 female), 3–5 weeks old; and (c) LSL-BrafV600E/Pten+/−/Cre+ (1 male and 2 female), 10–12 weeks old; were maintained in a barrier facility on high efficiency particulate air-filtered racks.
All surgical procedures were performed with the mice under general anesthesia with 2.5% isoflurane followed by immediate euthanasia. Euthanasia was achieved by 100% carbon dioxide insufflation, followed by cervical dislocation.
2.2 Lymph node mapping
We used these models as a first step to examine the involvement of the lymphatic system in dissemination of PTC. To trace the regional lymphatic drainage, a 25-microliter Hamilton syringe (Hamilton Company, Reno, NV) and a 26-gauge standard double-wing needle (pointed in a rostral and medial direction) was used to inject (depth 2–3 mm; velocity approximately 40–50 microliters/minute) a mixture of 1% methylene blue (Akorn, Lake Forest, IL) and 10 microliters phosphate-buffered saline into the left thyroid lobe/tumor approximately 15 minutes before euthanasia. Alternatively, the footpad was injected subcutaneously with the needle pointed in a caudal direction. The total volume of dye injected in each case was 2 microliters. Distilled water was used to flush any extravasation of dye away from the surrounding soft tissue.
Lymph nodes were classified based on their anatomic location using the nomenclature summarized by Van den Broeck et al19 and Shao et al20: mandibular lymph nodes, accessory mandibular lymph nodes, superficial parotid lymph nodes, cranial deep cervical lymph nodes, caudal deep cervical lymph nodes, proper axillary lymph nodes, accessory axillary lymph nodes, cranial mediastinal lymph nodes, tracheobronchial lymph nodes, and popliteal lymph nodes.
2.3 Tissue preparation and histology
At necropsy, the primary tumor was resected en bloc with the respiratory system for processing and analysis. The neck and lung tissue specimens were fixed in 10% formalin-buffered acetate overnight, dehydrated, and embedded in paraffin. The embedded specimens were cut into 5-μm serial sections, stained with hematoxylin-eosin, and reviewed by a high-volume thyroid pathologist (N.M.).
2.4 Subcapsular sinus width
The width (μm) of the marginal or subcapsular sinus (SCS) was measured in hematoxylin-eosin section images at ×40–×200 magnification using ImageJ (National Institutes of Health, Bethesda, MD). Statistical analysis was performed using GraphPad Prism 7 software (La Jolla, CA). Differences between groups were assessed by paired Student’s t test. All data were presented as the mean ± SD, and P values of ≤.05 were considered statistically significant.
3 RESULTS
3.1 Primary tumor
BrafV600E mice were significantly runted compared with their WT littermates, consistent with previous reports because of hypothyroidism in BrafV600 animals.15 Histologic examination of thyroid tumors obtained from 3-week-old LSL-BrafV600E/TPO-Cre and 12-week-old LSL-BrafV600E/Pten+/−/TPO-Cre mice demonstrated multifocal PTC involving both thyroid lobes. Compared to WT (Figure 1A), the BrafV600E/Pten−/−/TPO-Cre tumor sections demonstrated an extensive stromal component that was encompassed by a thick fibrotic capsule (Figure 1B–E), consistent with reports that these tumors secrete tumor-derived factors that induce fibroblast recruitment and abundant stromal deposition of fibrillar collagen.18 BrafV600E/TPO-Cre tumor sections exhibited highly cellular, florid PTC with areas of central necrosis, extrathyroidal invasion, and tumor-reactive stroma (Figure 1F). None of the tumors progressed to poorly differentiated thyroid carcinoma or ATC during the observed time course (21–84 days).
FIGURE 1.

Thyroid pathology. A, Normal thyroid parenchyma composed of colloid (CO)-rich follicles in an 8-week-old wild-type mouse (original magnification ×100). B, Intrathyroidal papillary thyroid carcinoma (PTC) encompassing the majority of the thyroid lobe, which is surrounded by a thick fibrotic capsule (CAP), in a 3-week-old BrafV600E/TPO-Cre mouse (original magnification ×100). C, Papillae lined by tumor cells with nuclear overlapping and crowding in a 3-week-old BrafV600E/TPO-Cre mouse (original magnification ×200). D, PTC and tumor-reactive stroma adjacent to skeletal muscle (SkM) and cartilage (CAR) in a 3-week-old BrafV600E/TPO-Cre mouse (original magnification ×100). E, PTC tumor composed of a large stromal component in a 12-week-old BrafV600E/Pten+/−/TPO-Cre mouse (original magnification ×100). D, High-grade PTC exhibiting central necrosis (NEC), extrathyroidal invasion, and tumor reactive stroma (TRS) in a 12-week old BrafV600E/Pten+/−/TPO-Cre mouse (original magnification ×40). All sections were stained with hematoxylin-eosin [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Lymph node mapping
Accurate identification of murine lymph nodes (<2 mm) is challenging because of their small size (<2 mm). Methylene blue and other dyes, which preferentially drain via the lymphatic system, have been used in the past to distinguish lymph nodes from the surrounding fat and connective tissue.21 To date, however, most descriptions of blue-dye injection techniques in the mouse have focused on the hindfoot and lateral tail base.22,23 The locoregional lymph nodes that are responsible for draining the thyroid gland are poorly defined. Therefore, we sought to identify the lymph nodes that drain the thyroid in the mouse and determine whether this technique could be used to identify and isolate tumor-draining lymph nodes.
To expose the thyroid gland, a 1.0-cm longitudinal midline cervical incision was made in the anterior neck. The salivary glands were retracted laterally, and the underlying superficial strap muscles were then elevated away from midline, revealing the trachea and thyroid lobes (Figure 2A–C). The deeper strap muscles were left intact on the trachea.
FIGURE 2.
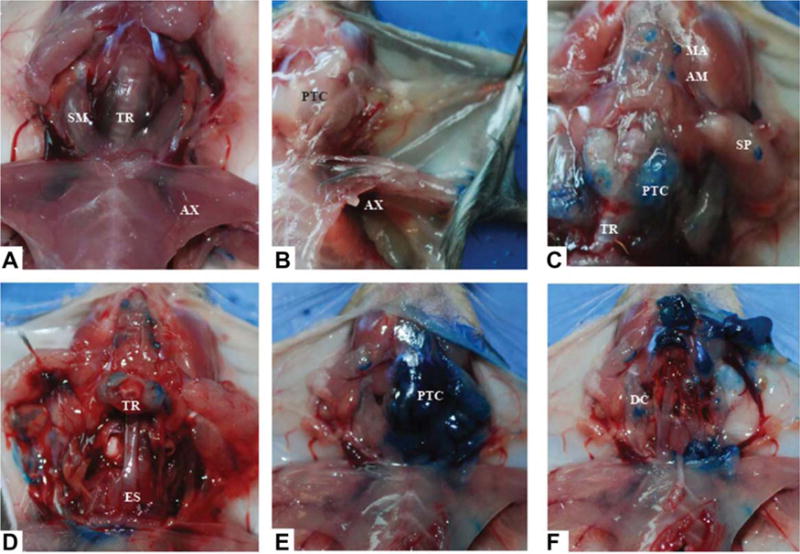
Lymph node mapping. A, Thyroid draining lymph nodes were not observed in the wild-type mouse, regardless of whether the dye was injected into the thyroid bed while the mouse was alive or dead. B, Only the ipsilateral axillary (AX) lymph nodes and the axillary vein were stained when dye was injected into the mouse footpad, and this had to be performed while the animal was alive. C, After injection of the left thyroid lobe/tumor (papillary thyroid carcinoma [PTC]), the mandibular (MA), accessory mandibular (AM), and superficial parotid (SP) lymph nodes were stained. D, The esophagus (Es) was easily visualized after sectioning of the trachea (TR), which is reflected caudally with the overlying tumor intact. E, Large tumor burden (PTC) occupying the left side of the neck. F, Subsequent dissection and removal of all nonlymphatic contents from the tracheoesophageal groove to the sternomastoid muscle (SM) revealed the deep cervical (DC) lymph nodes [Color figure can be viewed at wileyonlinelibrary.com]
Despite intrathyroidal injection and adequate time for systemic distribution (15–20 minutes), the salivary and deep cervical lymph nodes could not be visualized in WT mice (Figure 1A). In contrast, the blue-labeled mandibular (located rostromedial to the mandibular and sublingual salivary glands), accessory mandibular (located dorsolateral to the mandibular lymph nodes), and superficial parotid lymph nodes (located cranioventral to the parotid salivary gland) were easily located after intratumoral injection in the BrafV600E-positive models (Figure 2C). The cranial deep cervical lymph nodes (located adjacent to the thyroid gland capsule at the level of the second and third tracheal rings) and caudal deep cervical lymph nodes (located adjacent to the cervical thymus at the level of the third and fourth tracheal rings) were not visible until the thyroid tumor and surrounding soft tissue were removed (Figure 2D–F). Staining of the salivary gland lymph nodes and deep cervical lymph nodes occurred regardless of whether the dye was injected when the animal was alive or dead (Figure 2C).
After injection of the left frontal footpad, the underlying axillary lymph nodes were visualized (Figure 2A,B). A similar relationship could not be demonstrated after injection of the right footpad. This relationship is consistent with previous reports and is likely a result of the poorly connected lymphatic vasculature that connects the right and left lymphatic drainage systems in the mouse.22 In order to demonstrate the ipsilateral axillary lymph nodes, injection at the mouse foot-pad had to be performed while the mouse was still alive.
3.3 Tumor-reactive lymphadenopathy
Although no tumors demonstrated lymphatic or distant metastasis, the deep cervical lymph nodes in the BrafV600E-positive models, especially in the setting of concurrent Pten insufficiency, were enlarged and inflamed compared with the corresponding lymph nodes in WT mice (Figure 3A–F). The tumor-draining lymph nodes demonstrated increased and enlarged lymphatic sinuses that were distributed throughout the cortex and medulla (Figure 3C,E). In contrast, popliteal lymph nodes isolated from WT and BrafV600E/PtenHet/TPO-Cre animals were indistinguishable (Figure 3G,H) demonstrating the specificity of this response to tumor-draining lymph nodes.
FIGURE 3.

Tumor reactive lymphadenopathy. A, Normal thyroid parenchyma, tracheal cartilage (TC), and caudal (CA) deep cervical lymph node in a wild-type (WT) mouse (original magnification ×40). B, Cranial (CR) deep cervical lymph node draining a high-grade papillary thyroid carcinoma (PTC) tumor with extrathyroidal extension in a BrafV600E/TPO-Cre mouse (original magnification ×100). C, Cranial deep cervical lymph node draining an intrathyroidal PTC in a BrafV600E/TPO-Cre mouse (original magnification ×200). D, Caudal deep cervical lymph node with a subcapsular sinus (SCS) width measuring 8.6 μm in a wild-type mouse (original magnification ×200). E, Cranial deep cervical lymph node with a SCS measuring 41.3 μm in a BrafV600E/Pten+/−/TPO-Cre mouse (original magnification ×200). SCS is measured in micro-meters (black bars) in D and E. F, Cranial deep cervical lymph node demonstrating subcapsular histiocytes in a BrafV600E/Pten+/−/TPO-Cre mouse (original magnification ×100). G, Popliteal lymph node in an 8-week-old wild-type mouse (original magnification ×100). H, Popliteal lymph node in a 3-week-old BrafV600E/TPO-Cre mouse (original magnification ×100). I, Tumors in mice with oncogenic BrafV600E expression with and without Pten insufficiency had larger SCS widths compared with wild-type animals. Each dot represents an individual lymph node. Student’s t test, *P <.05. All sections were stained with hematoxylin-eosin [Color figure can be viewed at wileyonlinelibrary.com]
On average, the SCS of the deep cervical lymph nodes were significantly (P 5 0.045) dilated in mice harboring BrafV600E mutations (LSL-BrafV600E 44.7 ± 20.8 μm; range 20.7–57.1 μm); LSL-BrafV600E/Pten+/−/Cre+ 45.3 ± 5.8 μm (range 41.3–49.4 μm) compared to WT (10.8 ± 1.9 μm; range 8.8–12.6 μm; Figure 3I). These findings indicate that PTC promotes expansion of lymphatic sinuses in the locoregional lymph nodes, and these effects are restricted to the tumor-draining lymph nodes, as the nondraining lymph nodes in tumor-bearing mice showed normal lymphatic sinuses (Figure 3D). Finally, the size of the lymph nodes and the SCSs were irrespective of age differences. No evidence of lung metastases was found in any of the animals.
4 DISCUSSION
4.1 Nodal mapping
Although the anatomy of murine lymph nodes has been described previously, detailed descriptions of the cervical lymph nodes and drainage patterns of the thyroid gland are notably absent. In particular, confusion exists regarding the precise location and proper nomenclature of the cranial and caudal deep cervical lymph nodes.19 In a prior study, Van den Broeck et al19 developed an anatomic chart with the locations and terminology of 22 different lymph nodes in BALB/cAnNCrl mice. In their review, they could not find any evidence of the caudal deep cervical lymph nodes, which have been previously been described ventral to the trachea and dorsal to the sternum at the level of the first 2 ribs.19
In our study, a pair (right and left) of lymph nodes was consistently observed immediately adjacent to the thyroid capsule at the level of the second and third tracheal rings. A second pair of lymph nodes was also observed at the level of the third and fourth tracheal rings. The slightly lower positioning of the caudal deep lymph nodes observed in our study is consistent with the anatomic description described in the MXH10/Mo/lpr mouse strains.20 Because the cranial deep cervical lymph nodes are located immediately adjacent to the thyroid capsule, it is possible that these lymph nodes are often overlooked when removing the thyroid during deeper dissections (Table 1). Alternatively, there may be strain-specific murine variations in the number and positioning of the deep cervical lymph nodes. Future studies should elaborate on the anatomy of the cranial and caudal deep cervical lymph nodes to help explain the differences reported in the literature.
TABLE 1.
Literature review of major BRAFV600E-positive papillary and/or poorly differentiated thyroid carcinoma genetically engineered mouse models
| Reference | Line | Strain | Age, weeks | Metastasis | ||
|---|---|---|---|---|---|---|
| Local | Lymph node | Distant | ||||
| Knauf et al14 2005 | Tg-BRAF2 | FVB/N | 22 | Y | Y | N |
| Knauf et al14 2005 | Tg-BRAF3 | FVB/N | 22 | N | Y | N |
| Franco et al15 2011 | LSL-BrafV600E/TPO-Cre | C57BL/6 | 5 | Y | N | N |
| Chakravarty et al17 2011 | Tg-rtTA/tetO-BRAFV600E | FVB/N | 12DI | Y | N | N |
| Charles et al10 2011 | Thyro:CreER/BRAFCA/+ | C57BL/6 | 48TI | N | N | N |
| McFadden et al16 2014 | Thyro:CreER/BRAFCA/+/Trp53F/F | C57BL/6 | 24TI | Y | N | Y |
| Charles et al40 2014 | Thyro:CreER/BRAFCA/+/Pik3caLat/+ | FVB/N | 48TI | Y | N | N |
| Jolly et al18 2016 | BrafV600E/Pten−/−/TPO-Cre | C57BL/6 | <1 | Y | N | N |
| Hinson et ala 2016 | LSL-BrafV600E/TPO-Cre | C57BL/6 | 5 | N | N | N |
| Hinson et ala 2016 | LSL-BrafV600E/Pten+/−/TPO-Cre | C57BL/6 | 12 | Y | N | N |
Abbreviations: DI, doxycycline inducible; TI, tamoxifen inducible.
Presented herein.
4.2 Tumor-reactive lymphadenopathy
In the past, lymph node metastasis has been thought of as a relatively passive process, in which detached tumor cells drain via preexisting lymphatic vasculature to locoregional lymph nodes.24,25 However, recent studies have demonstrated that tumor dissemination is a highly regulated process that is mediated by a complex array of molecular signals.26,27 For example, vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor D (VEGF-D), which bind to and stimulate vascular endothelial growth factor 3 (VEGF-3) expressed on the lymphatic endothelium, are potent inducers of lymphatic endothelial proliferation and vessel enlargement.28 In both human and animal models, the expression of VEGF-C is significantly correlated with lymph node metastasis in a number of tumor types (eg, thyroid,29 prostate,30 gastric,31 colorectal,32 esophageal,33 lung,34 and breast35).36 The PTC tumor cells express VEGF-3, the major receptor for VEGF-C, and is hypothesized to benefit from VEGF-C autocrine signaling.26,36
Features of tumor-reactive lymphadenopathy include increased lymph flow, dilated lymphatic sinuses, and reorganization of the lymphatic channels.28,37 Importantly, alterations in the lymphatic architecture arise even before tumor cells reach the draining lymph nodes, indicating that the primary tumor can induce these alterations at a distance.28,37,38 In a study involving implanted melanoma cells in syngeneic C57B1/6 mice, lymph node lymphangiogenesis began before melanoma cells reached the draining lymph nodes.37 Interestingly, the primary footpad tumors, which were infiltrated primarily by macrophages and other leukocytes, showed no lymphatic or blood vessel growth. In contrast, the tumor-draining popliteal lymph nodes, which were infiltrated primarily by lymphocytes, featured enhanced lymphatic flow and enlarged lymphatic sinuses.37 These findings indicate that macrophage infiltration of the footpad tumor was not sufficient to induce lymph node lymphangiogenesis; in contrast, B-cell accumulation of B-lymphocytes within the draining lymph nodes is required to increase lymph flow and for expansion of the lymphatic sinuses in response to tumor growth.37 Based on their observations, the authors concluded that B-lymphocytes mediate the tumor-reactive changes, as these alterations were not observed in mice deficient for B cells.37
In our study, the width of the SCS in the deep cervical lymph nodes of WT animals was, on average, <15 μm (Figure 3D,F). Notably, this is approximately the same diameter as a circulating tumor cell. In contrast, the SCS of the deep cervical lymph nodes that were draining PTC tumors were 3 to 5 times larger (Figure 3E,F). Size restriction may represent a physical barrier that prevents a tumor cell or a cluster of tumor cells from entering the draining lymph nodes. Moreover, dilation of the SCS, which is lined by endothelial cells, is likely a prerequisite for tumor cell entry into the draining lymph nodes.39
As mentioned previously, lymph node mapping reports from various murine strains are not always consistent, and not all lymph nodes have been identified in various murine strains.19,20,37 It is possible that observed differences in lymph node size and structure are because of genetic differences rather than tumor drainage patterns. To test this possibility, we identified and collected the popliteal lymph nodes from thyroid tumor-bearing BrafV600E/PtenHet/TPO-Cre mice and their WT littermates. Of note, popliteal lymph nodes have been previously shown to have tumor-specific reactive changes in an implanted melanoma model.37 Similarly, in our study, no differences in popliteal lymph node architecture and/or immune infiltrate was observed between the tumor-bearing and WT animals (Figure 3G,H), indicating the observed changes in the cervical, tumor-draining lymph nodes are indeed a specific reaction to the tumor and not because of genetic differences.
5 CONCLUSION
The increasing incidence of thyroid cancer combined with the lack of treatment options for those patients with dedifferentiated, invasive, or metastatic disease makes genetically engineered mouse models invaluable for the development of effective treatment strategies. Our finding that PTC induces increased flow through the tumor-draining lymph nodes and enlargement of the lymphatic sinuses suggests that these alterations may actively promote lymphatic metastasis of tumor cells to regional lymph nodes. Examination of additional murine and human cancers should determine whether these alterations are a feature of thyroid cancers (eg, follicular thyroid cancer) in general or if these alterations are predictive of those cancers that metastasize specifically via the lymphatics.
Acknowledgments
Funding information
This work was supported by the University of Arkansas for Medical Sciences grant NIH UL1TR000039; The American Thyroid Association/Thyca research grant (A. Franco); UAMS Envoys Seeds of Science Award (A. Franco).
References
- 1.Aschebrook-Kilfoy B, Schechter RB, Shih YC, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1252–1259. doi: 10.1158/1055-9965.EPI-13-0242. [DOI] [PubMed] [Google Scholar]
- 2.Kim CS, Zhu X. Lessons from mouse models of thyroid cancer. Thyroid. 2009;19(12):1317–1331. doi: 10.1089/thy.2009.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu FH, Kuo SF, Hsueh C, Chao TC, Lin JD. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol. 2015;112(2):149–154. doi: 10.1002/jso.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118(7):1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 5.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD. Natl Cancer Inst. https://seer.cancer.gov/archive/csr/1975_2009_pops09/results_merged/sect_26_thyroid.pdf. Accessed March 1, 2016.
- 7.Kirschner LS, Qamri Z, Kari S, Ashtekar A. Mouse models of thyroid cancer: a 2015 update. Mol Cell Endocrinol. 2016;421:18–27. doi: 10.1016/j.mce.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 9.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13(4):1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 10.Charles RP, Iezza G, Amendola E, Dankort D, McMahon M. Mutationally activated BRAF(V600E) elicits papillary thyroid cancer in the adult mouse. Cancer Res. 2011;71(11):3863–3871. doi: 10.1158/0008-5472.CAN-10-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Borre P, McFadden DG, Gunda V, et al. The next generation of orthotopic thyroid cancer models: immunocompetent orthotopic mouse models of BRAF V600E-positive papillary and anaplastic thyroid carcinoma. Thyroid. 2014;24(4):705–714. doi: 10.1089/thy.2013.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer. 2014;21(3):R85–R103. doi: 10.1530/ERC-13-0431. [DOI] [PubMed] [Google Scholar]
- 14.Knauf JA, Ma X, Smith EP, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65(10):4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 15.Franco AT, Malaguamera R, Refetoff S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci U S A. 2011;108(4):1615–1620. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFadden DG, Vernon A, Santiago PM, et al. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci U S A. 2014;111(16):E1600–E1609. doi: 10.1073/pnas.1404357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolly LA, Novitskiy S, Owens P, et al. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 2016;76(7):1804–1813. doi: 10.1158/0008-5472.CAN-15-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312(1–2):12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Shao L, Mori S, Yagishita Y, et al. Lymphatic mapping of mice with systemic lymphoproliferative disorder: usefulness as an inter-lymph node metastasis model of cancer. J Immunol Methods. 2013;389(1–2):69–78. doi: 10.1016/j.jim.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 21.McMaster PD. The lymphatics and lymph flow in the edematous skin of human beings with cardiac and renal disease. J Exp Med. 1937;65(3):373–392. doi: 10.1084/jem.65.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods. 2008;332(1–2):170–174. doi: 10.1016/j.jim.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4(4):217–228. doi: 10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- 24.Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res. 2001;7(3):462–468. [PubMed] [Google Scholar]
- 25.Ozasa R, Ohno J, Iwahashi T, Taniguchi K. Tumor-induced lymphangiogenesis in cervical lymph nodes in oral melanoma-bearing mice. J Exp Clin Cancer Res. 2012;31:83. doi: 10.1186/1756-9966-31-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu XM, Lo CY, Chan WF, Lam KY, Leung P, Luk JM. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res. 2005;11(22):8063–8069. doi: 10.1158/1078-0432.CCR-05-0646. [DOI] [PubMed] [Google Scholar]
- 27.Witte MH, Jones K, Wilting J, et al. Structure function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 2006;25(2):159–184. doi: 10.1007/s10555-006-8496-2. [DOI] [PubMed] [Google Scholar]
- 28.Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225–234. doi: 10.1196/annals.1413.020. [DOI] [PubMed] [Google Scholar]
- 29.Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999;155(6):1967–1976. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsurusaki T, Kanda S, Sakai H, et al. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer. 1999;80(1–2):309–313. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonemura Y, Endo Y, Fujita H, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cencer Res. 1999;5(7):1823–1829. [PubMed] [Google Scholar]
- 32.Akagi K, Ikeda Y, Miyazaki M, et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83(7):887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitadai Y, Amioka T, Haruma K, et al. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001;93(5):662–666. doi: 10.1002/ijc.1379. [DOI] [PubMed] [Google Scholar]
- 34.Kilvaer TK, Paulsen EE, Hald SM, et al. Lymphangiogenic markers and their impact on nodal metastasis and survival in non-small cell lung cancer – a structured review with meta-analysis. PLoS One. 2015;10(8):e0132481. doi: 10.1371/journal.pone.0132481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao YC, Ni XJ, Wang MH, Zha XM, Zhao X, Wang S. Tumor-derived VEGF-C, but not VEGF-D, promotes sentinel lymph node lymphangiogenesis prior to metastasis in breast cancer patients. Med Oncol. 2012;29(4):2594–2600. doi: 10.1007/s12032-012-0205-0. [DOI] [PubMed] [Google Scholar]
- 36.Yu XM, Lo CY, Lam AK, Leung P, Luk JM. Serum vascular endothelial growth factor C correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Ann Surg. 2008;247(3):483–489. doi: 10.1097/SLA.0b013e31815fa447. [DOI] [PubMed] [Google Scholar]
- 37.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170(2):774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaya M, Castello A, Montaner B, et al. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347(6222):667–672. doi: 10.1126/science.aaa1300. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Sarrou E, Podgrabinska S, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210(8):1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles RP, Silva J, Iezza G, Phillips WA, McMahon M. Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol Cancer Res. 2014;12(7):979–986. doi: 10.1158/1541-7786.MCR-14-0158-T. [DOI] [PMC free article] [PubMed] [Google Scholar]


