Abstract
Metazoan cells translate adhesive events with neighbors into anti-proliferative signals in the nucleus. The cadherin–catenin adhesion complex has long been suspected of playing a key role in this process, and three recent papers suggest that it does so by modulating subcellular localization of the Hippo pathway component Yap1.
Most metazoan cells spend their lives in close apposition with adjacent cells and must use a variety of communication tools to organize group activities and set standards for communal living. One key to a stable cellular neighborhood is preventing cells from over-proliferating and crowding out neighbors. A model of cellular crowd control has evolved, termed ‘contact inhibition’, in which intercellular adhesion events block proliferation. Cancer cells are rogues that by and large fail to abide by this rule. An understanding of the mechanisms underlying contact inhibition, and why certain mutations allow cancer cells to avoid it, has, however, remained incomplete. Three recent papers [1–3] now help to fill this gap by identifying E-cadherin and α-catenin, two protein components of the cadherin–catenin adhesion complex, as regulators of Yes-associated protein-1 (Yap1), a major oncogenic component of the Hippo tumor suppressor network. In doing so, these groups have illuminated what could be a central element of the contact inhibition mechanism.
Yap1 and Hippo
Yap1 and its homolog TAZ (transcriptional co-activator with PDZ-binding motif) are the main targets of the vertebrate Hippo growth regulatory pathway, which was first identified in Drosophila and shown to be a key regulator of organ size and tumorigenesis in other organisms, including vertebrates [4]. Canonical Hippo signals in vertebrate cells are transduced through two sequentially acting sets of kinases —Mst1 and Mst2 (Mst1/2) and Lats1 and Lats2 (Lats1/2) — to regulate phosphorylation of Yap1. Phosphorylated Yap1 (p-Yap1) is sequestered in the cytoplasm by 14-3-3 proteins [4,5], while unphosphorylated Yap1 shuttles into the nucleus where it interacts with context-specific partners to drive expression of pro-proliferative genes.
The growing list of Hippo pathway components that are mutated in cancers provides strong impetus to understand its regulation. In Drosophila epithelia, Hippo signaling responds to extrinsic cues, such as morphogen gradients, local regenerative signals, cell adhesion, and the acquisition of apicobasal polarity [4–6]. Accordingly, key Hippo components can be found at cell junctions, including the atypical cadherin Fat and the polarity protein Crumbs [4]. Vertebrate Yap1 also responds to cell density in culture [7] and inhibition of Yap1 is required for efficient contact inhibition [8]; however, cell-surface receptors regulating vertebrate Hippo signaling were not particularly well understood prior to the work summarized here. These recent studies show that E-cadherin and α-catenin can sequester Yap1 in the cytoplasm where it is transcriptionally inert, but provide different insights into how this mechanism operates.
Yap1 and α-Catenin
The Schlegelmilch et al. [1] and Silvis et al. [2] groups used epidermal keratinocytes as a model of contact inhibition and made the shared discovery of a direct physical interaction between Yap1 and α-catenin that sequesters Yap1 in the cytoplasm. The two groups arrived at this discovery from opposing sides of the Yap1–α-catenin complex. Schlegelmilch et al. [1] used immunoprecipitation/mass-spectrometry to identify α-catenin in a search for Yap1 regulators. This was spurred by their observation that Yap1 is non-responsive to depletion of the Mst1/2 or Lats1/2 kinases in epidermal cells, implying that an undefined Yap1 inhibitory mechanism must predominate in this cell type. Reciprocally, Silvis et al. [2] identified Yap1 as a key driver of keratinocyte overproliferation induced by α-catenin loss. Both groups found that α-catenin depletion or deletion in keratinocytes relocalizes Yap1 from the cytoplasm to the nucleus and elevates nuclear Yap1 activity. Keratinocyte overproliferation following α-catenin loss was suppressed by depletion of Yap1, providing functional evidence that Yap1 acts downstream of α-catenin. Interestingly Schlegelmilch et al. [1] also found that Yap1 was non-responsive to the depletion of E-cadherin and/or P-cadherin, implying that another membrane receptor mediates contact inhibition in this system.
α-Catenin Blocks Yap1 Dephosphorylation
The discovery of a cytoplasmic Yap1–α-catenin complex led Schlegelmilch et al. [1] to examine interactions between Yap1 and 14-3-3 proteins, which bind and retain phospho-Yap1 in the cytoplasm [9]. This revealed that p-Yap1, α-catenin, and 14-3-3 can form a complex in the cytoplasm, and that disruption of the complex following α-catenin loss exposes p-Yap1 to the PP2A protein phosphatase. Subsequent dephosphorylation drives Yap1 into the nucleus and promotes cell proliferation. This simple model provides a direct link between α-catenin and p-Yap1, and predicts that α-catenin status is a critical determinant of Yap1 nuclear activity, as its loss may blunt the effect of inhibitory kinases. Indeed, Schlegelmilch et al. [1] found an inverse correlation between α-catenin levels and nuclear Yap1 protein in squamous cell carcinoma cell lines, indicating that dysregulation of the Yap1–α-catenin complex contributes to this disease. However, the data also raise the intriguing issue of which kinase actually phosphorylates Yap1 in keratinocytes. If it is not the Mst or Lats kinases, then which kinase(s) puts the phosphate group(s) on Yap1 that must then be protected by α-catenin? Moreover, if α-catenin binds Yap1, could E-cadherin act through α-catenin to regulate Yap1 in cell types other than keratinocytes?
Yap1 and E-Cadherin
The third paper in the series by Kim et al. [3] presents evidence from non-keratinocyte breast epithelial cells that E-cadherin acts as an adhesion receptor that regulates Yap1 localization via catenins and the canonical Hippo pathway. This link is defined using beads coated with purified E-cadherin to selectively engage E-cadherin on the surface of cells. While cells in situ engage in many types of adhesive interaction with neighbors, this system has the advantage of parsing the downstream effects of E-cadherin ligation. Moderate reduction of proliferation following exposure to E-cadherin beads could be blocked by small interfering RNA depletion of either α- or β-catenin, or by depletion of the Hippo components Merlin/NF2, Lats1/2, and Kibra. E-cadherin overexpression led to cytoplasmic retention of Yap1, and this required the E-cadherin catenin-binding domain. Reciprocally, depletion of β-catenin or Lats1/2 from dense cultures induced nuclear accumulation and reduced phosphorylation of Yap1. This suggests a possible tumor suppressor role for β-catenin that is rather surprising considering its role in the Wnt pathway [10]. Notably, Kim et al. [3] found that depletion of Mst1/2 does not abrogate the anti-proliferative effect of E-cadherin ligation, suggesting that relocalization of Yap1 to the cytoplasm following E-cadherin ligation occurs via a pathway requiring the α/β-catenins and canonical Hippo components, but that an additional kinase acts redundantly or in place of Mst1/2.
Future Directions
These three studies provide important new insight into the Hippo pathway by identifying E-cadherin and α-catenin as upstream regulators of Yap1 (Figure 1). They also raise quite a few interesting follow-on questions. First, as E-cadherin can associate with α-catenin, could the Yap1–α-catenin complex be part of a common density-sensing pathway that begins with homotypic E-cadherin interactions? Schlegelmilch et al. [1] did observe cadherin peptides in proteomic analysis of Yap1 interactors, but saw no obvious change in Yap1 reporters following depletion of E-cadherin or β-catenin depletion from cultured keratinocytes. However calcium depletion, which disrupts E-cadherin homotypic interactions, did trigger redistribution of Yap1 into the nucleus. The inability of E-cadherin depletion to robustly activate Yap1 in keratinocytes thus raises the interesting possibility that the Yap1–α-catenin complex is regulated by adhesion inputs in a cell-type-specific manner, with the emphasis on E-cadherin in breast epithelial cells and on other transmembrane proteins in keratinocytes. Thus, a second important issue is whether multiple adhesion complexes serve overlapping roles upstream of Yap1–α-Catenin. Crb3, the vertebrate homolog of fly Crumbs, regulates density-dependent growth through Yap1/TAZ and may be a candidate for this role [7]. Fly Crumbs controls Hippo signals by interacting with the cytoskeletal linker protein Expanded, which localizes to apical adherens junctions [11–14] and binds Merlin and Kibra [15]. Vertebrate Merlin/NF2 in turn promotes adherens junction formation and binds to α-catenin in keratinocytes [16]. As this Merlin/NF2–α-catenin complex also includes the Par3 protein, apical polarity complexes could provide additional input into α-catenin regulation. Yap1 can also be found in a complex with the tight junction proteins Amot and ZO-2 [17], and Amot is required to recruit Yap1 to the tight junctions and for contact inhibition in cultured MDCK cells [18]. Yap1/TAZ proteins may then receive regulatory input from tight junction and polarity complexes, as well as from proteins at the adherens junction. Finally, in what may be a case of turnabout being fair play, Crumbs, Expanded and the E-cadherin homolog Shotgun can be induced in fly epithelial cells by mutations that activate the Yap1 ortholog Yki [19,20] (and see [7,14]), suggesting an inhibitory feedback loop in which decreased Hippo signals upregulate junctional proteins. The potential complexity of this network, and the role of α-catenin in it, will surely keep researchers hard at work for some time unraveling additional molecular links between cellular adhesion and Hippo signaling.
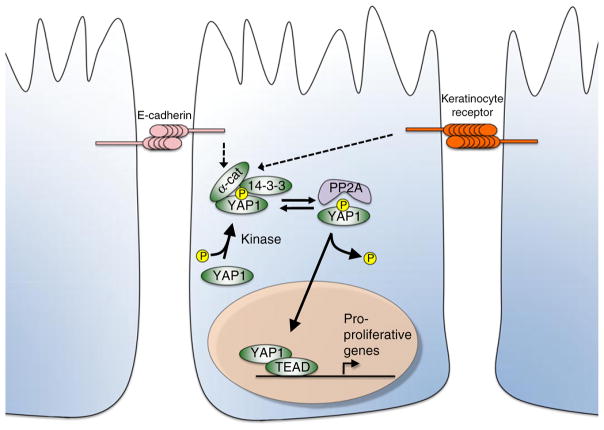
Figure 1. Model of E-cadherin and α-catenin regulation of Yap1.
α-Catenin and 14-3-3 proteins bind phospho-Yap1 and localize it to the cytoplasm. This α-catenin–14-3-3–phospho-Yap1 complex protects Yap1 from the protein phosphatase PP2A, which will otherwise dephosphorylate Yap1 and promote its nuclear entry. In breast epithelial cells, E-cadherin appears to act as an adhesion receptor upstream of α-catenin to control the α-catenin–14-3-3–phospho-Yap1 complex and thus restrain Yap1 activity. A different adhesion receptor appears to fulfill this role in keratinocytes.
References
- 1.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–R582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci USA. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka T, Schmitt AP, Sudol M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene. 2011 doi: 10.1038/onc.2011.216. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J Cell Sci. 2009;122:2351–2359. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]