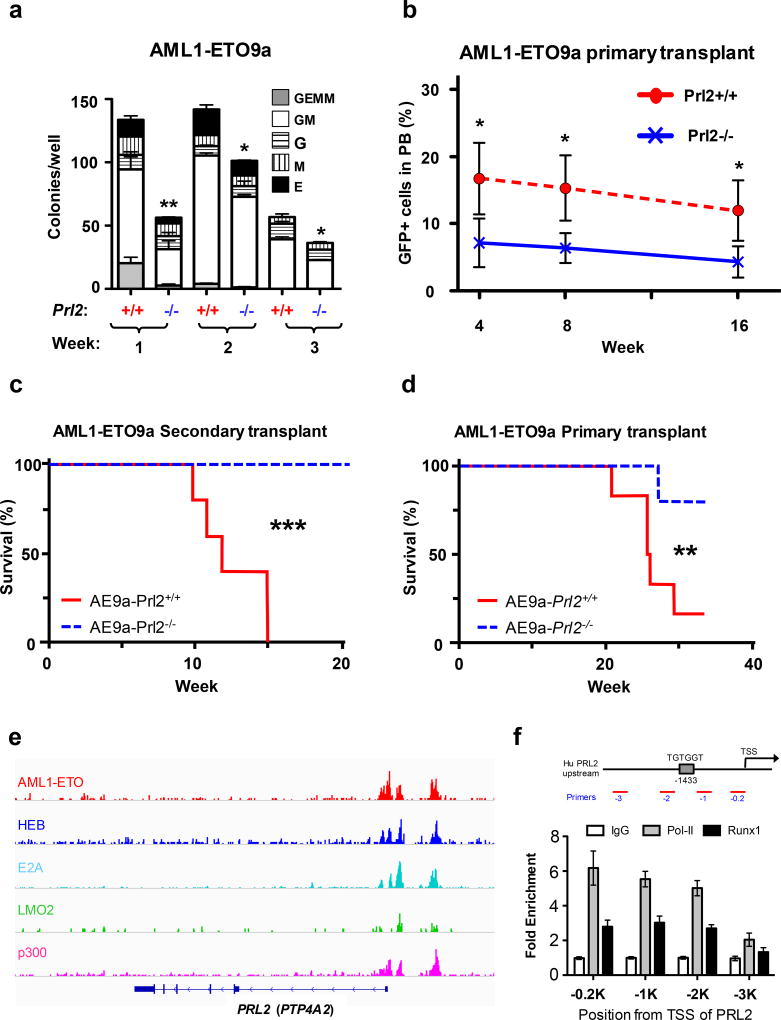
Figure 2.
PRL2 promotes AML1-ETO-induced leukemia in vivo. (a) Loss of PRL2 decreases the replating potential of AML1-ETO9a+ progenitor cells. Myeloid progenitors were quantified by methylcellulose culture using wild type and PRL2 null Lin− cells transduced with retroviruses expressing AML1-ETO9a. The methylcellulose cultures were serially replated, weekly, for 3 weeks. Data are means ± SD (*p<0.05, **p<0.01, n = 3 independent experiments). (b) Prl2+/+ and Prl2−/− hematopoietic progenitor cells (Lin−) were transduced with retroviruses expressing AML1-ETO9a and equivalent number of transduced cells (GFP+) were injected into lethally irradiated recipient mice. The frequency of donor-derived cells (GFP+) in peripheral blood was determined every 4 weeks for 16 weeks by flow cytometry analysis. Data are means ± SD (*p<0.05, n = 5). (c) Two million GFP+ cells isolated from the bone marrow of primary recipient mice were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing AML1-ETO9a for the period of observation (***p<0.001, n=5). (d) GFP+ fetal liver cells expressing AML1-ETO9a were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing AML1-ETO9a for the period of observation (**p<0.01, n=6). (e) AML1-ETO directly binds to PRL2 (PTP4A2) in Kasumi-1 cells revealed by genome-wide ChIP-seq analysis using an anti-ETO antibody.16 (f) AML1-ETO was associated with the promoter of PRL2 in human Kasumi-1 cells assayed by ChIP experiments using an anti-RUNX1 (AML1) antibody. RNA Pol-II antibody was used a positive control.