In periodontics and implant dentistry, traditional clinical criteria are often insufficient for determining sites of active disease, for monitoring quantitatively the response to therapy or for measuring the degree of susceptibility to future disease progression. Saliva as a mirror of oral and systemic health is a valuable source for clinically relevant information because it contains biomarkers specific for the unique physiological aspects of periodontal / peri-implant disease, and qualitative changes in the composition of these biomarkers could have diagnostic value by identifying patients with enhanced disease susceptibility, identifying sites with active disease, predicting sites that will have active disease in the future and / or serving as surrogate end points for monitoring the effectiveness of therapy. Although the diagnostic value of saliva has been recognized for some time (50, 51) and potential biomarkers of periodontal / peri-implant disease have been identified in saliva (39, 67, 74), most work carried out to date has failed to provide reliable aids to the clinician. However, the availability of more sophisticated analytic techniques give cause for optimism that saliva will eventually become the tool needed for more precise treatment planning.
Periodontal disease: background
Periodontal disease is a chronic disease of the oral cavity comprising a group of inflammatory conditions affecting the supporting structures of the dentition (3). The impact of dental plaque biofilms on the etiology of periodontal diseases has been studied in detail. However, it is the paradoxical impact of the susceptible host’s inflammatory response to the microbial challenge that ultimately leads to the destruction of periodontal tissues and subsequent tooth loss (60).
Periodontitis, the destructive category of periodontal disease, is a chronic nonreversible inflammatory state of the supporting structures. After its initiation, the disease progresses with the loss of collagen fibers and attachment to the root surface, apical migration of the pocket epithelium, formation of deepened periodontal pockets and the resorption of alveolar bone. If left untreated, the disease continues with progressive alveolar bone destruction, leading to increased tooth mobility and subsequent tooth loss (58).
Regarding the aforementioned microbiological challenge, an estimated 600 different bacteria are capable of colonizing the human mouth, with any individual typically harboring 150 to 200 of these species. Inherent virulence factors from these pathogenic species enable the bacteria to (i) colonize on the tooth surface and in the gingival sulcus, and (ii) cause tissue damage to the periodontal tissue by producing potent substances that subsequently trigger the host inflammatory response (45).
Periodontal / peri-implant diseases
Chronic periodontitis is the most prevalent form of destructive periodontal disease. From 1988 to 1994 approximately half of US adults (30 years and older) were found to have chronic periodontitis. Further breakdown of these findings indicated that 31% of the US population exhibited mild forms of periodontitis, 13% displayed periodontitis of moderate severity and 4% suffered from advanced periodontitis (2).
Approximately 800,000 dental implants are currently being placed each year in the USA alone. The yearly growth rate for implant dentistry is steadily increasing and reached, in the USA, a rate of 25% in 2006 (53). Long-term maintenance of osseointegration depends on the preservation of healthy soft and hard tissues surrounding oral implants. Unfortunately, regardless of the system types, implants may fail because of biological complications leading to peri-implantitis and risk variables that influence the occurrence of the disease (10, 18). Although prevalence data regarding peri-implantitis are controversial (7, 64–66), recent independent studies found that the condition affects at least 8.9%of patients (17) and that after 10 years in use peri-implant lesions adjacent to titanium implants represent a common clinical entity (66). As the number of implant placements is rapidly increasing, the potential occurrence of implant failure as a result of peri-implantitis is consequently also expected to increase.
Assessing the prognosis of periodontal / peri-implant diseases
Planning therapy is probably the most critical and difficult step in the treatment of patients with periodontal / peri-implant disease (41). The clinician must decide which teeth / implant to retain, which treatment strategy to follow and how to maintain or restore a functional and esthetically pleasing dentition. For making such decisions, it is pivotal to assess the prognosis of each tooth / implant in order to choose the treatment modality with the greatest probability of success (8). Assessing the prognosis of each tooth / implant is an integral part of the periodontal practice because it directly influences treatment planning. However, there is limited direct evidence in the literature regarding the assignment of periodontal / peri-implant prognosis (41) because patients are not equally at risk and teeth / implants are variably affected within the mouth (23). The reasons for this may lie in the multifactorial and not yet completely understood pathogenesis of both disease entities. The most compelling evidence indicates that periodontitis / peri-implantitis is not a typical infectious disease, but an inflammatory disease that is triggered by host immune responses to colonization with pathogenic periodontal microorganisms organized in biofilms and often modified by exogenous factors such as smoking (13). Intervening between the colonization and the affected hard and soft tissues is a dense mononuclear inflammatory infiltrate containing all cellular components, including T and B lymphocytes, which are necessary to control immunological interactive networks (5, 9, 19, 32, 77). However, it is still unclear how the variability in the progression rate of periodontal / peri-implant diseases can be linked to those factors. The emergence of new technologies to study the genomics, proteomics and metabolomics of the host and the pathogen will certainly increase the understanding of periodontal / peri-implant diseases, eventually resulting in the development of risk-assessment tools for better prediction of disease events. This is urgently needed because underestimating or overestimating the actual prognosis of a tooth / implant may result in both increased and unnecessary costs. For example, if the prognosis is underestimated, a tooth that could have been retained with a high probability by treating the existing disease and / or condition, may be extracted. The procedures required to replace the extracted tooth often cost more than treatment of the tooth’s problem. On the other hand, overestimating the prognosis of a tooth may lead to rendering treatment to a tooth with a low probability of survival. If the tooth is lost some time after treatment, further therapies become necessary, resulting in additional costs. A more accurate system of determining the prognosis (prior to periodontal / implant therapy and during supportive periodontal / implant therapy) would allow a more specific allocation of expenditure, thus improving the appropriateness and quality of dental care by minimizing under-utilization and over-utilization of therapeutic options.
Prospective periodontal / peri-implant healthcare
Today the greatest expenditure on periodontal / peri-implant diseases is focused on late disease stages that occur long after the initial pathological changes take place. As a result of increasing costs to treat periodontal / peri-implant disease, there is a need to implement an approach similar to that known as prospective healthcare in the medical field in order to reduce such costs. Prospective healthcare is a new approach that incorporates all the power of current disease-oriented medicine but is based on the concept of strategic health planning, a pro-active, prospective approach to care. In this system, individuals are evaluated to determine their baseline risk for a specific disease, their current health status and their likelihood of developing specific clinical problems given their risks (71–73). As mentioned before, allocation of resources to prevent periodontitis / peri-implantitis would be optimized and may help to reduce costs if diagnostic information would assist in identifying susceptible patients. In an interesting study by Higashi et al. (31), genetic testing for the interleukin-1 genotype was assessed for cost-effectiveness by using a disease-simulation model and by using decision analytic techniques over a 30-year time frame. Using different modelling scenarios, the genetic test produced results ranging from cost savings of $830,140 and 52.8 fewer cases of severe periodontitis to increased costs of $300,430 and 3.6 additional cases of severe periodontitis (per 1,000 patients). Irrespective of these conflicting results and the current controversial scientific discussion about the clinical value of testing for the interleukin-1 genoytype, the results suggest that the use of a susceptibility test could indeed result in significant savings in periodontal therapy (31). However, parallel to the identification of susceptible patients, more specific prevention / treatment strategies for high-risk and low-risk patients (e.g. immunomodulation or specific adjunctive antimicrobial strategies) need to be developed. Otherwise an obvious cost–benefit advantage of identifying susceptible patients cannot be expected. Saliva as a diagnostic and / or prognostic tool can improve and ease treatment planning in periodontics and implant dentistry, thus resulting in more predictable treatment outcomes and cost savings.
Early disease detection and evaluation of periodontal therapy
A periodontal diagnostic tool, in general, provides pertinent information for differential diagnosis, localization of disease and severity of infection. It serves as a basis for treatment planning and provides a means for assessing the effectiveness of periodontal therapy. Current clinical diagnostic parameters that were introduced more than half a century ago continue to function as the basic model for periodontal diagnosis in current clinical practice. They include various parameters such as probing pocket depths, bleeding on probing, clinical attachment levels, plaque index and radiographs quantifying alveolar bone levels (4). Albeit easy to use, cost-effective and relatively noninvasive, clinical attachment loss evaluation using the periodontal probe measures damage from past episodes of destruction and requires a 2– 3 mm threshold change before a site with significant breakdown can be identified. The use of subtraction radiography offers a means to detect minute changes in alveolar bone calcium content. These measures, however, are rarely seen in current dental clinical practice. Moreover, they lack the capacity to identify highly susceptible patients who are at risk for disease progression (22).
Need for a periodontal diagnostic indicator
The diagnosis of active phases of periodontal disease and the identification of patients at risk for active disease are challenges for clinical investigators and practitioners alike. Researchers are confronted with the need for innovative diagnostic tests that focus on the early recognition of the microbial challenge to the host. Optimal innovative approaches would correctly determine the presence of current disease activity, predict sites vulnerable for future breakdown and assess the response to periodontal interventions. A new paradigm for periodontal diagnosis would ultimately improve the clinical management of periodontal patients.
Salivary markers of periodontal diseases
Researchers involved in periodontal disease diagnostics are currently investigating the possible use of oral fluids, such as saliva, for disease assessment (49). Secretions from the major salivary glands (parotid, submandibular and sublingual), which have a large number of proteins and peptides, are responsible for maintaining the integrity of the oral cavity (Table 1). Also, because of its importance in oral biofilm formation and host defense, secreted saliva may have a significant role in the establishment and progression of periodontal disease.
Table 1.
Major salivary gland secretion mediators associated with periodontal diseases
| Marker | Relationship with periodontal disease | Type of periodontal disease |
|---|---|---|
|
| ||
| Specific | ||
| Immunoglobulins (IgA, IgM, IgG) | Interfere in adherence and bacterial metabolism / increased concentration in saliva of periodontal patients | Chronic and aggressive |
| Nonspecific | ||
| Mucins | Interfere with the colonization of Aggregatibacter actinomycetemcomitans | Aggressive |
| Lysozyme | Regulates biofilm accumulation | Chronic |
| Lactoferrin | Inhibits microbial growth / increased correlation with A. actinomycetemcomitans | Aggressive |
| Histatin | Neutralizes lipopolysaccharide and enzymes known to affect the periodontium | Chronic and aggressive |
| Peroxidase | Interferes with biofilm accumulation / increased correlation with periodontal patients | Chronic |
| Systemic | ||
| C-reactive protein | Increased concentration found in serum and saliva of periodontal patients | Chronic and aggressive |
Saliva (oral fluid) is a mirror of the body. It could be used to monitor the general health and the onset of specific diseases. Biomarkers, whether produced by normal healthy individuals or by individuals affected by specific systemic diseases, are tell-tale molecules that could be used to monitor health status, disease onset, treatment response and outcome. Informative biomarkers can further serve as early sentinels of disease, and this has been considered as the most promising alternative to classic environmental epidemiology (12).
Markers affecting the dental biofilm
Specific markers
Immunoglobulins (Ig) are important specific defense factors of saliva. Of the different classes of immunoglobulins, IgA, IgG and IgM influence the oral microbiota by interfering with the adherence of bacteria or by inhibiting bacterial metabolism, with IgA being the predominant immunoglobulin in this respect. Patients with periodontal disease are shown to have higher salivary concentrations of IgA, IgG and IgM specific to periodontal pathogens compared with healthy patients (68). Additionally, the levels of these immunoglobulins in saliva are greatly reduced after periodontal treatment (63). As a consequence, the screening of saliva, especially for IgA, has been previously discussed as a useful, noninvasive technique to identify individuals who have the potential to develop periodontal disease or those who are currently responding to a periodontopathogenic infection (51).
Nonspecific markers
Mucins are glycoproteins produced by submandibular and sublingual salivary glands and numerous minor salivary glands. The physiological functions of the mucins (MG1 and MG2) are cytoprotection, lubrication, protection against dehydration and maintenance of viscoelasticity in secretions. The mucin, MG2, affects the aggregation and adherence of bacteria and is known to interact with Aggregatibacter actinomycetemcomitans, and a decreased concentration of MG2 in saliva may increase colonization with this periodontopathogen (24).
Lysozyme is an antimicrobial enzyme with the ability to cleave chemical bonds in the bacterial cell wall. It can lyse some bacterial species by hydrolyzing glycosidic linkages in the cell wall peptidoglycan. It may also cause lysis of bacterial cells by interacting with monovalent anions and with proteases found in saliva. This combination leads to destabilization of the cell membrane, probably as a result of the activation and deregulation of endogenous bacterial autolysins. Patients with low levels of lysozyme in saliva are more susceptible to plaque accumulation, which is considered a risk factor for periodontal disease (38).
Lactoferrin is an iron-binding glycoprotein produced by salivary glands, which inhibits microbial growth by sequestering iron from the environment, thus depriving bacteria of this essential element. Lactoferrin is strongly up-regulated in mucosal secretions during gingival inflammation and is detected at a high concentration in saliva of patients with periodontal disease compared with healthy patients (25).
Histatin is a salivary protein with antimicrobial properties and is secreted from parotid and submandibular glands. It neutralizes the endotoxic lipopolysaccharides located in the membrane of gram-negative bacteria. Histatin is also an inhibitor of host and bacterial enzymes involved in the destruction of the periodontium. In addition to its antimicrobial activities, histatin is involved in the inhibition of the release of histamine from mast cells, affecting their role in oral inflammation (26, 28).
Peroxidase is a salivary enzyme produced by acinar cells in the salivary glands. This enzyme removes toxic hydrogen peroxide produced by oral microorganisms and reduces acid production in the dental biofilm, thereby decreasing plaque accumulation and the establishment of gingivitis and caries. Patients with periodontal disease have demonstrated high levels of this enzyme in saliva (27).
Systemic markers related to periodontal infection
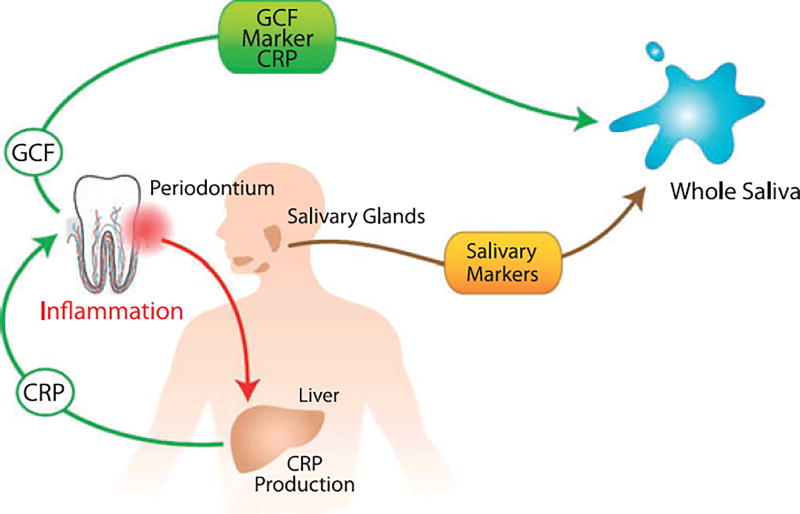
C-reactive protein is a systemic marker released during the acute phase of an inflammatory response. C-reactive protein is produced by the liver and is stimulated by circulating cytokines, such as tumor necrosis factor-a and interleukin-1, from local and / or systemic inflammation such as periodontal inflammation. Circulating C-reactive protein may reach saliva via gingival crevicular fluid or the salivary glands (Fig. 1). High levels of C-reactive protein have been associated with chronic and aggressive periodontal diseases and with other inflammatory biomarkers (15). Studies have demonstrated that periodontal patients display elevated concentrations of serum C-reactive protein when compared with healthy individuals (78). C-reactive protein has recently been shown to be measurable in saliva from periodontal patients using a ‘lab-on-a-chip’ method (14).
Fig. 1.
Schematic overview of the stimulation of CRP (C-reactive protein) in the liver by periodontal pathogens and its subsequent release into GCF (gingival crevice fluid) and whole saliva respectively. Reprinted with permission (62).
Markers of periodontal disease from whole saliva
Significance of gingival crevicular fluid
Easily collected and containing local and systemic-derived biomarkers of periodontal disease, oral fluids may offer the basis for patient-specific diagnostic tests for periodontal disease. Gingival crevicular fluid is both a physiological fluid as well as an inflammatory exudate, originating from the gingival plexus of blood vessels in the gingival corium, subjacent to the epithelium lining of the dentogingival space. As gingival crevicular fluid traverses through inflamed periodontal tissues en route to the sulcus, biological molecular markers are gathered from the surrounding areas and are subsequently eluted into whole saliva. Gingival crevicular fluid sampling methods have been shown to capture inflammatory and connective tissue breakdown mediators accurately. To date, more than 90 different components in gingival crevicular fluid have been evaluated for periodontal diagnosis (47). Of the numerous constituents in gingival crevicular fluid, however, the vast majority constitute soft-tissue inflammatory events, while only a few are regarded as specific biomarkers of alveolar bone destruction (39).
Markers of periodontal soft tissue inflammation
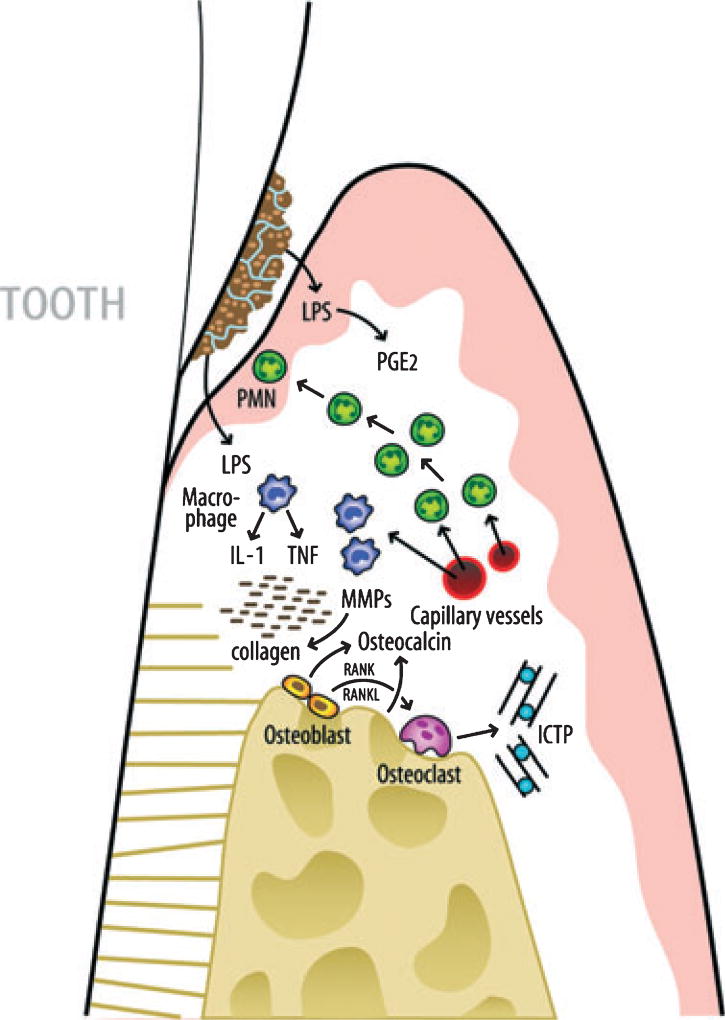
During the initiation of an inflammatory response in the periodontal connective tissue, numerous cytokines, such as prostaglandin E2, interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha are released from cells of the junctional epithelia and from connective tissue fibroblasts, macrophages and polymorphonuclear leukocytes (Fig. 2). Subsequently, enzymes such as matrix metalloproteinase (MMP)-8, MMP-9 and MMP-13 are produced by polymorphonuclear leukocytes and osteoclasts, leading to the degradation of connective tissue collagen and alveolar bone. During the inflammatory process, intercellular products are synthesized, released and diffuse towards the gingival sulcus or periodontal pocket.
Fig. 2.
Schematic overview of the pathogenic processes in periodontal disease. Initial events are triggered by lipopolysaccharide (LPS) from gram-negative bacteria on the tooth root surfaces. As a first line of defense, polymorphonuclear leukocytes (PMNs) are recruited to the site. Monocytes and activated macrophages respond to endotoxin by releasing cytokines [tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β)] which stimulate further tissue destruction. Matrix metalloproteinases (MMPs), powerful collagen destroying enzymes, are produced by fibroblasts and PMNs. TNF-α, IL-1β and receptor activator of NF-kB ligand (RANKL) are elevated in active sites and mediate osteoclastogenesis and bone breakdown. Bone-specific markers such as pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) are released into the surrounding area and transported by way of gingival crevice fluid (GCF) into the pocket and serve as potential biomarkers for periodontal disease detection. Reprinted with permission (62).
Prostglandins are arachidonic acid metabolites composed of 10 classes, of which D, E, F, G, H and I are of main importance. Of this group, prostaglandin E2 is one of the most extensively studied mediators of periodontal disease activity. During the host defense response to bacterial lipopolysaccharide, monocytes, polymorphonuclear leukocytes, macrophages and other cells release interleukin-1, tumor necrosis factor and prostaglandin E2. Prostaglandin E2 acts as a potent vasodilator and increases capillary permeability, which elicits clinical signs of redness and edema. Prostaglandin E2 also stimulates fibroblasts and osteoclasts to increase the production of MMPs (1).
Markers of alveolar bone loss
Many different biomarkers associated with bone formation, resorption and turnover, such as alkaline phosphatase, osteocalcin, osteonectin and collagen telopeptidases, have been evaluated in gingival crevicular fluid and saliva (39). These mediators are associated with local bone metabolism (in the case of periodontitis) as well as with systemic conditions (such as osteoporosis or metastatic bone cancers).
Matrix metalloproteinases are host proteinases responsible for both tissue degradation and remodeling. During progressive periodontal breakdown, gingival and periodontal ligament collagens are cleaved by host cell-derived interstitial collagenases. MMP-8 is the most prevalent MMP found in diseased periodontal tissue and gingival crevicular fluid. Elevated MMP-8 levels in active disease progression were observed in a longitudinal study of patients with gingivitis and with nonprogressive and progressive periodontitis (11, 54). Recently, the level of MMP-8 was demonstrated to be highly elevated in saliva from patients with periodontal disease using a rapid point-of-care microfluidic device (30). The MMP-8 level is also elevated in peri-implant sulcular fluid from peri-implantitis lesions (40). Collectively, these results show promise for the use of MMP-8 as a biomarker in the active phase of peri-implant disease. Longitudinal studies are required to evaluate MMP-8, either alone or in conjunction with other molecular biomarkers, to predict the risk of future disease occurrence and to monitor treatment interventions.
Gelatinase (MMP-9), another member of the collagenase family, is produced by neutrophils and degrades collagen intercellular ground substance. In a longitudinal study patients were asked to rinse and expectorate, providing subject-based instead of site-based gingival crevicular fluid samples (78). When analyzed, a twofold increase in mean MMP-9 levels was reported in patients with progressive attachment loss. Given these results, future use of MMP-9 in oral fluid diagnostics may serve as a guide in periodontal treatment monitoring.
Collagenase-3, referred to as MMP-13, is another collagenolytic MMP with an exceptionally wide substrate specificity. MMP-13 has also been implicated in peri-implantitis. It was concluded that elevated levels of both MMP-13 and MMP-8 correlated with irreversible perio-implant vertical bone loss around loosening dental implants (48). In the future, MMP-13 may be useful for diagnosing and monitoring the course of periodontal disease as well as for tracking the efficacy of therapy (29).
Given the specificity and sensitivity for bone resorption, pyridinoline cross-links, such as pyridinoline cross-linked carboxyterminal telopeptide of type I collagen represent a potentially valuable diagnostic aid for periodontal disease. Several investigations have explored the ability of pyridinoline cross-links to detect bone resorption in periodontitis and peri-implantitis, as well as in response to periodontal therapy (20, 59, 76). In brief, these studies assessing the role of gingival crevicular fluid carboxyterminal telopeptide of type I collagen levels as a diagnostic marker of periodontal disease activity have produced promising results to date. Carboxyterminal telopeptide of type I collagen has been shown to be a promising predictor of both future alveolar bone and attachment loss. Furthermore, the levels of carboxyterminal telopeptide of type I collagen were strongly correlated with clinical parameters and putative periodontal pathogens, and demonstrated significant reductions after periodontal therapy. Controlled human longitudinal trials are needed to establish fully the role of salivary carboxyterminal telopeptide of type I collagen as a predictor of periodontal tissue destruction, disease activity and response to therapy in periodontal patients.
Elevated serum osteocalcin levels have been found during periods of rapid bone turnover, such as in osteoporosis and multiple myeloma and during fracture repair. Therefore, studies have investigated the relationship between gingival crevicular fluid osteocalcin levels and periodontal disease. When a combination of the biochemical markers osteocalcin, collagenase, prostaglandin E2, alpha-2 macroglobulin, elastase and alkaline phosphatase was evaluated, increased diagnostic sensitivity and specificity values of 80 and 91%, respectively, were reported (55).
Osteopontin is a single-chain polypeptide with a molecular weight of approximately 32,600. In bone matrix, osteopontin is highly concentrated at sites where osteoclasts are attached to the underlying mineral surface (i.e. the clear zone attachment areas of the plasma membrane). Results from periodontal studies indicated that osteopontin concentrations in gingival crevicular fluid increased proportionally with the progression of disease; and when nonsurgical periodontal treatment was provided, the osteopontin levels in gingival crevicular fluid were significantly reduced. Although additional long-term prospective studies are needed, at this point osteopontin appears to hold promise as a possible salivary biomarker of periodontal disease progression (70).
Emerging salivary diagnostic tools for periodontal disease detection and therapeutic efficacy monitoring
There are compelling reasons to use saliva as diagnostic fluid to monitor the onset and progression of periodontal diseases. In the past 5 years, through a series of initiatives by the National Institute of Dental and Craniofacial Research, the use of saliva for translational and clinical application has emerged at the forefront. Most relevant to periodontal diseases are the emerging toolboxes of the salivary proteome and the salivary transcriptome for early detection, disease progression and therapeutic monitoring. Using these emerging technologies, we have shown that salivary proteins and RNAs can be used to detect oral cancer (36, 44) and Sjögren’s syndrome (34). The stage is now poised to use these technologies for translational and clinical applications in periodontal diseases.
Salivary proteome: human salivary proteome project
Human salivary proteome analysis is important for understanding oral health and disease pathogenesis. Three research groups were funded by the National Institute of Dental and Craniofacial Research / National Institutes of Health to decipher comprehensively the human salivary proteome. Significant progress has been made in cataloguing human saliva proteins and exploring their post-translational modifications. By using both two-dimensional gel electrophoresis / mass spectrometry and ‘shotgun’ proteomics approaches, we identified 309 distinct proteins in human whole saliva (33). In addition, after 3 years of collective identification and cataloguing of the salivary proteome by the three National Institute of Dental and Craniofacial Research-support salivary proteome projects, the first complete profile of the salivary secretory proteome has been completed (16). Collectively, 1,166 salivary proteins have been identified: 914 from the parotid fluid and 917 from the combined submandibular and sublingual fluids (16). The University of California at Los Angeles is the data-centralization site, harboring the entire database of the human salivary proteome known as the ‘Salivary Proteome Knowledge Base’ (http://www.skb.ucla.edu). Fig. 3 is a genome-wide view of the distribution of the parotid and combined submandibular and sublingual fluid proteomes.
Fig. 3.
Genome-wide view of the distribution of the salivary proteome (parotid and submandibular / sublingual). Note there is no salivary protein expressed by the Y chromosome.
Salivary transcriptome
We have found that RNA molecules elevated in oral cancer tissues are also elevated in saliva, which prompted us to examine the scope and complexity of RNA present in human saliva (42, 44). High-density oligonucleotide microarrays (Affymetrix HG U133A) were used to profile salivary mRNA and revealed that there are ~3,000 human mRNAs in the cell-free saliva supernatant of healthy subjects. Of particular interest is that there is a normal saliva transcriptome core signature of 185 mRNAs that is present in all normal subjects, providing the rationale to use the salivary transcriptome for disease detection.
The presence of human mRNA in saliva may seem surprising. However, we have shown that endogenous mRNA is protected from immediate degradation in a similar manner as cell-free RNA in plasma (56, 61). The utilization of cell-free RNA has been widely accepted. Two recent studies demonstrated that fetal cell-free RNA crossed the placenta and was detected in maternal serum. The first study involved the noninvasive determination of fetal aneuploidies from maternal plasma (46). The second study was a gene-expression microarray investigation of maternal and fetal whole blood that identified fetal mRNA markers in maternal blood that are independent of gender or polymorphism (52).
Other research groups, particularly from forensic sciences, are focusing on multiplex mRNA profiling for the identification of body fluids, including saliva (6, 57). Most recently a Dutch forensic group was able to perform a gene-expression profile on saliva stains from crime scenes, leading to the identification of five saliva RNA markers (SPRR3, SPRR1A, KRT4, KRT6A and KRT13), stable for up to 180 days, which can be used for the identification of blood and saliva stains in forensic practice (81). Of importance is that these five saliva RNA markers selected for forensic applications are all in the normal saliva transcriptome core of 185 mRNAs, substantiating and independently validating our data. Lastly, Shaw and co-workers have identified a biomarker, amylase, which is highly correlated with sleep drive. Importantly, both salivary amylase activity and mRNA levels are also responsive to extended waking in humans (69). These studies provide firm support for our work by showing that mRNA can be extracted from human saliva and used to develop standard tests.
We have recently advanced the ability to harness the salivary transcriptome to the exon level, increasing the diagnostic resolution of the salivary transcriptome by about sevenfold, from 185 to 851 diagnostic units (37). This enhanced capability increased the clinical utility of this diagnostic alphabet as it provides increased resolution to stratify the patient population, therapeutic responsiveness and disease recurrences.
Using the salivary proteome and transcriptome as diagnostic toolboxes, we have recently discovered highly clinical discriminatory panels of salivary proteins and mRNAs for detecting oral cancer (35, 36, 43, 44) and Sjögren’s syndrome (34). It is envisioned that this proteome-wide saliva tool can be used to identify markers for early detection, disease progression and therapy monitoring of patients with periodontal disease.
Clinical applications
Rapid point-of-care diagnostics for periodontal disease
Periodontal surveillance and disease diagnosis will greatly advance over the coming years via the use of rapid point-of-care oral diagnostics. The drug-discovery process has been an excellent catalyst to link together novel therapeutics to emerging diagnostic disease biomarkers. This connection in biomedicine has led to the development of prototype rapid, accurate and ‘real-time’ assessment of multiple diseases, including periodontal disease. Novel technologies such as ‘lab-on-a-chip’ and microfluidic devices have the potential to manage complex oral fluids such as saliva and gingival crevicular fluid, and to provide a determination of a patient’s periodontal disease-risk profile, current disease ‘activity’ and response to therapeutic interventions. This approach should accelerate clinical decision-making and monitoring of episodic disease progression in a chronic infectious disease such as periodontitis (21).
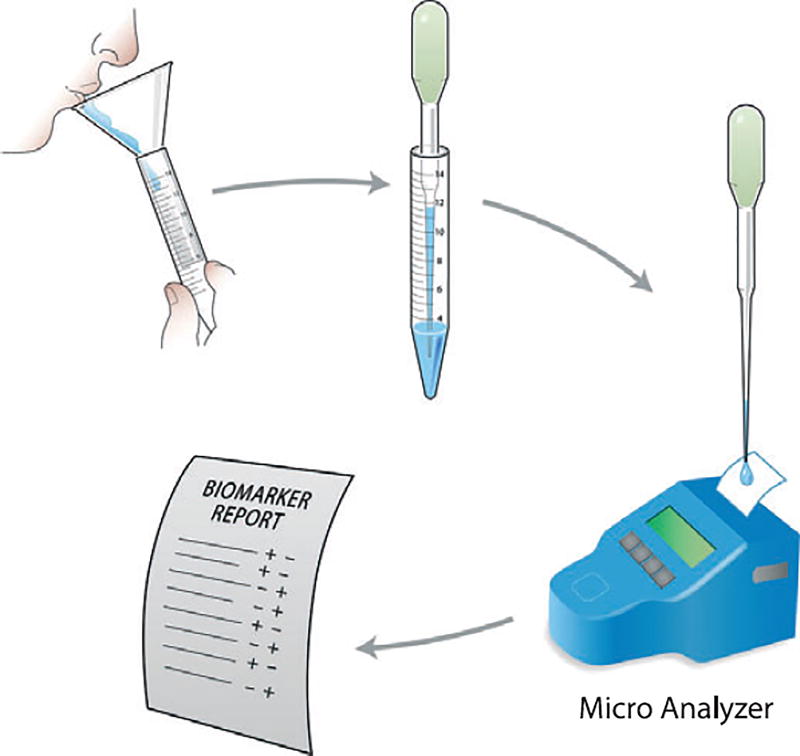
The use of optimized point-of-care devices for periodontal surveillance will probably require less training and fewer resources than current diagnostic tests, could lead to better utilization of skilled clinicians for simpler and less intensive treatment and may result in a more cost-effective healthcare delivery (Fig. 4). The portable, easy-to-use diagnostic tools will allow patients to be screened for periodontal disease in settings other than the dental practice, such as at a physician’s office or in the home, allowing patients to be screened for early disease detection and directed for possible treatment. Periodontal oral diagnostic devices will also enable screening of large populations (especially the underserved communities and resource-poor areas) around the globe more quickly and effectively than the current inefficient screening approaches. The potential to sample various populations will help to identify at-risk groups more effectively and increase access to treatment for those most at need, improving public health (80).
Fig. 4.
Strategy for oral fluid sampling and analysis with a rapid point-of-care or lab-on-a-chip device for the generation of a periodontal disease biomarker report. Reprinted with permission (62).
While the future of periodontal disease diagnosis using salivary diagnostics looks promising, obstacles to these approaches may appear in the clinical setting. Validation of novel periodontal diagnostics will need to be benchmarked with existing ‘gold standards’ of disease, such as alveolar bone levels and clinical attachment levels, in large patient populations. Acceptance by dentists and treatment clinicians is also necessary and may prove difficult. The dental community is not familiar with mass screening of populations for oral and systemic diseases. If more efficient periodontal therapy can be delivered, clinicians will be more likely to utilize new diagnostic approaches. Procedural reimbursement is also another issue that needs to be addressed. A greater emphasis must be placed on clinician education in diagnostics, disease-risk and disease prevention through the public health sector before diagnostics will be integrated into routine clinical periodontal practice (75).
Although challenges remain ahead, the use of saliva-based oral fluid diagnostics appear promising for future application to diagnose periodontal diseases and to prognosticate periodontal treatment outcomes.
Acknowledgments
This work was supported by NIH / NIDCR grant U01-DE14961 and by NCRR grant M01-RR000042 to WG; U01-DE16275; U01-DE17790 and R01-DE17170 to DTW.
References
- 1.Airila-Mansson S, Soder B, Kari K, Meurman JH. Influence of combinations of bacteria on the levels of prostaglandin E2, interleukin-1beta, and granulocyte elastase in gingival crevicular fluid and on the severity of periodontal disease. J Periodontol. 2006;77:1025–1031. doi: 10.1902/jop.2006.050208. [DOI] [PubMed] [Google Scholar]
- 2.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Armitage GC. The complete periodontal examination. Periodontol 2000. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. [DOI] [PubMed] [Google Scholar]
- 5.Azuma M. Fundamental mechanisms of host immune responses to infection. J Periodontal Res. 2006;41:361–373. doi: 10.1111/j.1600-0765.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne J. Validity of messenger RNA expression analyses of human saliva. Clin Cancer Res. 2007;13(1350) doi: 10.1158/1078-0432.CCR-06-2796. author reply 1351. [DOI] [PubMed] [Google Scholar]
- 7.Beikler T, Flemmig TF. Implants in the medically compromised patient. Crit Rev Oral Biol Med. 2003;14:305–316. doi: 10.1177/154411130301400407. [DOI] [PubMed] [Google Scholar]
- 8.Beikler T, Flemmig TF. Implant versus tooth retention from the periodontal perspective. Perio. 2006;3:167–176. [Google Scholar]
- 9.Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32(Suppl. 6):87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 10.Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29(Suppl. 3):197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 11.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64(5 Suppl):474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 12.Bonassi S, Neri M, Puntoni R. Validation of biomarkers as early predictors of disease. Mutat Res. 2001;481:349–358. doi: 10.1016/s0027-5107(01)00194-4. [DOI] [PubMed] [Google Scholar]
- 13.Burt B. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 14.Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, Ali MF, Bernard B, Romanovicz D, Anslyn E, Fox PC, McDevitt JT. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005;5:261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- 15.D’Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004;39:236–241. doi: 10.1111/j.1600-0765.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- 16.Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, Bassilian S, Bedi GS, Boontheung P, Cociorva D, Delahunty CM, Denny T, Dunsmore J, Faull KF, Gilligan J, Gonzalez-Begne M, Halgand F, Hall SC, Han X, Henson B, Hewel J, Hu S, Jeffrey S, Jiang J, Loo JA, Ogorzalek Loo RR, Malamud D, Melvin JE, Miroshnychenko O, Navazesh M, Niles R, Park SK, Prakobphol A, Ramachandran P, Richert M, Robinson S, Sondej M, Souda P, Sullivan MA, Takashima J, Than S, Wang J, Whitelegge JP, Witkowska HE, Wolinsky L, Xie Y, Xu T, Yu W, Ytterberg J, Wong DT, Yates JR, III, Fisher SJ. The proteomes of human parotid and submandibular / sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7:1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006;33:929–935. doi: 10.1111/j.1600-051X.2006.01001.x. [DOI] [PubMed] [Google Scholar]
- 18.Fransson C, Lekholm U, Jemt T, Berglundh T. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005;16:440–446. doi: 10.1111/j.1600-0501.2005.01137.x. [DOI] [PubMed] [Google Scholar]
- 19.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 20.Giannobile WV. C-telopeptide pyridinoline cross-links. Sensitive indicators of periodontal tissue destruction. Ann N Y Acad Sci. 1999;878:404–412. doi: 10.1111/j.1749-6632.1999.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannobile WV. Periodontal surveillance – implications in the promotion of public health. J Periodontol. 2007;78:1177. doi: 10.1902/jop.2007.077001. [DOI] [PubMed] [Google Scholar]
- 22.Goodson JM. Diagnosis of periodontitis by physical measurement: interpretation from episodic disease hypothesis. J Periodontol. 1992;63(4 Suppl):373–382. doi: 10.1902/jop.1992.63.4s.373. [DOI] [PubMed] [Google Scholar]
- 23.Grbic JT, Lamster IB, Celenti RS, Fine JB. Risk indicators for future clinical attachment loss in adult periodontitis. Patient variables. J Periodontol. 1991;62:322–329. doi: 10.1902/jop.1991.62.5.322. [DOI] [PubMed] [Google Scholar]
- 24.Groenink J, Ligtenberg AJ, Veerman EC, Bolscher JG, Nieuw Amerongen AV. Interaction of the salivary low-molecular-weight mucin (MG2) with Actinobacillus actinomycetemcomitans. Antonie Van Leeuwenhoek. 1996;70:79–87. doi: 10.1007/BF00393572. [DOI] [PubMed] [Google Scholar]
- 25.Groenink J, Walgreen-Weterings E, Nazmi K, Bolscher JG, Veerman EC, van Winkelhoff AJ, Nieuw Amerongen AV. Salivary lactoferrin and low-Mr mucin MG2 in Actinobacillus actinomycetemcomitans-associated periodontitis. J Clin Periodontol. 1999;26:269–275. doi: 10.1034/j.1600-051x.1999.260501.x. [DOI] [PubMed] [Google Scholar]
- 26.Gusman H, Travis J, Helmerhorst EJ, Potempa J, Troxler RF, Oppenheim FG. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect Immun. 2001;69:1402–1408. doi: 10.1128/IAI.69.3.1402-1408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guven Y, Satman I, Dinccag N, Alptekin S. Salivary peroxidase activity in whole saliva of patients with insulin-dependent (type-1) diabetes mellitus. J Clin Periodontol. 1996;23:879–881. doi: 10.1111/j.1600-051x.1996.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 28.Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J Dent Res. 2007;86:680–693. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez M, Valenzuela MA, Lopez-Otin C, Alvarez J, Lopez JM, Vernal R, Gamonal J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J Periodontol. 2006;77:863–870. doi: 10.1902/jop.2006.050461. [DOI] [PubMed] [Google Scholar]
- 30.Herr AE, Hatch AV, Throckmorton DJ, Tran HM, Brennan JS, Giannobile WV, Singh AK. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci U S A. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higashi MK, Veenstra DL, del Aguila M, Hujoel P. The cost-effectiveness of interleukin-1 genetic testing for periodontal disease. J Periodontol. 2002;73:1474–1484. doi: 10.1902/jop.2002.73.12.1474. [DOI] [PubMed] [Google Scholar]
- 32.Houri-Haddad Y, Wilensky A, Shapira L. T-cell phenotype as a risk factor for periodontal disease. Periodontol 2000. 2007;45:67–75. doi: 10.1111/j.1600-0757.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 33.Hu S, Xie Y, Ramachandran P, Ogorzalek Loo RR, Li Y, Loo JA, Wong DT. Large-scale identification of proteins in human salivary proteome by liquid chromatography / mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. 2005;5:1714–1728. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- 34.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, Zhou H, Henry S, Vissink A, Pijpe J, Kallenberg C, Elashoff D, Loo JA, Wong DT. Salivary proteomic and genomic biomarkers for primary Sjogren’s syndrome. Arthritis Rheum. 2007;56:3588–3600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu S, Yu T, Xie Y, Yang Y, Li Y, Zhou X, Tsung S, Loo RR, Loo JR, Wong DT. Discovery of oral fluid biomarkers for human oral cancer by mass spectrometry. Cancer Genomics Proteomics. 2007;4:55–64. [PubMed] [Google Scholar]
- 36.Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, Elashoff D, Wei R, Loo JA, Wong DT. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 2008;14:6246–6252. doi: 10.1158/1078-0432.CCR-07-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Z, Zimmermann BG, Zhou H, Wang J, Henson BS, Yu W, Elashoff D, Krupp G, Wong DT. Exon-level expression profiling: a comprehensive transcriptome analysis for oral fluids. Clin Chem. 2008;54:824–832. doi: 10.1373/clinchem.2007.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jalil RA, Ashley FP, Wilson RF. The relationship between 48-h dental plaque accumulation in young human adults and the concentrations of hypothiocyanite, ‘free’ and ‘total’ lysozyme, lactoferrin and secretory immunoglobulin A in saliva. Arch Oral Biol. 1992;37:23–28. doi: 10.1016/0003-9969(92)90148-2. [DOI] [PubMed] [Google Scholar]
- 39.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–251. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kivela-Rajamaki M, Maisi P, Srinivas R, Tervahartiala T, Teronen O, Husa V, Salo T, Sorsa T. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J Periodontal Res. 2003;38:583–590. doi: 10.1034/j.1600-0765.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- 41.Kwok V, Caton JG. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol. 2007;78:2063–2071. doi: 10.1902/jop.2007.070210. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zhou X, St John MA, Wong DT. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83:199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Elashoff D, Oh M, Sinha U, St John MA, Zhou X, Abemayor E, Wong DT. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol. 2006;24:1754–1760. doi: 10.1200/JCO.2005.03.7598. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC, Eisele D, Abemayor E, Elashoff D, Park NH, Wong DT. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 45.Listgarten MA. Structure of surface coatings on teeth. A review. J Periodontol. 1976;47:139–147. doi: 10.1902/jop.1976.47.3.139. [DOI] [PubMed] [Google Scholar]
- 46.Lo YM, Tsui NB, Chiu RW, Lau TK, Leung TN, Heung MM, Gerovassili A, Jin Y, Nicolaides KH, Cantor CR, Ding C. Plasma placental RNA allelic ratio permits noninvasive prenatal chromosomal aneuploidy detection. Nat Med. 2007;13:218–223. doi: 10.1038/nm1530. [DOI] [PubMed] [Google Scholar]
- 47.Loos BG, Tjoa S. Host-derived diagnostic markers for periodontitis: do they exist in gingival crevice fluid? Periodontol 2000. 2005;39:53–72. doi: 10.1111/j.1600-0757.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Kitti U, Teronen O, Sorsa T, Husa V, Laine P, Ronka H, Salo T, Lindqvist C, Konttinen YT. Collagenases in different categories of peri-implant vertical bone loss. J Dent Res. 2000;79:1870–1873. doi: 10.1177/00220345000790110901. [DOI] [PubMed] [Google Scholar]
- 49.Malamud D. Salivary diagnostics: the future is now. J Am Dent Assoc. 2006;137:284–286. doi: 10.14219/jada.archive.2006.0158. [DOI] [PubMed] [Google Scholar]
- 50.Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 51.Mandel ID. Markers of periodontal disease susceptibility and activity derived from saliva. In: Johnson NW, editor. Periodontal diseases – markers of disease susceptibility and activity. Cambridge, New York: Cambridge University Press; 1991. pp. 228–253. [Google Scholar]
- 52.Maron JL, Bianchi DW. Prenatal diagnosis using cell-free nucleic acids in maternal body fluids: a decade of progress. Am J Med Genet C Semin Med Genet. 2007;145:5–17. doi: 10.1002/ajmg.c.30115. [DOI] [PubMed] [Google Scholar]
- 53.Millenium Research Group. Global markets for dental implants 2007. Millenium Research Group; Toronto, Canada: 2008. [Google Scholar]
- 54.Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 55.Nakashima K, Giannopoulou C, Andersen E, Roehrich N, Brochut P, Dubrez B, Cimasoni G. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23:832–838. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 56.Ng EK, Tsui NB, Lau TK, Leung TN, Chiu RW, Panesar NS, Lit LC, Chan KW, Lo YM. mRNA of placental origin is readily detectable in maternal plasma. Proc Natl Acad Sci USA. 2003;100:4748–4753. doi: 10.1073/pnas.0637450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nussbaumer C, Gharehbaghi-Schnell E, Korschineck I. Messenger RNA profiling: a novel method for body fluid identification by Real-Time PCR. Forensic Sci Int. 2006;157:181–186. doi: 10.1016/j.forsciint.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 59.Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, Giannobile WV. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin Oral Implants Res. 1998;9:365–373. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 61.Park NJ, Li Y, Yu T, Brinkman BM, Wong DT. Characterization of RNA in saliva. Clin Chem. 2006;52:988–994. doi: 10.1373/clinchem.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramseier CA, Morelli T, Kinney JS, Dubois M, Rayburn L, Giannobile WB. Periodontal disease. In: Wong DT, editor. Salivary diagnostics. Ames, Iowa, USA: Wiley-Blackwell; 2008. [Google Scholar]
- 63.Reiff RL. Serum and salivary IgG and IgA response to initial preparation therapy. J Periodontol. 1984;55:299–305. doi: 10.1902/jop.1984.55.5.299. [DOI] [PubMed] [Google Scholar]
- 64.Roos-Jansaker AM. Long time follow up of implant therapy and treatment of peri-implantitis. Swed Dent J Suppl. 2007;188:7–66. [PubMed] [Google Scholar]
- 65.Roos-Jansaker AM, Renvert S, Egelberg J. Treatment of peri-implant infections: a literature review. J Clin Periodontol. 2003;30:467–485. doi: 10.1034/j.1600-051x.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 66.Roos-Jansaker AM, Lindahl C, Renvert H, Renvert S. Nine-to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33:290–295. doi: 10.1111/j.1600-051X.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 67.Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann N Y Acad Sci. 2007;1098:496–497. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seemann R, Hagewald SJ, Sztankay V, Drews J, Bizhang M, Kage A. Levels of parotid and submandibular / sublingual salivary immunoglobulin A in response to experimental gingivitis in humans. Clin Oral Investig. 2004;8:233–237. doi: 10.1007/s00784-004-0280-5. [DOI] [PubMed] [Google Scholar]
- 69.Seugnet L, Boero J, Gottschalk L, Duntley SP, Shaw PJ. Identification of a biomarker for sleep drive in flies and humans. Proc Natl Acad Sci USA. 2006;103:19913–19918. doi: 10.1073/pnas.0609463104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma CG, Pradeep AR. Gingival crevicular fluid osteopontin levels in periodontal health and disease. J Periodontol. 2006;77:1674–1680. doi: 10.1902/jop.2006.060016. [DOI] [PubMed] [Google Scholar]
- 71.Snyderman R, Williams RS. Prospective medicine: the next health care transformation. Acad Med. 2003;78:1079–1084. doi: 10.1097/00001888-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Snyderman R, Langheier J. Prospective health care: the second transformation of medicine. Genome Biol. 2006;7:104. doi: 10.1186/gb-2006-7-2-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snyderman R, Yoediono Z. Prospective care: a personalized, preventative approach to medicine. Pharmacogenomics. 2006;7:5–9. doi: 10.2217/14622416.7.1.5. [DOI] [PubMed] [Google Scholar]
- 74.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–571. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tabak LA. Point-of-care diagnostics enter the mouth. Ann N Y Acad Sci. 2007;1098:7–14. doi: 10.1196/annals.1384.043. [DOI] [PubMed] [Google Scholar]
- 76.Talonpoika JT, Hamalainen MM. Type I collagen carboxy-terminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. J Clin Periodontol. 1994;21:320–326. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 77.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76(11 Suppl):2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 78.Teng YT, Sodek J, McCulloch CA. Gingival crevicular fluid gelatinase and its relationship to periodontal disease in human subjects. J Periodontal Res. 1992;27:544–552. doi: 10.1111/j.1600-0765.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 79.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 80.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 81.Zubakov D, Hanekamp E, Kokshoorn M, van Ijcken W, Kayser M. Stable RNA markers for identification of blood and saliva stains revealed from whole genome expression analysis of time-wise degraded samples. Int J Legal Med. 2008;122:135–142. doi: 10.1007/s00414-007-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]