Acute myeloid leukemia (AML) is a markedly heterogeneous hematological malignancy with poor prognosis.1 Core-binding factor AML is cytogenetically defined by the presence of t(8;21)(q22;q22) or inv(16)(p13q22)/t(16;16)(p13;q22), commonly abbreviated as t(8;21) and inv(16), respectively.2–3 In both subtypes, the cytogenetic rearrangements disrupt genes that encode subunits of core-binding factor, a transcription factor that functions as an essential regulator of normal hematopoiesis.2–3 The t(8;21) translocation, which generates the AML1-ETO fusion gene, is one of the most common chromosomal abnormalities detected in AML.4 The AML1-ETO fusion transcription factor is present in approximately 4%–12% of adult and 12%–30% of pediatric acute myeloid leukemia (AML) patients.4 AML1-ETO+ AML remains a significant clinical problem, with 30% of patients relapsing and long-term survival rates ranging between 30 and 60%, indicating the need for improved therapeutic approaches.2–4
The phosphatase of regenerating liver (PRL) family of phosphatases, consisting of PRL1, PRL2, and PRL3, represents an intriguing group of proteins being validated as biomarkers and therapeutic targets in human cancer.5 Notably, recent findings indicate that PRLs may play important roles in the pathogenesis of hematological malignancies.6 Both PRL2 and PRL3 are highly expressed in some hematological malignancies, including acute myeloid leukemia (AML), chronic myeloid leukemia (CML), multiple myeloma (MM) and acute lymphoblastic leukemia (ALL).6 High PRL3 mRNA expression is associated with FLT3-ITD mutations and poor prognosis in AML patients with normal karyotype.7 We have identified PRL2 to be important for the proliferation and self-renewal of hematopoietic stem cells (HSCs) through the regulation of KIT signaling.8 Recently, we found that PRL2 mediates NOTCH and KIT signals in early T cell progenitors and that PRL2 is essential for oncogenic NOTCH1-induced T cell leukemia in vivo.9–10 An improved understanding of how PRLs function and how they are regulated may establish PRLs as novel therapeutic targets in acute myeloid leukemia.
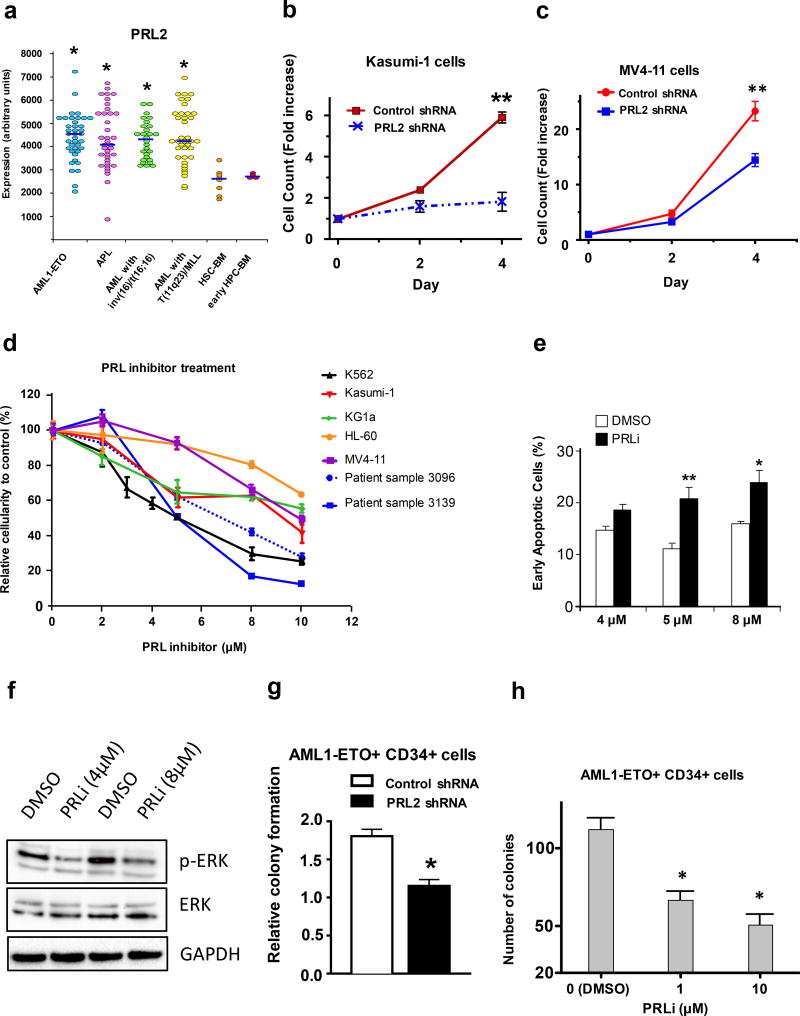
PRL2 is highly expressed in some subtypes of AML, including AML1-ETO+ AML and AML with mixed lineage leukemia (MLL) translocations (Figure 1a). The PRL2 expression profiling data were obtained from the HemaExplorer, a Web server for easy and fast visualization of gene expression in normal and malignant hematopoiesis.11 We observed that the levels of PRL2 protein were elevated in several human AML cell lines compared to cord blood and peripheral blood mononuclear cells from healthy donors (Supplementary Figure S1a). However, the role of PRL2 in the proliferation and survival of human AML cells is largely unknown. Kasumi-1 is a human AML cell line with AML1-ETO and MV4-11 is a human B-myelomonocytic leukemia cell line with MLL translocation. We found that knockdown of PRL2 decreased the proliferation of both Kasumi-1 and MV4-11 cells (Figures 1b, 1c and S1b). We also found that ectopic expression of a PRL2 dominant-negative mutant PRL2/C101S-D69A (PRL2/CS-DA) reduced the proliferation of Kasumi-1 cells (Figure S1c).
Figure 1.
PRL2 promotes the proliferation and survival of human AML cells. (a) PRL2 is highly expressed in some subtypes of human AML compared to normal human bone marrow HSPCs (*P<0.05). (b) Kasumi-1 cells were transduced with lentiviruses expressing control or PRL2 shRNA. The proliferation of transduced cells (GFP+) was measured over time (**p<0.01, n=3). (c) MV4-11 cells were transduced with lentiviruses expressing control or PRL2 shRNA. The proliferation of transduced cells (GFP+) was measured over time (**p<0.01, n=3). (d) Inhibiting of PRL2 activity with a small molecule PRL inhibitor (PRLi) decreases the viability of human AML cell lines and primary human AML cells in a dosage-dependent manner. Patient sample 3096 is from an AML patient positive for FLT3ITD and FLT3TKD mutations. Patient sample 3139 is from an AML patients negative for FLT3ITD and NPM mutations. (e) Human K562 cells were treated with PRL inhibitor (PRLi) for 24 hours and apoptosis was determined by Annexin V and DAPI staining (*p<0.05, **p<0.01, n = 3). (f) Immunoblot analysis of ERK phosphorylation in human K562 cells following DMSO or a small molecule inhibitor (PRLi) treatment. Representative Western blot analysis of indicated proteins is shown. (g) Human cord blood CD34+ cells expressing AML1-ETO were transduced with lentiviruses expressing control or PRL2 shRNA. Myeloid progenitors were quantified by using the methylcellulose culture (*p<0.05, n=3). (h) PRL inhibitor (PRLi) treatment decreases the colony formation of human cord blood CD34+ cells expressing AML1-ETO in a dosage dependent manner (*p<0.05, n = 3).
Recently, we identified a small molecule PRL inhibitor (PRLi) using computer-based virtual screening.12 Intraperitoneally injection of 15 mg/kg of PRLi into wild type mice daily for 3 weeks exhibited no toxicity and body weight and weights of major organs (liver, spleen, and kidney) were comparable to DMSO treated mice.12 PRLi did not affect the viability of human cord blood mononuclear cells and CD34+ cells.10 Further, we found that pharmacological inhibition of PRL2 function decreased the proliferation and survival of human T-ALL cells in a dosage-dependent manner.10 To determine the role of PRL2 in the proliferation and survival of human AML cells, we treated several human AML cell lines with PRL inhibitor (PRLi) and monitored cell proliferation and survival. We found that PRLi treatment of PRL2-expressing human AML cell lines resulted in decreased proliferation (Figure 1d). Furthermore, we found that primary human AML cells (FLT3-ITD+ and FLT3-ITD−) were sensitive to PRL2 inhibitor treatment in a dose-dependent manner (Figure 1d). Inhibition of PRL2 activity with PRLi resulted in apoptotic cell death of K562 cells that highly expresses PRL2 (Figure 1e). Importantly, we found that PRLi treatment significantly decreased ERK phosphorylation in human AML cells (Figure 1f), suggesting that PRL2 is a key mediator of signaling pathways in human leukemia cells.
Human leukemia-initiating cells (LICs) are enriched in the CD34+ population of leukemia blasts. Human CD34+ cells expressing AML1-ETO have nearly unlimited potential for multilineage cell generation, and represent a valuable tool for studying AML1-ETO-positive AML.13 We found that knockdown of PRL2 decreased the proliferation and colony formation of CD34+ cells expressing AML1-ETO (Figures 1g and S1d). In addition, ectopic expression of a PRL2 dominant-negative mutant PRL2/CS-DA, but not the wild type PRL2, reduced colony formation of human CD34+ cells expressing AML1-ETO (Figure S1e). While PRLi treatment does not affect the colony formation of human cord blood CD34+ cells,10 blocking PRL2 function with PRLi decreased the colony formation of human CD34+ cells expressing AML1-ETO (Figure 1h). These data demonstrate that PRL2 is important for the proliferation and survival of human AML cells bearing AML1-ETO.
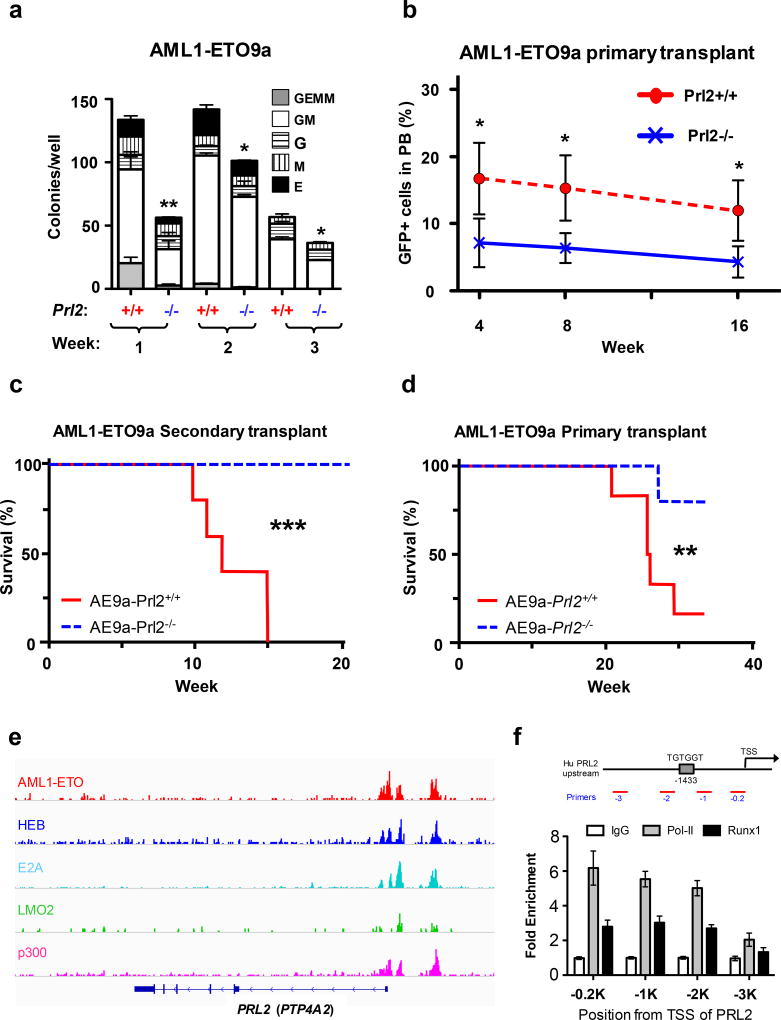
Both human and mouse models of AML have demonstrated that AML1-ETO is insufficient for leukemogenesis in the absence of secondary events.13–14 However, a truncated form of the AML1-ETO fusion protein, AML1-ETO9a, is sufficient to cause leukemia in mice.15 To determine the role of PRL2 in AML1-ETO-induced leukemia in vivo, we introduced AML1-ETO9a (AE9a) into Lin− cells isolated from WT and Prl2 null mice using a retrovirus carrying cDNA that encodes the leukemia associated oncogene AML1-ETO9a (MSCV-AML1-ETO9a-IRES-GFP). We found that loss of PRL2 decreased the proliferation of hematopoietic progenitor cells expressing AML1-ETO9a in vitro (Figures S2a). We also found that loss of PRL2 decreased the replating potential of hematopoietic progenitor cells expressing AML1-ETO9a in vitro (Figures 2a and S2b), suggesting that PRL2 may be essential for LIC maintenance.
Figure 2.
PRL2 promotes AML1-ETO-induced leukemia in vivo. (a) Loss of PRL2 decreases the replating potential of AML1-ETO9a+ progenitor cells. Myeloid progenitors were quantified by methylcellulose culture using wild type and PRL2 null Lin− cells transduced with retroviruses expressing AML1-ETO9a. The methylcellulose cultures were serially replated, weekly, for 3 weeks. Data are means ± SD (*p<0.05, **p<0.01, n = 3 independent experiments). (b) Prl2+/+ and Prl2−/− hematopoietic progenitor cells (Lin−) were transduced with retroviruses expressing AML1-ETO9a and equivalent number of transduced cells (GFP+) were injected into lethally irradiated recipient mice. The frequency of donor-derived cells (GFP+) in peripheral blood was determined every 4 weeks for 16 weeks by flow cytometry analysis. Data are means ± SD (*p<0.05, n = 5). (c) Two million GFP+ cells isolated from the bone marrow of primary recipient mice were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing AML1-ETO9a for the period of observation (***p<0.001, n=5). (d) GFP+ fetal liver cells expressing AML1-ETO9a were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing AML1-ETO9a for the period of observation (**p<0.01, n=6). (e) AML1-ETO directly binds to PRL2 (PTP4A2) in Kasumi-1 cells revealed by genome-wide ChIP-seq analysis using an anti-ETO antibody.16 (f) AML1-ETO was associated with the promoter of PRL2 in human Kasumi-1 cells assayed by ChIP experiments using an anti-RUNX1 (AML1) antibody. RNA Pol-II antibody was used a positive control.
We then transduced Lin− cells isolated from WT and Prl2 null mice with a retrovirus expressing AML1-ETO9a and transplanted transduced cells (GFP+) into lethally irradiated recipient mice. Prl2 null HSPCs expressing AML1-ETO9a show decreased repopulating potential in primary transplantation assays (Figure 2b). The frequency of GFP+ cells in the bone marrow of recipient mice repopulated with Prl2 null cells was significantly decreased compared to that of the WT cells 20 weeks following transplantation (Figure S2c), suggesting that PRL2 is important for the repopulating potential of HSPCs expressing AML1-ETO9a. At this point, none of the recipient mouse developed leukemia. To determine the role of PRL2 in leukemogenesis, we transplanted equal number of GFP+ cells isolated from primary recipient mice into lethally irradiated secondary recipient mice and monitored leukemia development. While the frequency of GFP+ cells in the peripheral blood was comparable between wild type and Prl2 null groups 40 days after transplantation (Figure S2d), the development of AML in host that received wild-type cells transduced with AML1-ETO9a retrovirus was rapid, with all control animals succumbing to disease within 15 weeks following transplantation (Figure 2c). In contrast, animals repopulated with Prl2 null cells displayed significant protection from disease, with all animals remaining disease free 20 weeks following transplantation (Figure 2c), indicating that PRL2 is essential for the maintenance of leukemia-induced by AML1-ETO9a.
To determine the role of PRL2 in leukemia initiation and progression, we transduced wild type and Prl2 null fetal liver cells, which are enriched for hematopoietic stem and progenitor cells, with a retrovirus expressing AML1-ETO9a and transplanted infected cells (GFP+) into lethally irradiated recipient mice. Recipient mice repopulated with wild type fetal liver cells expressing AML1-ETO9a developed leukemia within 20 weeks following transplantation (Figure 2d). However, recipient mice repopulated with Prl2 null fetal liver cells expressing AML1-ETO9a show delayed leukemia development and majority of Prl2 null recipients are still alive 40 weeks following transplantation (Figure 2d). Prl2 wild type recipients show splenomegaly and lethargic phenotype compared to Prl2 null recipients (Figures S2e and S2f). These data demonstrate that PRL2 is important for leukemia progression.
PRL2 is highly expressed in AML-ETO positive AML cells (Figure S1a) and we found that ectopic AML1-ETO9a expression in mouse HSPCs increased the levels of both PRL2 mRNA and protein (Figures S3a and S3b). There is a putative AML1(RUNX1) binding site (TGTGGT) in the promoter region of PRL2, which is conserved between mouse and human. We examined the genome-wide occupancy of AML1-ETO in AML1-ETO+ Kasumi-1 cells by performing the ChIP-seq assays using an anti-ETO antibody.16 We found that AML1-ETO is associated with the PRL2 promoter in Kasumi-1 cells (Figure 2e). We also found hematopoietic transcription cofactors, including E2A, LMO2, and p300, are associated with the PRL2 promoter (Figure 2e), indicating that the AML1-ETO-transcription factor complex (AETFC) binds the PRL2 promoter.16 We then examined the occupancy of AML1-ETO on PRL2 promoter regions in Kasumi-1 cells by using an anti-RUNX1 (AML1) antibody in the ChIP assays. Using primer pairs that cover the putative AML1 binding site in the promoter region of PRL2, we found that AML1-ETO was associated with the promoter region of PRL2 in Kasumi-1 cells (Figure 2f). These findings suggest that AML1-ETO may directly activate PRL2 expression in hematopoietic cells.
To date, very little information exists in the literature on the role of PRL2 in human AML.6–8 In this study, we have identified a critical role for PRL2 phosphatase in the proliferation and survival of human AML cells. Further, we demonstrated that PRL2 is essential for the leukemogenic potential of AML1-ETO9a in vivo. Given that some human AML cells are sensitive to PRLi treatment, PRL2 may play a general role in human AML. Our findings suggest that pharmacological inhibition of PRL2 holds potential as a novel therapy for acute myeloid leukemia, and might also be applicable to the treatment of other hematological malignancies.10
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grant CA69202 (ZYZ), Department of Defense Grant W81XWH-13-1-0187 (YL), a St. Baldrick’s Foundation Scholar Award (YL), an Elsa Pardee Foundation New Investigator Award (YL), an Alex’s Lemonade Stand Foundation Grant (YL), a Children’s Leukemia Research Association Grant (YL), a Leukemia Research Foundation grant (YL), and American Cancer Society Institutional Research Grants (YL and MK). This work was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108. We like to thank Marilyn Wales and John Spence for helping the preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declared that no conflict interest exists.
AUTHOR CONTRIBUTIONS
MK, SC, YB, ZYZ, and YL. Designed the research. MK, SC, YB, CY, RG, XJS, CM, TAT, and ZHY. Performed the research; MK, and YL. Analyzed the data and performed the statistical analysis. HSB, MCY, RK, and JCM. Provided reagents to the study. ZYZ and YL. Wrote the manuscript. All authors read, comment on, and approved the manuscript.
References
- 1.Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24:711–9. doi: 10.1097/CCO.0b013e328358f62d. [DOI] [PubMed] [Google Scholar]
- 2.Paschka P. Core binding factor acute myeloid leukemia. Semin Oncol. 2008;35:410–7. doi: 10.1053/j.seminoncol.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Sinha C, Cunningham LC, Liu PP. Core Binding Factor Acute Myeloid Leukemia: New Prognostic Categories and Therapeutic Opportunities. Semin Hematol. 2015;52:215–22. doi: 10.1053/j.seminhematol.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatlen MA, Wang L, Nimer SD. AML1-ETO driven acute leukemia: insights into pathogenesis and potential therapeutic approaches. Front Med. 2012;6:248–62. doi: 10.1007/s11684-012-0206-6. [DOI] [PubMed] [Google Scholar]
- 5.Bessette DC, Qiu D, Pallen CJ. PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev. 2008;27:231–252. doi: 10.1007/s10555-008-9121-3. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Chen S, Gao R, Bai Y, Zhang ZY, Liu Y. Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies. Cell Cycle. 2014;13:2827–35. doi: 10.4161/15384101.2014.954448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Bi C, Chng WJ, Cheong LL, Liu SC, Mahara S, et al. PRL-3, a metastasis associated tyrosine phosphatase, is involved in FLT3-ITD signaling and implicated in anti-AML therapy. PLoS One. 2011;6:e19798. doi: 10.1371/journal.pone.0019798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi M, Bai Y, Dong Y, Yu H, Chen S, Gao R, et al. PRL2/PTP4A2 phosphatase is important for hematopoietic stem cell self-renewal. Stem Cells. 2014;32:1956–67. doi: 10.1002/stem.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M, Nabinger SC, Bai Y, Yoshimoto M, Gao R, Chen S, et al. Protein Tyrosine Phosphatase PRL2 Mediates Notch and Kit Signals in Early T Cell Progenitors. Stem Cells. 2016 Dec 23; doi: 10.1002/stem.2559. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi M, Bai Y, Chen S, Gao R, Yao C, Cai W, et al. Phosphatase PRL2 promotes oncogenic NOTCH1-induced T cell leukemia. Leukemia. 2016 Nov 22; doi: 10.1038/leu.2016.340. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagger FO, Rapin N, Theilgaard-Mönch K, Kaczkowski B, Jendholm J, Winther O, et al. HemaExplorer: a Web server for easy and fast visualization of gene expression in normal and malignant hematopoiesis. Blood. 2012;119:6394–5. doi: 10.1182/blood-2012-05-427310. [DOI] [PubMed] [Google Scholar]
- 12.Bai Y, Yu ZH, Liu S, Zhang L, Zhang RY, Zeng LF, et al. Novel Anticancer Agents Based on Targeting the Trimer Interface of the PRL Phosphatase. Cancer Res. 2016;76:4805–15. doi: 10.1158/0008-5472.CAN-15-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulloy JC, Cammenga J, MacKenzie KL, Berguido FJ, Moore MA, Nimer SD. The AML1-ETO fusion protein promotes the expansion of human hematopoietic stem cells. Blood. 2002;99:15–23. doi: 10.1182/blood.v99.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Zhou L, Miyamoto T, Iwasaki H, Harakawa N, Hetherington CJ, et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci USA. 2001;98:10398–403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan M, Kanbe E, Peterson LF, Boyapati A, Miao Y, Wang Y, et al. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med. 2006;12:945–9. doi: 10.1038/nm1443. [DOI] [PubMed] [Google Scholar]
- 16.Sun XJ, Wang Z, Wang L, Jiang Y, Kost N, Soong TD, et al. A stable transcription factor complex nucleated by oligomeric AML1-ETO controls leukaemogenesis. Nature. 2013;500:93–7. doi: 10.1038/nature12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.