Abstract
Sex influences risk for opioid dependence (OD). We hypothesized that sex might interact with genetic loci that influence the risk for OD. Therefore we performed an analysis to identify sex-specific genomic susceptibility regions for OD using linkage. Over 6000 single nucleotide polymorphism (SNP) markers were genotyped for 1758 African- and European-American (AA and EA) individuals from 739 families, ascertained via affected sib-pairs with OD and/or cocaine dependence. Autosomewide non-parametric linkage scans, stratified by sex and population, were performed. We identified one significant linkage region, segregating with OD in EA men, at 71.1 cM on chromosome 4 (LOD=3.29; point-wise p=0.00005; empirical autosome-wide p=0.042), which significantly differed from the linkage signal at the same location in EA women (empirical p=0.002). Three suggestive linkage signals were identified at 181.3 cM on chromosome 7 (LOD=2.18), 104 cM on chromosome 11 (LOD=1.85), and 60.9 cM on chromosome 16 (LOD=1.93) in EA women. In AA men, four suggestive linkage signals were detected at 201.1 cM on chromosome 3 (LOD=2.32), 152.9 cM on chromosome 6 (LOD=1.86), 16.8 cM on chromosome 7 (LOD=1.95), and 36.1 cM on chromosome 17 (LOD=1.99). The significant region, mapping to 4q12-4q13.1, harbors several OD candidate genes with interconnected functionality, including VEGFR, CLOCK, PDCL2, NMU, NRSF, and IGFBP7. In conclusion, these results provide an evidence for the existence of sex-specific and population-specific differences in OD. Furthermore, these results provide positional information that will facilitate the use of targeted next-generation sequencing to search for genes that contribute to sex-specific differences in OD.
Keywords: Opioid dependence, sex-specific, linkage analysis, 4q12
INTRODUCTION
Sex differences are present in all phases of drug dependence, including drug acquisition, escalation of use, addiction, withdrawal, relapse, and treatment response (Becker and Hu, 2008; Greenfield et al., 2011). In general, females, both animals and humans, will initiate self-administration of abused drugs at lower doses than males, will escalate more rapidly to compulsive use and dependence, and are more vulnerable to relapse following abstinence (Becker and Hu, 2008; Becker and Koob, 2016). Specifically for opioid dependence (OD), evidence from methadone maintenance treatment programs shows that women with OD had greater illness severity, elevated rates of medical and other psychiatric comorbidity, and less satisfactory treatment outcomes than men (Bawor et al., 2015; Chatham et al., 1999). In a retrospective study from 1960 to 2010 on heroin use in the United States, the gap between women and men in the prevalence of combining heroin use with prescription opioids has been closing and, in 2010 it was slightly higher for women (Cicero et al., 2014). The heritability of OD has been well established via adoption, twin and family studies (reviewed in (Gelernter and Kranzler, 2010)). The estimated heritability of drug dependence traits, including OD, differs substantially for men and women: 0.73 versus 0.55, respectively (Kendler et al., 2014).
Recent genome-wide association studies (GWAS) for OD have identified promising genetic variants in KCNG2 and several other loci (Gelernter et al., 2014) and CNIH3 (Nelson et al., 2015). However, these variants account for only a small proportion of OD risk. Rare variants were not included on the GWAS arrays used for the cited OD GWAS studies (or were removed in the quality control process), and most of the loci are non-coding common variants with unknown effects. These limitations can be overcome by whole-genome sequencing (WGS), although the very large quantity of data generated by WGS poses analytical challenges. Another approach is to use linkage to identify rare risk variants in genes of interest. Because linkage detects segregation within families, different rare variants at the same locus can coalesce to create a positive linkage finding; and variants that have a large effect but are rare in the population may still be shared by affected family members. Therefore, in conjunction with WGS filtering approaches, linkage analysis, which had been largely supplanted by newer methods, has begun to re-emerge as an analytical method for the identification of genes in disease etiology (Ott et al., 2015).
Linkage scans for OD have been reported for a few populations including African-Americans (AA) and European Americans (EA) (Gelernter et al., 2006), Han Chinese (Glatt et al., 2008), and an ethnically mixed population of Hispanic, non-Hispanic AA, non-Hispanic EA and others (Lachman et al., 2007). The main linkage signals identified by these studies are, for the most part, located in different chromosomal regions, indicative of the genetic complexity of OD and the likely population specificity of many, or even most, susceptibility variants. To minimize trait heterogeneity a priori, Gelernter and colleagues used cluster analysis to identify OD-related symptom clusters in a genomewide linkage study of >250 families using a panel of short tandem repeat (STR) markers. They identified two significant linkage signals on different regions of chromosome 17 for the “heavy-opioid-use” cluster and the “non-opioid-use” cluster (Gelernter et al., 2006).
There have been no prior studies of sex specific linkage analysis in OD. We used genetic linkage analysis to identify the chromosomal location of genes that increase sex-specific risk of OD by focusing on sex-specific linkage signals. We collected a set of small nuclear families suitable for linkage analyses of OD. Most of the subjects (82% of AAs and 90% of EAs) were included in our previous report (Gelernter et al., 2006), which did not include sex-specific analyses. The instrument used to evaluate these subjects was the Semi Structured Assessment for Drug Dependence and Alcoholism (SSADDA) (Pierucci-Lagha et al., 2005). We used a microarray linkage panel of single nucleotide polymorphism (SNP) markers for the current study, identified the male sib-pairs and female sib-pairs, and describe here the results of an autosome-wide sex-specific linkage analysis for the categorical trait of DSM-IV OD.
MATERIALS and METHODS
Subject recruitment
Subjects were recruited because they were thought to be affected with OD and/or cocaine dependence (CD), and to have at least one sibling affected by the same kind of substance dependence. Subjects were recruited at the Yale University School of Medicine (APT Foundation; New Haven, Connecticut), University of Connecticut Health Center (UConn; Farmington, Connecticut), Medical University of South Carolina (MUSC; Charleston, South Carolina), and McLean Hospital (Harvard Medical School; Belmont, Massachusetts). Probands were excluded from the study if they had ever received a clinical diagnosis of a major psychotic disorder (e.g. schizophrenia or bipolar disorder). Probands’ other siblings and parents were recruited whenever available regardless of their affection status to increase the power to detect linkage. After screening, 384 AA and 355 EA families were included in this analysis. Each subject provided written informed consent. The institutional review boards at all sites approved the study, and certificates of confidentiality for the work were issued by the National Institute on Drug Abuse.
Subject assessment
Subjects were interviewed and assessed using the SSADDA for DSM-IV substance dependence (SD, including OD) and other psychiatric disorders as described elsewhere (Gelernter et al., 2005; Pierucci-Lagha et al., 2005). We have previously reported on this study cohort for linkage analyses (Yang et al., 2012; Yang et al., 2011), and approximately 82% of the AA and 90% of the EAs (in this current study) included in our previous reports (Gelernter et al., 2005; Gelernter et al., 2007; Gelernter et al., 2006), depending on the phenotype.
Subject genotyping and quality control
DNA was obtained from immortalized cell lines for most subjects, but directly from blood or saliva for a small proportion of the subjects. The Center for Inherited Disease Research (CIDR) genotyped the 1,492 subjects using the 6,008 SNP Illumina Linkage IVb Marker Panel (“first panel”, http://www.cidr.jhmi.edu), while the Yale Keck Center genotyped the other 266 individuals using the 6,090 SNP Illumina Infinium-12 Human Linkage Marker Panel (“second panel”). Only autosomal SNPs were analyzed (n=5,636 for the first panel and n=5,735 for the second panel). Among the SNPs in the two panels, 4,518 SNPs were shared across the two platforms; only these SNPs were considered in the subsequent quality control (QC) step.
We used PLINK software (Purcell et al., 2007) for the QC step, using a randomly selected subset of unrelated subjects (384 AA and 355 EA subjects, one per family). Individual SNPs were excluded from further analysis if the genotyping rate ≤ 0.95, minor allele frequency (MAF) ≤ 0.1, or the SNP was not in Hardy-Weinberg equilibrium (HWE) (p ≤ 0.01). Mendelian inconsistencies were identified using PedCheck (O’Connell and Weeks, 1998) and Merlin (Abecasis et al., 2002). The Merlin “--error” option was used to detect likely-erroneous genotypes based on the estimated probability of double-crossover events. Family relationships were verified using the Pedigree Relationship Statistical Test (PREST) (McPeek and Sun, 2000).
For the AA subjects the QC filters removed 1,366, 322 and 46 SNPs for genotyping rate, MAF and HWE, respectively. For the EA subjects the QC filters removed 1,360, 70 and 40 SNPs for genotyping rate, MAF and HWE, respectively. The remaining autosomal SNP markers (4,133 for AAs and 4,395 for EAs) were used for the linkage analysis. SNPs excluded by QC were set to missing in the linkage analysis. The average marker spacing was 0.946 cM for AAs and 0.917 cM for EAs for the remaining markers. We used the Marshfield human genetic maps (Broman et al., 1998).
Family relationships were corrected in one AA family and five EA families based on the shared IBD patterns. The re-assigned family relationships were validated by rerunning PREST. One AA family was excluded from the linkage analysis because of unresolvable relationship problems identified by PREST. Family structure, including number of half-sibs, is given in Table 1.
Table 1.
Numbers of affected families with opioid dependence by sex among the African-American and European-American samples.
| Opioid Dependence | ||||
|---|---|---|---|---|
|
| ||||
| Number of affecteds | African American | European American | ||
|
| ||||
| Female | Male | Female | Male | |
| 0 affecteds | 286 | 275 | 183 | 146 |
| 1 affecteds | 76 | 79 | 128 | 126 |
| 2 affecteds | 20 | 27 | 40 | 76 |
| 3 affecteds | 2 | 3 | 3 | 7 |
| 4 affecteds | 0 | 0 | 1 | 0 |
| Total affecteds | 98 | 109 | 172 | 209 |
| Total participants | 384 | 384 | 355 | 355 |
| Number of half-sib pairs | 9 | 9 | 3 | 5 |
| Number of full-sib pairs | 16 | 22 | 38 | 83 |
| Merlin informative families | 25 | 31 | 41 | 88 |
A set of 1,574 SNP ancestry informative markers (AIMs) from the SNP linkage panel was used to estimate the genetic ancestry proportions in STRUCTURE for each subject (Pritchard et al., 2000). The selection of AIMs was based on the marker characteristics inferred from the Hapmap samples: CEU (Utah residents with Northern and Western European ancestry; European) and YRI (Yoruba in Ibadan, Nigeria; African) samples. The AIM selection criteria for each SNP included: 1) the absolute allele-frequency difference (δ) between CEU and YRI > 0.2; 2) pair-wise SNP correlation r2 < 0.1 within each population; and 3) HWE testing with p-value > 0.01 within each population. Bayesian clustering implemented in the STRUCTURE analysis combined 10,000 burn-ins and 10,000 collected iterations to estimate ancestry for each population. The inferred population for each individual was classified on the basis of >50% estimated ancestry in that population. We re-classified families as either EA or AA based on the predominant classification for each family.
Defining male and female sib-pair groups
If the affected sibs within each nuclear family were either all males or all females, the families were classified accordingly. If the affected sibs within a nuclear family were both male and female within a nuclear family, either only male affected or only female affected sibs were retained for analysis (with opposite sex individuals set to missing). All parents were retained in the sample.
Linkage analyses
We used Merlin software (Abecasis et al., 2002) to implement model-free, nonparametric, penetrance-independent, affected-only and allele-sharing models to detect linkage. Allele frequencies were calculated by counting all genotyped individuals and the Kong and Cox linear allele-sharing model (Kong and Cox, 1997) was used to estimate the logarithm of odds (LOD) score. To reduce the computational burden attributable to modeling maker-marker linkage disequilibrium (LD), we pruned the markers in selection of those with low LD (3675 SNPs in AAs and 3760 SNPs in EAs), that is, r2<0.1 for each pair of markers. To assess the empirical thresholds for genomewide suggestive and significant linkages, we conducted Monte Carlo simulations under the null hypothesis of random linkage between phenotype and genotype. Simulation of 1,000 data sets was performed in Merlin using a gene-dropping algorithm, and was based on the observed family structure, marker spacing, allele frequencies, and missing data pattern. The same analytical approaches used to analyze the observed data were implemented in each simulated data set, from which the highest LOD score for each chromosome was recorded.
The genomewide suggestive linkage threshold was characterized as the highest LOD score expected once by chance per genome scan (Lander and Kruglyak, 1995). Operationally this genomewide suggestive linkage threshold was set as the 1,000th highest LOD score out of 22,000 LOD scores for each of the 22 autosomal chromosomes from the 1,000 simulations. The genomewide significant threshold was set as the 95th percentile of the distribution of 22,000 LOD scores. The empirical sex-specific genome-wide linkage “suggestive” and “significant” thresholds are 1.7 and 2.89 for AA females, 1.78 and 3.16 for AA males, 1.77 and 3.16 for EA females and 1.73 and 3.11 for EA males, respectively (Table 2). The genomewide empirical significance of an observed LOD score was assessed from the same simulation. It was estimated by calculating the proportion of the entire genome which had a maximum LOD score greater than or equal to the observed LOD score across the 1,000 simulated replicates.
Table 2.
Summary of sex-specific autosomewide linkage analysis results for LOD scores exceeding the empirically derived significance linkage thresholds for opioid dependence in African- and European-Americans (AA and EA).
| Population | African American (AA) | European American (EA) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Sex | Male | Female | Male | |||||
| Chromosome | 3 | 6 | 7 | 17 | 7 | 11 | 16 | 4 |
| Cytogenetic location | 3q27.3 | 6q25.1 | 7p21.3 | 17p12 | 7q36.3 | 11q22.3 | 16q12.1 | 4q12-4q13.1 |
| Peak location (cM)* | 201.1 | 152.9 | 16.8 | 36.1 | 181.3 | 104 | 60.9 | 71.7 |
| Peak LOD score** | 2.32 | 1.86 | 1.95 | 1.99 | 2.18 | 1.85 | 1.93 | 3.29 |
| Pointwise p -value | 0.0005 | 0.002 | 0.0014 | 0.0012 | 0.0008 | 0.002 | 0.0014 | 0.00005 |
| Empirical autosome-wide p -value | 0.3 | 0.62 | 0.54 | 0.52 | 0.36 | 0.58 | 0.53 | 0.042 |
| LOD score for the opposite sex at the same location as the peak’s*** | −0.41 | 0.12 | −0.08 | 0.02 | −0.63 | 0.28 | 0.02 | 0.01 |
| Difference of the male-female LOD scores | 2.73 | 1.74 | 2.03 | 1.97 | 2.81 | 1.57 | 1.91 | 3.28 |
| Empirical p-value of the male-female LOD score difference | - | - | - | - | - | - | - | 0.002 |
| Number of families | 384 | 384 | 384 | 384 | 350 | 350 | 350 | 350 |
| Number of Merlin informative families | 30 | 30 | 30 | 30 | 44 | 44 | 44 | 83 |
| Suggestive Threshold | 1.78 | 1.78 | 1.78 | 1.78 | 1.77 | 1.77 | 1.77 | 1.73 |
| Significant Threshold | 3.16 | 3.16 | 3.16 | 3.16 | 3.16 | 3.16 | 3.16 | 3.11 |
Note:
cM, centiMorgan;
LOD, the logarithm of odd score;
for example, in the EA male column, the peak LOD score is 3.29 at 71.7 cM; then the LOD score for female at 71.7 cM is 0.01 (These numbers are in bold).
Empirical evaluation of the differences between male-specific and female-specific linkage signals
The empirical p-value of the differences between linkage signals from the male and female parts of the sample was based on 1,000 simulations using the same number of informative families for linkage analysis regardless of sex information (Weiss et al., 2006). For example, the highest LOD score for the EA subgroup was observed for the male sib-pair group on chromosome 4q13.1. The comparison interval is centered on the peak observed in the data and extends 1 centiMorgan (cM) up- and down-stream from the linkage peak. In brief, there are 83 and 44 families informative for linkage analysis of OD in the male and female sib-pair group, respectively. We combined these families and randomly split the resulting set of 127 families into 44 and 83 families for linkage analysis of OD. In other words, we randomized the sex information. The difference between the maximum LOD score (lod1) obtained from the 44 families within this comparison interval centered on the chromosome 4q12 peak and the LOD score (lod2) was derived from the remaining 83 families at the same location as that from which lod1 was obtained. The LOD score difference, lod1-lod2, was generated 1,000 times by simulation. Each simulation used the same parameters for linkage analysis as those used in the male sib-pair only analysis. We counted the proportion of the simulated “lod1-lod2” scores that were greater or equal to the observed difference (3.28) between the peak LOD score for males (3.29) and the LOD score for female (0.01) at the same location across 1,000 simulations. There were two simulations where the difference exceeded 3.28; therefore, the empirical p-value is 0.002 for the difference of OD linkage signals between the male and female EAs.
RESULTS
The clinical sample with the family count for the distribution of affected members within each family is presented in Table 1. There were more informative male sib-pairs for both AAs and EAs. The probands’ ages ranged from 21 to 58 (mean±sd=41.7±6.3) for the AA male sample, 20 to 58 (40.2±6.1) for the AA female sample, 18 to 61 (36.3±8.6) for the EA male sample, and 18 to 59 (36.6±8.1) for the EA female sample. On average, probands were younger in the EA than the AA sample and the ages were similar for males and females within each population.
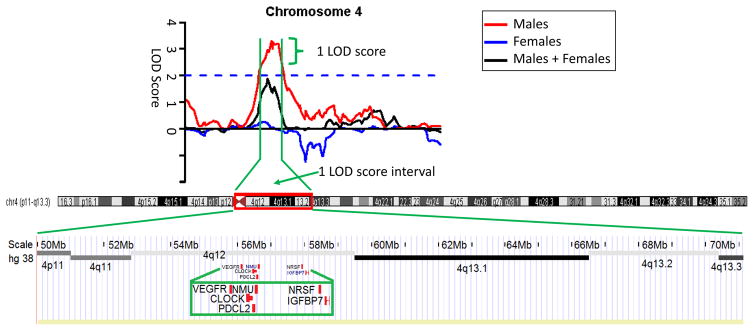
We identified one linkage peak in EA males that satisfied empirical autosome-wide significant criteria (i.e., on the basis of simulation) (Figure 1 and Table 2), on chromosome 4 at 71.7 cM, for OD (rs1509062, maximum LOD=3.29, point-wise p=0.00005; empirical autosome-wide p=0.042). This SNP marker with the maximum LOD score maps to the border between 4q12 and 4q13.1. In the EA female sample, we detected three suggestive linkage signals at 181.3 cM on chromosome 7 (LOD=2.18, point-wise p=0.0008), 104 cM on chromosome 11 (LOD=1.85, point-wise p=0.002), and 60.9 cM on chromosome 16 (LOD=1.93, point-wise p=0.0014) (Supplemental Figure S1). In the AA male sample (Supplemental Figure S2), four suggestive linkage signals were detected at 201.1 cM on chromosome 3 (LOD=2.32, point-wise p=0.0005), 152.9 cM on chromosome 6 (LOD=1.86, point-wise p=0.002), 16.8 cM on chromosome 7 (LOD=1.95, point-wise p=0.0014), and 36.1 cM on chromosome 17 (LOD=1.99, point-wise p=0.0012).
Figure 1.
Sex-differences linkage analysis on opioid dependence. The linkage peak at 71.7 cM on chromosome 4 satisfied empirical autosome-wide linkage significance criteria for Opioid Dependence (rs1509062, maximum LOD=3.29, point-wise p=0.00005; empirical autosome-wide p=0.042) with 1 LOD score support harboring six opioid-related candidate genes.
We also performed linkage scans for the OD trait using the entire sample, results of which are presented in Supplemental Figures S1 and S2.
Additional simulations were conducted to evaluate whether there was a significant sex-specific effect for the empirically-significant linkage region at 71.7 cM. The difference between male- and female-specific LOD scores was 3.28, which yielded an empirical male-female difference of p=0.002.
DISCUSSION
To the best of our knowledge, this is the first sex-specific autosome-wide linkage scan for OD or any closely related trait. We observed one sex-specific autosome-wide significant linkage signal in EA males at the chromosomal region 4q12-4q13.1 and three suggestive linkage signals in EA females. There were also four suggestive linkage signals in AA males. These results support the existence of sex-specific genetic influences on OD.
In a previous report, we identified significant linkage to the 4q12 region in EAs for a trait derived by fuzzy clustering for comorbid dependence on multiple substances (Yang et al., 2012). This region was also implicated for linkage to bipolar disorder in the Wellcome Trust UK-Irish bipolar affective disorder sibling-pair study (Lambert et al., 2005). Here, the significant linkage region at 4q12-4q13.1 for EA males included several OD candidate genes (in chromosomal order, Figure 1): VEGFR (vascular endothelial growth factor receptor), CLOCK (Clock circadian regulator gene), PDCL2 (phosducin like 2), NMU (neuromedin U), NRSF (neuron-restrictive silence factor), and IGFBP7 (insulin like growth factor binding protein 7). NMU, of particular interest, encodes neuromedin U (NMU), a hypothalamic neuropeptide, which plays a role in pain, stress, immune-mediated inflammatory diseases, and feeding regulation [summarized from RefSeq, July 2015]. NMU has been shown to have a pro-nociceptive role in mice (Cao et al., 2003). Its two receptors, NMU-1 and NMU-2, serve different physiological roles, with vasoconstriction with nociception mediated via NMU-1 (Mitchell et al., 2009). A recent report showed that the administration of the anorexigenic peptide NMU decreases alcohol intake and attenuates alcohol-induced reward in rodents (Vallof et al., 2016).
Another interesting candidate mapped to this linkage peak, NRSF, encodes the neuron-restrictive silence factor, NRSF. NRSF is a transcriptional repressor in neuronal cells that regulates the mu-opioid receptor gene (Kim et al., 2006; Kim et al., 2004). IGFBP7, also in the linkage region 4q12 and 95 kb downstream of NRSF, co-regulates the mu-opioid receptor gene. IGFBP7 encodes a member of the insulin-like growth factor (IGF) family, which transcriptionally activates the mu-opioid receptor gene; this activation is modulated by NRSF (Bedini et al., 2008). NRSF also represses the expression of PDYN (prodynorphin), a member of the dynorphin kappa-opioid receptor system, which is expressed in the adult human brain (Henriksson et al., 2014).
This linkage peak also contains VEGFR, which encodes one of the two vascular endothelial growth factor (VEGF) receptors. Opioids inhibit VEGF expression in endothelial cells and cardiac myocytes (Balasubramanian et al., 2001; Roy et al., 2003; Yamamizu et al., 2011). OD subjects showed deficits of circulating stem progenitor cells and this deficit appeared to accelerate aging (Reece and Davidson, 2007). Furthermore, the duration of opioid exposure is a determinant of arterial stiffness and vascular age, specifically in males with OD (Reece and Hulse, 2014). Estrogen has been shown to mediate the VEGF system (Duckles and Krause, 2007; Jesmin et al., 2004), which could account for the sex-specificity of the observation. VEGFR plays a role in angiogenesis and neurogenesis (Elfving et al., 2015; Erskine et al., 2011; Kim et al., 2007), and is necessary for the behavioral effects of 5-HT selective reuptake inhibitors (SSRIs) and norepinephrine selective reuptake inhibitors (Greene et al., 2009).
Finally, another candidate gene in this region, CLOCK, which encodes a circadian regulator, has long been studied in addiction (Malison et al., 2006) and psychiatric disorders (Desan et al., 2000). In rats, morphine withdrawal produces circadian rhythm alterations of clock genes, including CLOCK, in mesolimbic brain areas and peripheral blood mononuclear cells (Li et al., 2009). PDCL2, also in the 4q12 linkage region, encodes a member of the phosducin-like protein family. Opioid-induced phosphorylation of phosducin-like protein was impaired by inhibiting the activity of Ca2+/calmodulin-dependent protein kinase II, and subsequently was linked to the recovery of G protein regulation by the mu opioid receptor, leading to a diminution of morphine antinociceptive tolerance (Sanchez-Blazquez et al., 2008). Our previous GWAS identified Ca2+ signaling as important for OD (Gelernter et al., 2014).
Given these strong and functionally interconnected strands of evidence for the candidate genes at chromosome 4q12 in relation to OD or OD-related traits, follow-up of the region associated with OD or OD-related traits with targeted sequencing is indicated. Because several of these genes appear to be functionally related, it is possible that the coincidence of effects at several of these loci combined to generate the autosome-wide-significant result.
Limitations of the present study include exclusion of the sex chromosomes in our sex-specific linkage scans, which was precluded by the relatively low marker quality for SNP in the sex chromosomes on the arrays that we used. Another important limitation was the comparatively small sample analyzed; by design, opposite sex sib-pairs were excluded in the analysis. Collection of families with multiple members affected with OD is arduous and there are few such samples available.
In conclusion, we identified at least one sex-specific OD linkage, and provided suggestive evidence for several others. The main linkage peaks identified were not discernable in the entire, combined-sex samples. These results demonstrate the importance of taking sex effects into consideration in investigating the genetic basis of OD, and suggest that this may be important for other substance dependence traits as well.
Supplementary Material
European American–Opioid Dependence (EA-OD) linkage results. Logarithm of odd (LOD) scores on 22 autosomal chromosomes resulting from non-parametric linkage analyses including EA families with OD for the male, female and combined samples. The dashed lines denote the thresholds of LOD scores for the three samples for genomewide-‘suggestive’ linkage. (Some of these thresholds appear as one dashed line because they are very close).
African-American–Opioid Dependence (AA-OD) linkage results. Logarithm of odd (LOD) scores on 22 autosomal chromosomes resulting from non-parametric linkage analyses including AA families with OD for the male, female and combined samples. The dashed lines denote the thresholds of LOD scores for the three samples for genomewide-‘suggestive’ linkage. (Some of these thresholds appear as one dashed line because they are very close).
Acknowledgments
We are appreciative to the participants in this research study. Genotyping services were provided by the Center for Inherited Disease Research (CIDR) and the Keck Center at Yale. CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University (contract number N01-HG-65403). Genotyping was supported in part by a Yale CTSA and NIH Neuroscience Microarray Consortium award U24 NS051869-02S1. We thank Ann Marie Lacobelle and Greg Kay for their excellent technical assistance. This study was supported by National Institute on Drug Abuse (NIDA) Grants K01 DA24758, R01 DA12690, R01 DA12849, R01 DA18432, R01 AA11330, the VA Medical Research Service (VA MERIT grant to JG), and the VA Connecticut and VISN 4 MIRECCs, and a Brain and Behavior Research NARSAD Young Investigator award (Yang).
Footnotes
AUTHORS CONTRIBUTION
BY, SH and JG were responsible for the study concept and design. JG and HK designed the overall study and supervised subject recruitment; and JG supervised the laboratory aspects of the study. AP discussed methods for analyses of sex-difference evaluation. SH and BY performed the data analysis and all authors contributed to interpretation of findings. BY drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content and reviewed and approved the final version for publication.
DISCLOSURE
Dr. Kranzler has been an advisory board member, consultant, or continuing medical education speaker for Indivior, Lundbeck, and Otsuka. He is a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which is sponsored by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Pfizer, and Xenoport.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nature genetics. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Ramakrishnan S, Charboneau R, Wang J, Barke RA, Roy S. Morphine sulfate inhibits hypoxia-induced vascular endothelial growth factor expression in endothelial cells and cardiac myocytes. Journal of molecular and cellular cardiology. 2001;33:2179–2187. doi: 10.1006/jmcc.2001.1480. [DOI] [PubMed] [Google Scholar]
- Bawor M, Dennis BB, Varenbut M, Daiter J, Marsh DC, Plater C, Worster A, Steiner M, Anglin R, Pare G, Desai D, Thabane L, Samaan Z. Sex differences in substance use, health, and social functioning among opioid users receiving methadone treatment: a multicenter cohort study. Biol Sex Differ. 2015;6:21. doi: 10.1186/s13293-015-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological reviews. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedini A, Baiula M, Spampinato S. Transcriptional activation of human mu-opioid receptor gene by insulin-like growth factor-I in neuronal cells is modulated by the transcription factor REST. J Neurochem. 2008;105:2166–2178. doi: 10.1111/j.1471-4159.2008.05303.x. [DOI] [PubMed] [Google Scholar]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL. Comprehensive human genetic maps: individual and sex-specific variation in recombination. American journal of human genetics. 1998;63:861–869. doi: 10.1086/302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao CQ, Yu XH, Dray A, Filosa A, Perkins MN. A pro-nociceptive role of neuromedin U in adult mice. Pain. 2003;104:609–616. doi: 10.1016/S0304-3959(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Chatham LR, Hiller ML, Rowan-Szal GA, Joe GW, Simpson DD. Gender differences at admission and follow-up in a sample of methadone maintenance clients. Substance use & misuse. 1999;34:1137–1165. doi: 10.3109/10826089909039401. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Desan PH, Oren DA, Malison R, Price LH, Rosenbaum J, Smoller J, Charney DS, Gelernter J. Genetic polymorphism at the CLOCK gene locus and major depression. American journal of medical genetics. 2000;96:418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clinical and experimental pharmacology & physiology. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- Elfving B, Jakobsen JL, Madsen JC, Wegener G, Muller HK. Chronic restraint stress increases the protein expression of VEGF and its receptor VEGFR-2 in the prefrontal cortex. Synapse. 2015;69:190–194. doi: 10.1002/syn.21808. [DOI] [PubMed] [Google Scholar]
- Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, Ruhrberg C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70:951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of drug dependence. Dialogues in clinical neuroscience. 2010;12:77–84. doi: 10.31887/DCNS.2010.12.1/jgelernter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Koesterer R, Almasy L, Zhao H, Farrer LA. Genome-wide association study of opioid dependence: multiple associations mapped to calcium and potassium pathways. Biological psychiatry. 2014;76:66–74. doi: 10.1016/j.biopsych.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits: significant linkages for a cocaine-related trait and cocaine-induced paranoia. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2005;136b:45–52. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, Farrer L, Kranzler HR. Genomewide linkage scan for nicotine dependence: identification of a chromosome 5 risk locus. Biological psychiatry. 2007;61:119–126. doi: 10.1016/j.biopsych.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, Kranzler HR. Genomewide linkage scan for opioid dependence and related traits. American journal of human genetics. 2006;78:759–769. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Lasky-Su JA, Zhu SC, Zhang R, Zhang B, Li J, Yuan X, Li J, Lyons MJ, Faraone SV, Tsuang MT. Genome-wide linkage analysis of heroin dependence in Han Chinese: results from Wave Two of a multi-stage study. Drug Alcohol Depend. 2008;98:30–34. doi: 10.1016/j.drugalcdep.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Rosa C, Putnins SI, Green CA, Brooks AJ, Calsyn DA, Cohen LR, Erickson S, Gordon SM, Haynes L, Killeen T, Miele G, Tross S, Winhusen T. Gender research in the National Institute on Drug Abuse National Treatment Clinical Trials Network: a summary of findings. Am J Drug Alcohol Abuse. 2011;37:301–312. doi: 10.3109/00952990.2011.596875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson R, Backman CM, Harvey BK, Kadyrova H, Bazov I, Shippenberg TS, Bakalkin G. PDYN, a gene implicated in brain/mental disorders, is targeted by REST in the adult human brain. Biochimica et biophysica acta. 2014;1839:1226–1232. doi: 10.1016/j.bbagrm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesmin S, Sakuma I, Hattori Y, Kitabatake A. Regulatory molecules for coronary expressions of VEGF and its angiogenic receptor KDR in hypoestrogenic middle-aged female rats. Molecular and cellular biochemistry. 2004;259:189–196. doi: 10.1023/b:mcbi.0000021372.99727.b3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Maes HH, Sundquist K, Ohlsson H, Sundquist J. Genetic and family and community environmental effects on drug abuse in adolescence: a Swedish national twin and sibling study. Am J Psychiatry. 2014;171:209–217. doi: 10.1176/appi.ajp.2013.12101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Choi HS, Hwang CK, Song KY, Lee BK, Law PY, Wei LN, Loh HH. Evidence of the neuron-restrictive silencer factor (NRSF) interaction with Sp3 and its synergic repression to the mu opioid receptor (MOR) gene. Nucleic acids research. 2006;34:6392–6403. doi: 10.1093/nar/gkl724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Hwang CK, Choi HS, Song KY, Law PY, Wei LN, Loh HH. Neuron-restrictive silencer factor (NRSF) functions as a repressor in neuronal cells to regulate the mu opioid receptor gene. The Journal of biological chemistry. 2004;279:46464–46473. doi: 10.1074/jbc.M403633200. [DOI] [PubMed] [Google Scholar]
- Kim HY, Choi JS, Cha JH, Choi JY, Lee MY. Expression of vascular endothelial growth factor receptors Flt-1 and Flk-1 in embryonic rat forebrain. Neuroscience letters. 2007;425:131–135. doi: 10.1016/j.neulet.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Fann CS, Bartzis M, Evgrafov OV, Rosenthal RN, Nunes EV, Miner C, Santana M, Gaffney J, Riddick A, Hsu CL, Knowles JA. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Human molecular genetics. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- Lambert D, Middle F, Hamshere ML, Segurado R, Raybould R, Corvin A, Green E, O’Mahony E, Nikolov I, Mulcahy T, Haque S, Bort S, Bennett P, Norton N, Owen MJ, Kirov G, Lendon C, Jones L, Jones I, Holmans P, Gill M, Craddock N. Stage 2 of the Wellcome Trust UK-Irish bipolar affective disorder sibling-pair genome screen: evidence for linkage on chromosomes 6q16-q21, 4q12-q21, 9p21, 10p14-p12 and 18q22. Molecular psychiatry. 2005;10:831–841. doi: 10.1038/sj.mp.4001684. [DOI] [PubMed] [Google Scholar]
- Li SX, Liu LJ, Jiang WG, Lu L. Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J Neurochem. 2009;109:1668–1679. doi: 10.1111/j.1471-4159.2009.06086.x. [DOI] [PubMed] [Google Scholar]
- Malison RT, Kranzler HR, Yang BZ, Gelernter J. Human clock, PER1 and PER2 polymorphisms: lack of association with cocaine dependence susceptibility and cocaine-induced paranoia. Psychiatric genetics. 2006;16:245–249. doi: 10.1097/01.ypg.0000242198.59020.ca. [DOI] [PubMed] [Google Scholar]
- McPeek MS, Sun L. Statistical tests for detection of misspecified relationships by use of genome-screen data. American journal of human genetics. 2000;66:1076–1094. doi: 10.1086/302800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JD, Maguire JJ, Davenport AP. Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. British journal of pharmacology. 2009;158:87–103. doi: 10.1111/j.1476-5381.2009.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Heath AC, Bogdan R, Sherva R, Zhang B, Al-Hasani R, Bruchas MR, Chou YL, Demers CH, Carey CE, Conley ED, Fakira AK, Farrer LA, Goate A, Gordon S, Henders AK, Hesselbrock V, Kapoor M, Lynskey MT, Madden PA, Moron JA, Rice JP, Saccone NL, Schwab SG, Shand FL, Todorov AA, Wallace L, Wang T, Wray NR, Zhou X, Degenhardt L, Martin NG, Hariri AR, Kranzler HR, Gelernter J, Bierut LJ, Clark DJ, Montgomery GW. Evidence of CNIH3 involvement in opioid dependence. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. American journal of human genetics. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nature reviews Genetics. 2015;16:275–284. doi: 10.1038/nrg3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80:303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Davidson P. Deficit of circulating stem--progenitor cells in opiate addiction: a pilot study. Substance abuse treatment, prevention, and policy. 2007;2:19. doi: 10.1186/1747-597X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Duration of opiate exposure as a determinant of arterial stiffness and vascular age in male opiate dependence: a longitudinal study. Journal of clinical pharmacy and therapeutics. 2014;39:158–167. doi: 10.1111/jcpt.12121. [DOI] [PubMed] [Google Scholar]
- Roy S, Balasubramanian S, Wang J, Chandrashekhar Y, Charboneau R, Barke R. Morphine inhibits VEGF expression in myocardial ischemia. Surgery. 2003;134:336–344. doi: 10.1067/msy.2003.247. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Rodriguez-Munoz M, Montero C, de la Torre-Madrid E, Garzon J. Calcium/calmodulin-dependent protein kinase II supports morphine antinociceptive tolerance by phosphorylation of glycosylated phosducin-like protein. Neuropharmacology. 2008;54:319–330. doi: 10.1016/j.neuropharm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Vallof D, Ulenius L, Egecioglu E, Engel JA, Jerlhag E. Central administration of the anorexigenic peptide neuromedin U decreases alcohol intake and attenuates alcohol-induced reward in rodents. Addiction biology. 2016 doi: 10.1111/adb.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nature genetics. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Furuta S, Katayama S, Narita M, Kuzumaki N, Imai S, Nagase H, Suzuki T, Narita M, Yamashita JK. The kappa opioid system regulates endothelial cell differentiation and pathfinding in vascular development. Blood. 2011;118:775–785. doi: 10.1182/blood-2010-09-306001. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Han S, Kranzler HR, Farrer LA, Elston RC, Gelernter J. Autosomal linkage scan for loci predisposing to comorbid dependence on multiple substances. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2012;159b:361–369. doi: 10.1002/ajmg.b.32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Han S, Kranzler HR, Farrer LA, Gelernter J. A genomewide linkage scan of cocaine dependence and major depressive episode in two populations. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:2422–2430. doi: 10.1038/npp.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
European American–Opioid Dependence (EA-OD) linkage results. Logarithm of odd (LOD) scores on 22 autosomal chromosomes resulting from non-parametric linkage analyses including EA families with OD for the male, female and combined samples. The dashed lines denote the thresholds of LOD scores for the three samples for genomewide-‘suggestive’ linkage. (Some of these thresholds appear as one dashed line because they are very close).
African-American–Opioid Dependence (AA-OD) linkage results. Logarithm of odd (LOD) scores on 22 autosomal chromosomes resulting from non-parametric linkage analyses including AA families with OD for the male, female and combined samples. The dashed lines denote the thresholds of LOD scores for the three samples for genomewide-‘suggestive’ linkage. (Some of these thresholds appear as one dashed line because they are very close).