Abstract
OBJECTIVE
To estimate the rate of acute respiratory distress syndrome (ARDS) in pregnant patients as well as to investigate clinical conditions associated with mortality.
METHODS
We used the Nationwide Inpatient Sample from 2006 to 2012 to identify a cohort of pregnant patients who underwent mechanical ventilation for ARDS. A multivariate model predicting in-hospital mortality was created.
RESULTS
A total of 55,208,382 hospitalizations from the 2006–2012 Nationwide Inpatient Samples were analyzed. There were 2,808 pregnant patients with ARDS who underwent mechanical ventilation included in the cohort. The overall mortality rate for the cohort was 9%. The rate of ARDS requiring mechanical ventilation increased from 36.5 cases (95% confidence interval [CI] 33.1–39.8) per 100,000 live births in 2006 to 59.6 cases (95% CI 57.7–61.4) per 100,000 live births in 2012. Factors associated with a higher risk of death were prolonged mechanical ventilation (adjusted odds ratio [OR] 1.69, 95% CI 1.25–2.28), renal failure requiring hemodialysis (adjusted OR 3.40, 95% CI 2.11–5.47), liver failure (adjusted OR 1.71, 95% CI 1.09–2.68), amniotic fluid embolism (adjusted OR 2.31, 95% CI 1.16–4.59), influenza infection (OR 2.26, 95% CI 1.28–4.00), septic obstetric emboli (adjusted OR 2.15, 95% CI 1.17–3.96), and puerperal infection (adjusted OR 1.86, 95% CI 1.28–2.70). Factors associated with a lower risk of death were: insurance coverage (adjusted OR 0.56, 95% CI 0.37–0.85), tobacco use (adjusted OR 0.53, 95% CI 0.31–0.90), and pneumonia (adjusted OR 0.70, 95% CI 0.50–0.98).
CONCLUSION
In this nationwide study, the overall mortality rate for pregnant patients mechanically ventilated for ARDS was 9%. The rate of ARDS requiring mechanical ventilation increased from 36.5 cases (95% CI 33.5–41.8) per 100,000 live births in 2006 to 59.6 cases (95% CI 54.3–65.3) per 100,000 live births in 2012.
Acute respiratory distress syndrome (ARDS) is a state of diffuse inflammatory lung injury characterized by acute-onset, noncardiogenic pulmonary edema with hypoxemia as defined by an arterial partial pressure of oxygen-to-inspired oxygen ratio of under 300.1 Acute respiratory distress syndrome is a rare occurrence in the pregnant patient with an estimated incidence of 16–70 per 100,000 pregnancies.2,3
Most of the published information on pregnancy-related ARDS comes from case reports or small series in which pregnancy-related ARDS was attributed to pyelonephritis, tocolytic use, sepsis, placental abruption, disseminated intravascular coagulopathy, chorioamnionitis, sickle cell disease, or preeclampsia–eclampsia.2–5 One of the largest series available involved 64 patients with ARDS during the 2009 H1N1 Influenza pandemic with an overall mortality rate of 11%.6 The case mortality for ARDS in pregnancy has been reported to range from 11% to more than 50%.2,5,6 Many of these historical figures reflect a prior era of ARDS management without low-tidal volume ventilation and likely do not reflect the outcomes of current patients.
Given the lack of data on ARDS in pregnant patients, we preformed a national analysis of ARDS among pregnant patients in the United States. The primary objective of this analysis is to estimate the rate of ARDS in pregnant patients as well as to investigate clinical conditions associated with patient outcomes.
MATERIALS AND METHODS
This nationwide cohort study is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology statement.7 A deidentified data set was used for this analysis, for which a waiver of consent was obtained from the University of British Columbia institutional review board. The Nationwide Inpatient Sample was used for this analysis; this robust database captures approximately 20% of all U.S. in-patient hospitalizations.8 The Nationwide Inpatient Sample is a complex survey produced by the Agency for Healthcare Quality and Research. Through the use of weighting, the survey is powered to estimate 95% of all inpatient care delivered across the United States.8 The individual patient weights are supplied by the Agency for Healthcare Quality and Research and were used in all analyses to maintain the integrity of the complex survey design.
All patients with a discharge diagnosis of ARDS who were mechanically ventilated and also pregnant from the 2006–2012 Nationwide Inpatient Samples were included in the analysis (Fig. 1). Our definition of ARDS was consistent with previous authors and included 1) International Classification of Diseases, 9th Revision codes for acute respiratory failure after trauma and surgery (518.51); 2) other pulmonary insufficiency, not elsewhere classified, after trauma and surgery (518.52); 3) acute and chronic respiratory failure after trauma and surgery (518.53); 4) acute respiratory failure (518.81); and 5) other pulmonary insufficiency, not elsewhere classified (518.82).9 Pregnancy was defined as the presence of any of the following codes: 640–649, 650–659, 660–669, V22, V23, and V27.10 Mechanical ventilation was isolated by the presence of the following International Classification of Diseases, 9th Revision codes: 96.70, 96.71, 96.72, and 96.04. Those patients with code 96.72 were defined as having prolonged mechanical ventilation (greater than 96 hours). We also excluded patients who had a diagnosis of peripartum cardiomyopathy.
Fig. 1.
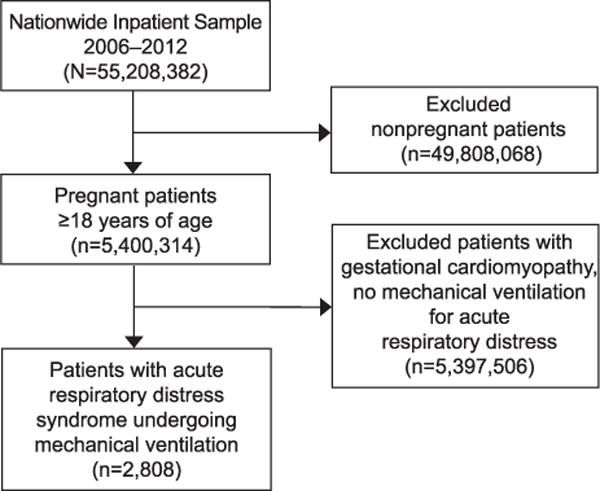
Flowchart of study participants.
Rush. ARDS in Pregnancy. Obstet Gynecol 2017.
Patient-level variables obtained from the data set included age, length of stay, race (white, black, Hispanic, other, missing), in-hospital mortality, total hospital charges, and insurance coverage (yes compared with no). We also collected the presence of the following comorbidities: renal failure requiring hemodialysis, obesity, tobacco use, gestational diabetes, liver failure, peripartum cardiomyopathy, thermal injury, trauma, influenza, pneumonia, septic obstetric emboli, puerperal infection, amniotic fluid embolism, multiparity, eclampsia, gestational hypertension, and multiple gestational pregnancy. In addition, we collected data on the rates of prolonged mechanical ventilation (greater than 96 hours) and delivery by cesarean. Hospital-level characteristics included in the analysis were: size (small, medium, large as defined by the Agency for Healthcare Quality and Research8), location (rural compared with urban), region of the country (Northeast, South, Midwest, West), and academic teaching status.
To calculate the rate of ARDS requiring mechanical ventilation as a function of live births in the United States, yearly birth data from the National Center for Health Statistics were obtained.11 The weighted number of pregnant patients with ARDS for each study year was divided by the number of live births in that year with the results expressed in number of patients requiring mechanical ventilation for ARDS per 100,000 live births.
All analyses were performed in SAS 9.4 using proper complex survey procedures and weights. All percentages displayed in tables and figures are estimates of national projections using proper weights. Multivariate logistic regression modeling was used to predict the outcome of death in the cohort of pregnant patients with ARDS. Variables in the model were selected a priori and included age, insurance coverage, prolonged mechanical ventilation, renal failure requiring hemodialysis, gestational diabetes, obesity, tobacco use, eclampsia or preeclampsia, gestational hypertension, multiparity, liver failure, and peripartum cardiomyopathy. Linear regression was used for trend analysis.
RESULTS
A total of 55,208,382 hospitalizations from the 2006–2012 Nationwide Inpatient Samples were analyzed. Of the 5,400,314 pregnant patients in the cohort, there were 2,808 pregnant patients with ARDS who underwent mechanical ventilation (0.05%; Table 1). The mean age of patients was 28.7 years (standard deviation 6.6), and the median hospital length of stay was 7.0 days (interquartile range 3.9–12.5). The overall in-hospital mortality rate for all patients was 9.0% (252 patients); however, the mortality rate for patients undergoing prolonged mechanical ventilation (greater than 96 hours) was 14.0%. The in-hospital mortality rate for patients with shorter durations of mechanical ventilation (less than 96 hours) was 6.9% with the highest rate of mortality during 2008 (Fig. 2; Appendix 1 [available online at http://links.lww.com/AOG/A927]).
Table 1.
Baseline Demographic and Patient Characteristics for Pregnant Patients With Acute Respiratory Distress Syndrome Requiring Mechanical Ventilation
| Characteristic | Pregnant Patients With ARDS Requiring Mechanical Ventilation (n=2,808) |
|---|---|
| Age (y) | 28.766.6 |
| Length of stay (d) | 7.0 (3.9–12.5) |
| Hospital mortality | 252 (9.0) |
| Race | |
| White | 1,009 (35.9) |
| Black | 612 (22.0) |
| Hispanic | 423 (15.0) |
| Other | 264 (9.3) |
| Missing | 500 (17.8) |
| Insurance coverage | 2,558 (91.2) |
| Hemodialysis need | 109 (3.9) |
| Liver failure | 165 (5.9) |
| Vaginal delivery | 412 (14.7) |
| Delivery by cesarean | 1,154 (41.1) |
| Mechanical ventilation greater than 72 h | 794 (28.4) |
| Gestational diabetes | 138 (4.9) |
| Puerperal infection | 268 (9.6) |
| Septic obstetric emboli | 76 (2.7) |
| Gestational hypertension | 288 (10.2) |
| Eclampsia | 620 (22.1) |
| Tobacco use | 314 (11.2) |
| Multiple gestations | 72 (2.5) |
| TRALI | 12 (0.4) |
| Amniotic fluid embolism | 64 (2.2) |
| Thermal injuries | 27 (1.0) |
| Traumatic injuries | 136 (4.8) |
| Pneumonia | 724 (25.9) |
| Influenza | 102 (3.6) |
| Hospital size | |
| Small | 185 (6.3) |
| Medium | 596 (21.5) |
| Large | 1,997 (72.2) |
| Hospital location and teaching status | |
| Rural | 133 (4.7) |
| Urban nonteaching | 908 (31.8) |
| Urban teaching | 1,767 (63.49) |
| Hospital region | |
| Northeast | 385 (14.3) |
| Midwest | 615 (22.2) |
| South | 1,163 (41.1) |
| West | 645 (22.4) |
ARDS, acute respiratory distress syndrome; TRALI, transfusion-related acute lung injury.
Data are mean±standard deviation, median (interquartile range), or n (%).
Fig. 2.
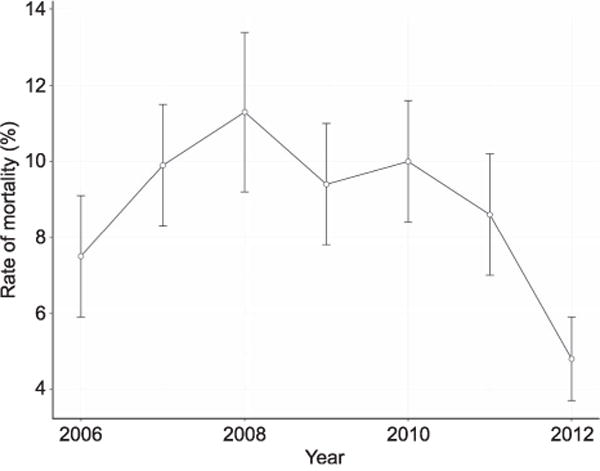
Rate of mortality for acute respiratory distress syndrome in pregnancy, 2006–2012.
Rush. ARDS in Pregnancy. Obstet Gynecol 2017.
There were 109 (3.9%) patients with acute renal failure requiring hemodialysis and 165 (5.9%) of patients with a diagnosis of liver failure. The majority of patients were treated at large (72.2%) urban teaching centers (63.5%). The rate of ARDS requiring mechanical ventilation increased from 36.5 cases (95% confidence interval [CI] 33.5–41.8) per 100,000 live births in 2006 to 59.6 cases (95% CI 54.3–65.3) per 100,000 live births in 2012 (Fig. 3).
Fig. 3.
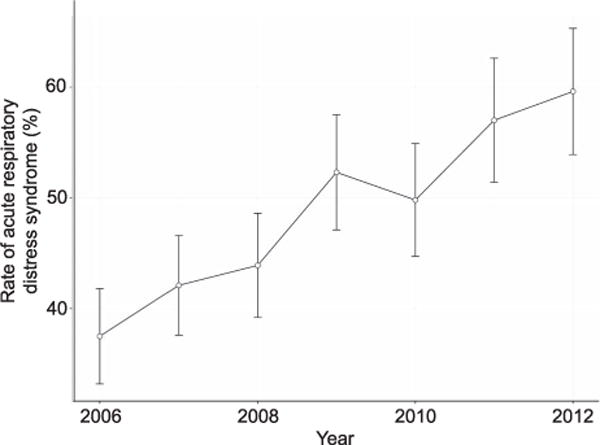
Rate of mechanical ventilation for acute respiratory distress syndrome in pregnancy, 2006–2012.
Rush. ARDS in Pregnancy. Obstet Gynecol 2017.
During their index admissions, 41.1% of patients underwent cesarean delivery and 14.6% of patients underwent vaginal delivery. The frequency of other pregnancy-related conditions were: amniotic fluid embolism: 64 (2.2%), preeclampsia or eclampsia: 620 (22.1), gestational hypertension: 288 (10.2%), septic obstetric embolism: 76 (2.7%), puerperal infection: 268 (9.6%), and gestational diabetes: 138 (4.9%).
Other nonpregnancy-related causes of ARDS occurred with the following frequency: thermal injuries 27 (1.0%), traumatic injuries 136 (4.8%), pneumonia 724 (25.9%), transfusion-related lung injury 12 (0.4%), and influenza 102 (3.6%). The case mortality rate for ARDS causes ranged from 0% in patients with transfusion-related lung injury to 20% in patients with septic obstetric emboli (Table 2).
Table 2.
Case Mortality Rates for Various Causes of Acute Respiratory Distress Syndrome in Mechanically Ventilated Pregnant Patients
| Mechanically Ventilated Pregnant Patients With ARDS
|
Mortality Rate for ARDS Precipitators in Mechanically Ventilated | ||
|---|---|---|---|
| Who Died In-Hospital | With Comorbidity Pregnant Patients |
||
| Pneumonia | 57 | 724 | 8.0 (6.0–9.9) |
| Influenza | 18 | 102 | 17.9 (11.1–24.7) |
| Traumatic injuries | 13 | 136 | 9.9 (4.4–15.4) |
| Thermal injuries | 4 | 27 | 15.3 (2.4–28.0) |
| Amniotic fluid embolism | 12 | 63 | 18.8 (10.7–27.0) |
| Septic obstetric emboli | 15 | 76 | 19.9 (12.3–27.4) |
| Eclampsia | 49 | 619 | 7.9 (5.7–10.0) |
| Puerperal infection | 45 | 268 | 16.8 (12.1–21.5) |
| TRALI | 0 | 12 | 0 (0–0) |
ARDS, acute respiratory distress syndrome; TRALI, transfusion-related acute lung injury. Data are n or % (95% confidence interval).
After for controlling for all variables in the multivariate model (Table 3), factors associated with a higher risk of death were prolonged mechanical ventilation (adjusted odds ratio [OR] 1.69, 95% CI 1.25–2.28), renal failure requiring hemodialysis (adjusted OR 3.40, 95% CI 2.11–5.47), liver failure (adjusted OR 1.71, 95% CI 1.09–2.68), amniotic fluid embolism (adjusted OR 2.31, 95% CI 1.16–4.59), influenza infection (adjusted OR 2.26, 95% CI 1.28–4.00), septic obstetric emboli (adjusted OR 2.15, 95% CI 1.17–3.96), and puerperal infection (adjusted OR 1.86, 95% CI 1.28–2.70). Factors associated with a lower risk of death were: insurance coverage (adjusted OR 0.56, 95% CI 0.37–0.85), tobacco use (adjusted OR 0.53, 95% CI 0.31–0.90), and pneumonia (adjusted OR 0.70, 95% CI 0.50–0.98).
Table 3.
Results of Multivariate Logistic Regression for Covariates Associated With Mortality in Mechanically Ventilated Pregnant Patients With Acute Respiratory Distress Syndrome
| Variable | OR | 95% CI | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|
| Age (per year) | — | — | 1.01 | 0.99–1.03 | .36 |
| Prolonged mechanical ventilation | 2.17 | 1.66–2.84 | 1.69 | 1.25–2.28 | <.01 |
| Renal failure requiring hemodialysis | 5.07 | 3.19–8.05 | 3.40 | 2.11–5.47 | <.01 |
| Insurance coverage | 0.65 | 0.44–0.95 | 0.56 | 0.37–0.85 | <.01 |
| Gestational diabetes | 0.70 | 0.32–1.50 | 0.75 | 0.37–1.52 | .42 |
| Tobacco use | 0.51 | 0.30–0.87 | 0.53 | 0.31–0.90 | .02 |
| Eclampsia | 0.83 | 0.60–1.16 | 0.82 | 0.58–1.16 | .27 |
| Gestational hypertension | 1.05 | 0.69–1.60 | 1.11 | 0.71–1.71 | .66 |
| Multiparity | 0.45 | 0.14–1.47 | 0.47 | 0.15–1.55 | .22 |
| Liver failure | 2.72 | 1.78–4.15 | 1.71 | 1.09–2.68 | .02 |
| Amniotic fluid embolism | 2.42 | 1.28–4.58 | 2.31 | 1.16–4.59 | .02 |
| Thermal injury | 1.83 | 0.64–5.27 | 2.11 | 0.59–7.63 | .25 |
| Trauma | 1.11 | 0.59–2.10 | 0.93 | 0.45–1.89 | .84 |
| Influenza | 2.30 | 1.37–3.86 | 2.26 | 1.28–4.00 | <.01 |
| Pneumonia | 0.84 | 0.63–1.13 | 0.70 | 0.50–0.98 | .04 |
| Septic obstetric emboli | 2.60 | 1.46–4.64 | 2.15 | 1.17–3.96 | .01 |
| Puerperal infection | 2.27 | 1.57–3.28 | 1.86 | 1.28–2.70 | <.01 |
All variables displayed were included in the final model.
DISCUSSION
In this large study of pregnant patients undergoing mechanical ventilation for ARDS, mortality was between 9% and 14%, depending on the duration of mechanical ventilation. Our analysis revealed numerous obstetric and patient factors that were associated with a higher risk of in-hospital mortality including renal failure, puerperal infection, septic obstetric emboli, and influenza.
The results of our analysis extend the findings of previous authors.2,3,12 We were able to capture patients from across the United States treated in a variety of hospital settings. The lower overall mortality rate observed in our cohort compared with contemporary reports of nonpregnant patients with ARDS likely reflects the younger age of pregnant patients.13 It is also possible that the lower incidence of chronic illness and medical comorbidities in the pregnant population allows for improved survival in the setting of respiratory failure. Our findings of differential mortality rates for the various causes of ARDS in pregnancy are not unexpected. Patients with acute catastrophic clinical events such as amniotic fluid embolism or septic pulmonary emboli in the setting of puerperal infections would be expected to have higher mortality rates than patients with pneumonia. The higher mortality rate in patients with influenza is in accordance with the severe respiratory distress seen during the 2009 H1N1 influenza pandemic.6 In addition, many of the case reports of extracorporeal membrane oxygenation support with pregnant patients with ARDS involved patients with influenza.14–16
Our observation of lower rates of mortality for pregnant patients with pneumonia and tobacco exposure warrants explanation. Pneumonia could be associated with lower rates of mortality compared with other cases of ARDS resulting from the reversible and treatable nature of pneumonia. Tobacco smoking has been associated with increased risks of pneumonia.17 In our cohort, 33% of tobacco smokers had a codiagnosis of pneumonia compared with 24% of nonsmokers. Although both variables were controlled for in our modeling, there was still a signal of decreased mortality in the tobacco smokers.
The observation of increasing rates of ARDS across the study period warrants examination. There was a sharp increase for cases during the 2009 calendar year, which could reflect the H1N1 pandemic of that year.6,18 Differences in coding and the year-to-year composition of the Nationwide Inpatient Sample may have influenced the observed increase in patients with ARDS. However, the linear trend is suggestive of a genuine trend of increasing diagnoses of ARDS in this population. Future studies will be needed to examine whether this trend in increasing incidence continues and to explore causal factors.
The curve for case rate mortality displayed in Figure 2 may be explained by several factors. The influenza pandemic of 2008 and 2009 may have played a role in the increased mortality observed. Improvements in the case fatality rate seen in 2010–2012 could reflect improved techniques in the management of patients with ARDS with increased use of low tidal volume ventilation and overall improvements in the care of patients with severe lung injury. However, the increased rate of diagnosis observed in the later years may explain some of the decreased mortality observed with the possibility that patients with less severe ARDS are being recognized. Future research is needed to explore these trends in mortality for this patient population.
The results of this analysis must be interpreted in the context of its study design. The use of large administrative databases comes at the costs of inherent uncertainty and bias. We were unable to use the Berlin definition of ARDS in establishing our cohort because this requires very detailed patient-level factors not available in the Nationwide Inpatient Sample. Nevertheless, the definition of ARDS we used has been used by multiple other investigators in the study of ARDS using administrative data sets.9,19,20 The accuracy of discharge abstract coding, although generally accurate, does not escape information errors that would be minimized with prospectively collected data in a controlled trial.21 Nevertheless, the ability to capture the treatment of pregnant patients with ARDS across the United States adds valuable information to the sparse literature regarding these patients.
Supplementary Material
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–82. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 2.Catanzarite V, Willms D, Wong D, Landers C, Cousins L, Schrimmer D. Acute respiratory distress syndrome in pregnancy and the puerperium: causes, courses, and outcomes. Obstet Gynecol. 2001;97:760–4. doi: 10.1016/s0029-7844(00)01231-x. [DOI] [PubMed] [Google Scholar]
- 3.Mabie WC, Barton JR, Sibai BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol. 1992;167:950–7. doi: 10.1016/s0002-9378(12)80018-4. [DOI] [PubMed] [Google Scholar]
- 4.Schwaiberger D, Karcz M, Menk M, Papadakos PJ, Dantoni SE. Respiratory failure, and mechanical ventilation in the pregnant patient. Crit Care Clin. 2016;32:85–95. doi: 10.1016/j.ccc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Catanzarite VA, Willms D. Adult respiratory distress syndrome in pregnancy: report of three cases and review of the literature. Obstet Gynecol Surv. 1997;52:381–92. doi: 10.1097/00006254-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 6.ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–35. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 8.Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2011.pdf. Retrieved December 1, 2016.
- 9.Reynolds HN, McCunn M, Borg U, Habashi N, Cottingham C, Bar-Lavi Y. Acute respiratory distress syndrome: estimated incidence and mortality rate in a 5 million-person population base. Crit Care. 1998;2:29–34. doi: 10.1186/cc121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azar T, Longo C, Oddy L, Abenhaim HA. Motor vehicle collision-related accidents in pregnancy. J Obstet Gynaecol Res. 2015;41:1370–6. doi: 10.1111/jog.12745. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) About natality, 2007–2014. Available at: http://wonder.cdc.gov/natality-current.html. Retrieved May 23, 2016.
- 12.Cole DE, Taylor TL, McCullough DM, Shoff CT, Derdak S. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005;33(suppl):S269–78. doi: 10.1097/01.ccm.0000182478.14181.da. [DOI] [PubMed] [Google Scholar]
- 13.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 14.Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151:1154–60. doi: 10.1016/j.jtcvs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Zhou Y, Gong S, Dong H, Wu G, Xiang X, et al. A pregnant woman with avian influenza A (H7N9) virus pneumonia and ARDS managed with extracorporeal membrane oxygenation. Southeast Asian J Trop Med Public Health. 2015;46:444–8. [PubMed] [Google Scholar]
- 16.Anselmi A, Ruggieri VG, Letheulle J, Robert AL, Tomasi J, Le Tulzo Y, et al. Extracorporeal membrane oxygenation in pregnancy. J Card Surg. 2015;30:781–6. doi: 10.1111/jocs.12605. [DOI] [PubMed] [Google Scholar]
- 17.Almirall J, González CA, Balanzó X, Bolíbar I. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest. 1999;116:375–9. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 18.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 19.Rush B, Wiskar K, Berger L, Griesdale D. Trends in extracorporeal membrane oxygenation for the treatment of acute respiratory distress syndrome in the United States. J Intensive Care Med. 2016 Feb 17; doi: 10.1177/0885066616631956. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–62. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–9. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


