Acute lymphoblastic leukemia (ALL) is a heterogeneous group of hematologic malignancies that arise from clonal proliferation of immature lymphoid cells in the bone marrow, peripheral blood, and other organs.1 T-cell acute lymphoblastic leukemia (T-ALL) is characterized by outgrowth of immature T-cells.1 Gain-of-function mutations in NOTCH1 are common in T-ALL,2 making this receptor a promising target for drugs such as γ-secretase inhibitors (GSIs), which can block a proteolytic cleavage required for NOTCH1 activation.3 However, the enthusiasm for these therapies has been tempered by tumor resistance and paucity of information on the oncogenic programs regulated by oncogenic NOTCH1.3, 4 Therefore, the purpose of this study was to identify oncogenic NOTCH1-mediated molecular and genetic changes that contribute to T-ALL for future targeted therapies in order to improve leukemia treatment.
The phosphatase of regenerating liver (PRL) family of phosphatases, consisting of PRL1, PRL2, and PRL3, represents an intriguing group of proteins that are currently being validated as potential biomarkers and therapeutic targets in human cancer, including breast cancer, ovarian cancer, and lung cancer.5 Notably, recent findings indicate that PRLs play important roles in the pathogenesis of hematological malignancies.6 For instance, PRL3 is highly expressed in BCR-ABL positive ALL, which may be responsible for the aggressive phenotype of the disease.7 PRL2 is located on human chromosome 1p35, a region often rearranged or amplified in malignant lymphoma and B-cell chronic lymphocytic leukemia (B-CLL).5 PRL2 mRNA is also highly expressed in primary human acute myeloid leukemia (AML) and T-ALL cells, suggesting that PRL2 may play an important role in the pathogenesis of T-ALL.8, 9 We recently generated Prl2 deficient mice and found that PRL2 is required for extra-embryonic development by negatively regulating PTEN, thereby activating the PI3K-Akt pathway.10 In a previous study, we observed that PRL2 enhances hematopoietic stem and progenitor cell (HSPC) proliferation through mediating SCF/KIT signaling.11 These findings suggest that PRLs may represent novel therapeutic targets for the treatment of hematological malignancies.6–11
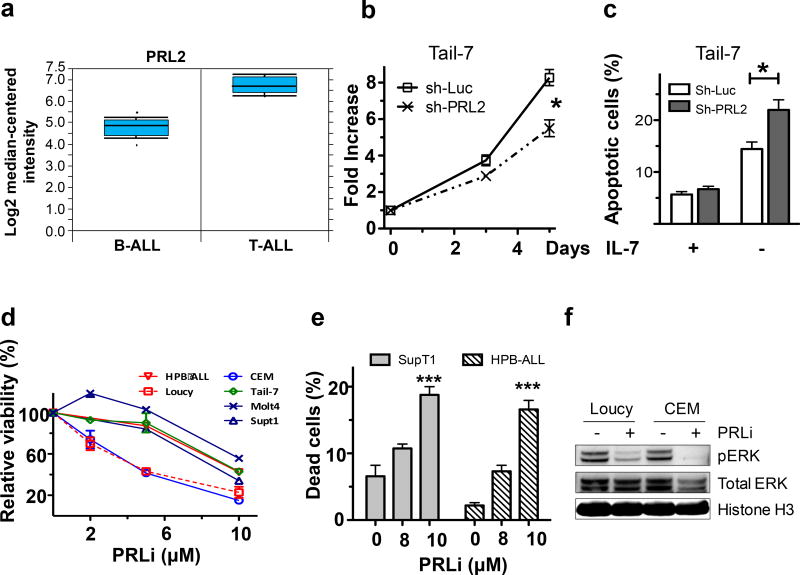
To determine the functional requirement for PRL2 in the pathogenesis of T-ALL, we analyzed PRL2 expression in a gene expression profiling data set reported by Haferlach and colleagues.9 We found that PRL2 is highly expressed in both human B-ALL and T-ALL patient cells compared to normal control cells (Figure 1a). Given that PRL2 is important for the proliferation and survival of hematopoietic stem and progenitor cells,11 we determined the effect of genetic inhibition of PRL2 on human T-ALL cell proliferation by introducing shRNA targeting PRL2 into human T-ALL cells using lentiviruses. We found that knockdown of PRL2 decreased the proliferation and survival of human T-ALL cells (Figures 1b and 1c; Supplementary Figures S1a and S1b). We also found that ectopic expression of a dominant-negative PRL2 mutant (PRL2-CSDA) decreased the proliferation of Molt4 cells compared to mock transduced cells (Supplementary Figure S1c).12
Figure 1.
PRL2 is important for the proliferation and survival of human T-ALL cells. (a) PRL2 is highly expressed in both primary human B-ALL and T-ALL cells (Normal control cells were set to 1).8 (b) Tail-7 cells were transduced with lentiviruses expressing control or PRL2 shRNA. The proliferation of transduced cells (GFP+) was measured over time (*p<0.05, n=3). (c) Tail-7 cells were transduced with lentiviruses expressing control or PRL2 shRNA were cultured with or without IL-7 for 2 days. The percentage of apoptotic cells was measured by Annexin V and PI staining (*p<0.05, n=3). (d) Inhibiting of PRL2 activity with a small molecule inhibitor (PRLi) decreases the viability of human T-ALL cell lines in a dosage-dependent manner. (e) Treatment of human T-ALL cell lines with PRL2 inhibitor (PRLi) induces apoptosis (***p<0.001, n = 3). (f) Immunoblot analysis of ERK phosphorylation in human T-ALL cells following DMSO or a small molecule inhibitor (PRLi) treatment. Representative Western blot analysis of indicated proteins is shown.
In a previous study, we conducted an unbiased high-throughput small-molecule library screen and identified several compounds capable of inhibiting PRL activity.13 The lead compound (Cmpd-43) displayed a respectable pharmacokinetic profile in mouse with a plasma compound exposure Cmax=0.3 mM and a half-life t1/2=15.8 h at a single 20 mg/kg intraperitoneal dosage. Intraperitoneally injection of 15 mg/kg of PRLi daily for 3 weeks exhibited no toxicity and body weight and weights of major organs (liver, spleen, and kidney) were comparable to control mice.13 We named the compound Cmpd-43 as PRL inhibitor (PRLi). To determine the effect of pharmacological inhibition of PRL2 on human T-ALL cells, we treated several human T-ALL cell lines with PRLi and measured cell viability. We found that pharmacological inhibition of PRL2 activity decreased proliferation and enhanced apoptosis in a dosage-dependent manner (Figures 1d and 1e). PRLi appears to be specific to leukemia cells and did not affect the viability of human cord blood mononuclear cells and HSPCs (Supplementary Figures S1d, S1e and S1f). Given that PRLi specifically blocks the PRL1-induced cell proliferation and migration through attenuation of both ERK1/2 and AKT activity,13 we treated two human T-ALL cell lines with PRLi and then measured the level of phosphorylated ERK using western blot analysis. We found that PRLi treatment resulted in decreased ERK phosphorylation in both Loucy and CEM cells (Figure 1f), suggesting that PRLi can inhibit ERK activation in leukemia cells. These results demonstrate that PRL2 is important for the proliferation and survival of human T-ALL cells.
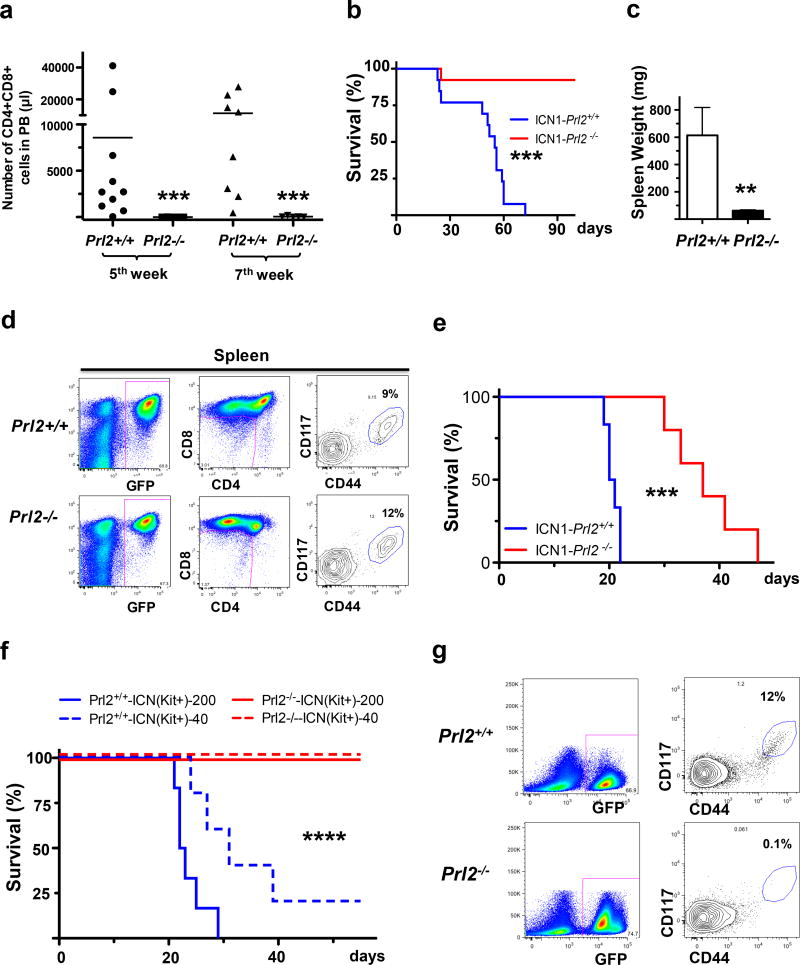
More than 90% of human T-ALL show signs of constitutive NOTCH1 pathway activation and majority of human T-ALL lines are addicted to NOTCH1 function.4 Indeed, we found that PRL2 is a direct target of NOTCH1 signaling in mouse T cell progenitors (M.K. and Y.L. unpublished data). In order to test whether PRL2 is important for oncogenic NOTCH1 induced T-ALL in vivo, we transduced lineage− bone marrow progenitors from wild-type and Prl2 null mice with retrovirus encoding the constitutively active intracellular domain of NOTCH1 (NOTCH1-ICN1-EGFP). These cells were then transplanted into lethally irradiated recipient mice, and we monitored for the presence of CD4+CD8+ double positive (DP) leukemic cells in the peripheral blood.14 We found that the peripheral blood of control animals contained high number of donor-derived CD4+CD8+ DP cells at 5 and 7 weeks after transplantation, while only a minute number of leukemic cells were observed in recipient of Prl2 null cells (Figure 2a). The development of T-ALL in host that received wild-type cells transduced with NOTCH1-ICN1 retrovirus was rapid, with all control animals succumbing to the disease and died 9 weeks following transplantation (Figure 2b). In contrast, animals repopulated with Prl2 null cells displayed significant protection from disease, with majority of animals remaining disease free 18 weeks following transplantation (Figure 2b). All control animals show enlarged spleen compared with recipient mice repopulated with PRL2 null cells (Figure 2c and Supplementary Figure S2a). Prl2−/− cells expressing NOTCH1-ICN1 showed long-term myeloid cell, B cell, CD4+, and CD8+ T cell engraftment in the surviving recipients (Supplementary Figure S2b). These results demonstrated that PRL2 is essential for the initiation and/or maintenance of T-ALL-induced by oncogenic NOTCH1.
Figure 2.
PRL2 promotes oncogenic NOTCH1 induced T-ALL in vivo. (a) Lethally irradiated B6.SJL mice were transplanted with Prl2+/+ or Prl2−/− progenitor cells expressing NOTCH1-ICN1, and peripheral blood was collected and the frequency of donor-derived (GFP+) CD4+CD8+ cells was determined by FACS analysis (n=10, ***p<0.001). (b) Lin− bone marrow cells isolated from Prl2+/+ and Prl2−/− mice were transduced with retroviruses expressing NOTCH1-ICN1 and equivalent number of transduced cells (GFP+) were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing NOTCH1-ICN1 for the period of observation (n=12, ***p<0.001). (c) Average spleen weight in recipient mice repopulated with Prl2+/+ or Prl2−/− cells expressing NOTCH1-ICN1 (n=10, **p<0.01). (d) Prl2+/+ and Prl2−/− fetal liver cells were transduced with retroviruses expressing NOTCH1-ICN1 and equivalent number of transduced cells (GFP+) were injected into lethally irradiated recipient mice. Representative flow cytometry blots showing the frequency of leukemia–initiating cells (Kit+CD44+CD25+) in the spleen of primary recipient mice 4 weeks following transplantation. (e) 20,000 GFP+ cells isolated from the spleens of primary recipient mice were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing NOTCH1-ICN1 for the period of observation (***p<0.001, n=5). (f) 40 or 200 leukemia–initiating cells (Kit+CD44+CD25+) purified from the spleens of primary recipient mice were injected into lethally irradiated recipient mice. Kaplan-Meier curve shows the survival of mice transplanted with cells expressing NOTCH1-ICN1 for the period of observation (****p<0.0001, n=5). (g) Representative flow cytometry blots showing the frequency of leukemia–initiating cells (Kit+CD44+CD25+) in the spleens of secondary recipient mice repopulated with leukemia–initiating cells (Kit+CD44+CD25+) expressing NOTCH1-ICN1.
To determine whether PRL2 is essential for T-ALL maintenance, we introduced NOTCH1-ICN1 into Prl2+/+ and Prl2−/− fetal liver cells using retroviruses and transplanted transduced cells (GFP+) into lethally irradiated recipient mice. All recipient mice repopulated with Prl2+/+ and Prl2−/− cells development leukemia with similar latency and penetrance (data not shown). Recently, the leukemia initiating cells (LICs) in a mouse model of T-ALL were characterized as c-Kit+ cells.15 We found that the frequency of leukemia-initiating cells (Kit+CD44+CD25+) in the spleen of leukemic mice are comparable between Prl2+/+ and Prl2−/− groups (Figure 2d). We then transplanted 20,000 GFP+ cells purified from the spleen of primary recipients into secondary recipients and monitor leukemia development. Recipient mice repopulated with Prl2−/− cells show delayed disease onset and extended survival compared to that of the Prl2+/+ cells (Figure 2e). To determine the role of PRL2 in leukemia-initiating cell maintenance, we performed limiting dilution transplantation assays and transplanted 40 or 200 leukemia-initiating cells (Kit+CD44+CD25+) purified from the spleen of primary recipients that have developed leukemia into lethally irradiated recipient mice. While most recipient mice repopulated with Prl2+/+ cells expressing NOTCH1-ICN1 developed leukemia and died within 40 days, none of the recipient mice repopulated with Prl2−/− cells developed leukemia (Figure 2f). Further, LICs were not detectable in the spleen of recipient mice repopulated with Prl2−/− LICs (Figure 2g). It appears that PRL2 is important for the ability of LICs, defined immunophenotypically by flow cytometry, to recapitulate leukemia in secondary hosts rather than maintaining the number of LICs. Thus, these data demonstrate that PRL2 is important for the maintenance of T-ALL mediated by oncogenic NOTCH1.
DELTA/ NOTCH1 association is one of the most important signals provided by the thymic environment to initiate T cell differentiation.16 Given that NOTCH1 signaling upregulates c-Kit expression in early T cell progenitors (ETPs) (M.K. and Y.L. unpublished data), we hypothesized that PRL2 may promote the pathogenesis of T-ALL through maintaining c-Kit+ LICs. To test this hypothesis, we introduced NOTCH1-ICN1 into Lin−Sca1+ BM cells isolated from Prl2+/+ and Prl2−/− mice and then cultured infected cells without stroma to examine cell differentiation and expansion. While Prl2+/+ Lin−Sca1+ cells expressing NOTCH1-ICN1 showed expansion, Prl2−/− Lin−Sca1+ cells failed to proliferate under this condition (Supplementary Figure S2c). We found the level of c-Kit proteins in Prl2+/+ cells expressing NOTCH1-ICN1 was significantly higher than that of the Prl2−/− cells both at day 11 and day 17 (Supplementary Figure S2d). Consistent with these observations, we observed high level of PRL2 mRNAs and proteins in the spleen of recipient mice transplanted with Lin− cells expressing NOTCH1-ICN1 (Supplementary Figures S2e and S2f). Further, treatment of some human T-ALL cells with γ-secretase inhibitor (GSI) decreased the level of PRL2 proteins (Supplementary Figure S2g). Thus, our data suggest that oncogenic NOTCH1 may promote T-ALL development through sustaining PRL2 and c-Kit expression.
To date, very little information exists in the literature on the role of PRL2 in human T-ALL.5,6 In this study, we have identified a critical role for PRL2 phosphatase in the proliferation and survival of human T-ALL cells. Further, we demonstrated that PRL2 is important for the leukemogenic potential of oncogenic NOTCH1 in vivo. Our findings suggest that pharmacological inhibition of PRL2 holds potential as a novel therapy for T cell acute lymphoblastic leukemia, and might also be applicable to the treatment of other human cancers.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grant CA69202 (ZYZ), Department of Defense Grant W81XWH-13-1-0187 (YL), a St. Baldrick’s Foundation Scholar Award (YL), an Elsa Pardee Foundation New Investigator Award (YL), an Alex’s Lemonade Stand Grant (YL), a Children’s Leukemia Research Association Grant (YL), a Leukemia Research Foundation grant (YL), and American Cancer Society Institutional Research Grants (YL and MK). This work was supported in part by a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108.
Footnotes
CONFLICT OF INTEREST
The authors declared that no conflict interest exists.
AUTHOR CONTRIBUTIONS
MK, YB, ZYZ, and YL. Designed the research. MK, YB, SC, RG, CY, and WC. Performed the research; MK and YL. Analyzed the data and performed the statistical analysis. AAC and JC. Provided reagents to the study. MK and YL. Wrote the manuscript. All authors read, comment on, and approved the manuscript.
References
- 1.Ribera JM, Oriol A. Acute lymphoblastic leukemia in adolescents and young adults. Hematol Oncol Clin North Am. 2009;23:1033–42. doi: 10.1016/j.hoc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 4.Paganin M, Ferrando A. Molecular pathogenesis and targeted therapies for NOTCH1-induced T-cell acute lymphoblastic leukemia. Blood Rev. 2011;25:83–90. doi: 10.1016/j.blre.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessette DC, Qiu D, Pallen CJ. PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev. 2008;27:231–252. doi: 10.1007/s10555-008-9121-3. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Chen S, Gao R, Bai Y, Zhang ZY, Liu Y. Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies. Cell Cycle. 2014;13:2827–35. doi: 10.4161/15384101.2014.954448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juric D, Lacayo NJ, Ramsey MC, Racevskis J, Wiernik PH, Rowe JM, et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol. 2007;25:1341–9. doi: 10.1200/JCO.2006.09.3534. [DOI] [PubMed] [Google Scholar]
- 8.Yagi T, Morimoto A, Eguchi M, Hibi S, Sako M, Ishii E, et al. Identification of a gene expression signature associated with pediatric AML prognosis. Blood. 2003;102:1849–56. doi: 10.1182/blood-2003-02-0578. [DOI] [PubMed] [Google Scholar]
- 9.Haferlach T, Kohlmann A, Wieczorek L, Basso G, et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 2010;28:2529–37. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Zhang L, Zhang S, et al. Phosphatase of Regenerating Liver 2 (PRL2) Is Essential for Placental Development by Down-regulating PTEN (Phosphatase and Tensin Homologue Deleted on Chromosome 10) and Activating Akt Protein. J Biol Chem. 2012;287:32172–9. doi: 10.1074/jbc.M112.393462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, Bai Y, Dong Y, Yu H, Chen S, Gao R, et al. PRL2/PTP4A2 phosphatase is important for hematopoietic stem cell self-renewal. Stem Cells. 2014;32:1956–67. doi: 10.1002/stem.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy S, Uetani N, Wong N, Kostantin E, Labbé DP, Bégin LR, et al. The protein tyrosine phosphatase PRL-2 interacts with the magnesium transporter CNNM3 to promote oncogenesis. Oncogene. 2015;34:986–95. doi: 10.1038/onc.2014.33. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Yu ZH, Liu S, et al. Novel Anticancer Agents Based on Targeting the Trimer Interface of the PRL Phosphatase. Cancer Res. 2016;76:4805–15. doi: 10.1158/0008-5472.CAN-15-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aster JC, Xu L, Karnell FG, Patriub V, Pui JC, Pear WS. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by notch1. Mol Cell Biol. 2000;20:7505–15. doi: 10.1128/mcb.20.20.7505-7515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubbert S, Cardenas A, Chen H, et al. Targeting the MYC and PI3K pathways eliminates leukemia-initiating cells in T-cell acute lymphoblastic leukemia. Cancer Res. 2014;74:7048–7059. doi: 10.1158/0008-5472.CAN-14-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothenberg EV. Transcriptional control of early T and B cell developmental choices. Annu Rev Immunol. 2014;32:283–321. doi: 10.1146/annurev-immunol-032712-100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.