Abstract
Background
Enhanced recovery after surgery (ERAS) program is an effective evidence-based multidisciplinary protocol of perioperative care, but its roles in thoracic surgery remain unclear. This systematic review of randomized controlled trials (RCTs) aims to investigate the efficacy and safety of the ERAS programs for lung cancer surgery.
Materials and methods
We searched the PubMed and EMBASE databases to identify the RCTs that implemented an ERAS program encompassing more than four care elements within at least two phases of perioperative care in lung cancer surgery. The heterogeneity levels between studies were estimated by the Cochrane Collaborations. A qualitative review was performed if considerable heterogeneity was revealed. Relative risk (RR) and weighted mean difference served as the summarized statistics for the meta-analyses. Additional analyses were also performed to perceive potential bias risks.
Results
A total of seven RCTs enrolling 486 patients were included. The meta-analysis indicated that the ERAS group patients had significantly lower morbidity rates (RR=0.64; p<0.001), especially the rates of pulmonary (RR=0.43; p<0.001) and surgical complications (RR=0.46; p=0.010), than those of control group patients. No significant reduction was found in the in-hospital mortality (RR=0.70; p=0.58) or cardiovascular complications (RR=1.46; p=0.25). In the qualitative review, most of the evidence reported significantly shortened length of hospital and intensive care unit stay and decreased hospitalization costs in the ERAS-treated patients. No significant publication bias was detected in the meta-analyses.
Conclusion
Our review demonstrates that the implementation of an ERAS program for lung cancer surgery can effectively accelerate postoperative recovery and save hospitalization costs without compromising patients’ safety. A worldwide consensus guideline is urgently required to standardize the ERAS protocols for elective lung resections in the future.
Keywords: enhanced recovery after surgery, lung cancer surgery, morbidity, systematic review, meta-analysis
Introduction
Rationale
Lung cancer is the leading cause of malignancy-related deaths worldwide and remains the most prevalent cancer in both developed and developing countries.1,2 Nowadays, surgical treatment is regarded not only as the optimal therapeutic measure for early-stage non-small-cell lung cancer (NSCLC), but also as a key component of multidisciplinary treatment for advanced-stage NSCLC.3,4 Advances in the radiographic techniques and the prevalent practice of cancer screening have substantially increased the opportunity for early detection, helping to offer more effective therapeutic options to lung cancer patients. However, despite advances in surgical techniques and perioperative care, the morbidity rate still remains at 20.8%–34.1% in patients undergoing lung cancer surgery, as reported in recent literature.5,6
Postoperative complications are commonly considered as the major cause of delayed recovery after surgery, resulting in the prolonged length of stay (LOS), increased hospitalization cost and poor life quality.7 Thoracic surgeons usually focus on the efficacy of specific interventions during the in-hospital period, although these individual interventions seem not to further improve the postoperative outcomes.8 Therefore, it is necessary to optimize the utilization of a variety of health care resources and implement a multidisciplinary care plan in the perioperative management of operable lung cancer.
Enhanced Recovery after Surgery (ERAS), an evidence-based multimodal protocol of perioperative care, was firstly introduced by Kehlet and Mogensen9 in colorectal surgery in the late 1990s, in order to minimize the perioperative stress responses, catabolism and morbidity rates, shorten the LOS, and fasten postoperative recovery to achieve an early return to normal life. Since then, the ERAS strategies have dramatically developed and encompassed an increasing number of elements within all phases of perioperative care. These care elements are believed to have synergistic effects on the attenuation of operative stress responses and the protection of baseline function status, leading to an accelerated recovery postoperatively.10,11
A recent systematic review with meta-analysis based on 38 randomized controlled trials (RCTs) has demonstrated the great efficacy of ERAS programs in terms of reducing the morbidity rate and shortening the LOS across surgical specialties.12 However, these findings could be hardly generalized to lung resections because of the limited supporting data for quantitative synthesis.12 A later evidence-based review reported by Fiore et al7 attempted to evaluate the clinical roles of ERAS programs in elective lung resections, but finally failed to draw a convincing conclusion. Only six comparative analyses were included in that review, while five of them were nonrandomized studies, resulting in high bias risks favoring the intervention. Given such concerns, the authors have appealed for more well-designed RCTs to investigate the effects of ERAS programs on pulmonary resections.7
Objectives
The primary purpose of our evidence-based literature review was to integrate the clinical data from RCTs and lung cancer cases to summarize the clinical significance of ERAS programs on a range of postoperative outcomes (in-hospital morbidity, in-hospital mortality, LOS and hospitalization cost) in patients undergoing lung cancer surgery.
Materials and methods
Protocol
This evidence-based review of published RCTs was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.13 Additional PRISMA checklist is shown in the Supplementary materials.
Search strategy
Complete procedures for a comprehensive literature retrieval ranged from July 25 to August 2, 2017. No publication date restriction was imposed during the retrieval.
Three researchers were assigned to search two universal electronic databases, the PubMed and EMBASE (via the Ovid interface), to recognize the eligible articles updated to July 25, 2017. We used the following 9 key words (5 “ERAS” terms and 4 “lung cancer” terms) and two Boolean Operators (“AND” and “OR”) to formulate four search strings in each selected database:
ERAS terms: “fast-track”, “enhanced recovery”, “intensive rehabilitation”, “accelerated rehabilitation” and “ERAS”
Lung cancer terms: “lung cancer”, “lung carcinoma”, “lung neoplasm” and “lung malignancy”
The search details are shown in the Supplementary materials. Moreover, we also manually searched the reference lists of retried publications to identify any possible study with no duplication.
Eligibility criteria
The following eligibility criteria were established to determine the appropriateness of studies for our review.
Study designs
Only RCTs were considered eligible. The sample size was not limited. The following studies were immediately excluded because of their irrelevant styles: cohort studies, case series, reviews, animal experiments and conference abstracts.
Participants
The target diseases were operable lung cancers, including both primary and secondary lesions. A small cohort of mixed malignancies was also included. No limitation was imposed for any basic characteristic of the patients.
Interventions
Elective pulmonary resections operated by traditional thoracotomy and video-assisted thoracoscopic surgery (VATS) approaches were considered eligible.
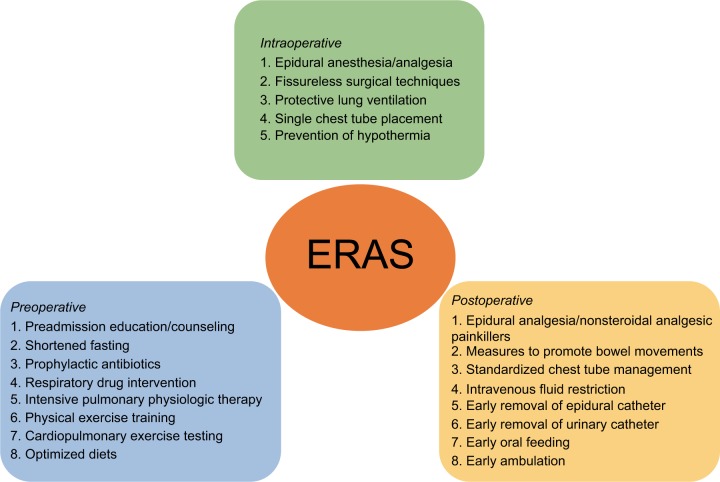
We recognized a total of 21 ERAS elements encompassing all phases of perioperative care (preoperative/intraoperative/postoperative) in the literature,7,10–12 as shown in Figure 1. An ERAS program involving more than four of these elements and encompassing at least two phases of perioperative care was considered eligible.
Figure 1.
Care elements implemented in the ERAS protocols for lung cancer surgery.
Abbreviation: ERAS, enhanced recovery after surgery.
Endpoints and outcome measures
The primary outcomes of interest were overall morbidity and in-hospital mortality. Overall morbidity was defined by the presence of any individual complication within 30 days after surgery or later during the same hospitalization. In-hospital mortality was defined as death within 30 days after surgery or later during the same hospitalization.
The secondary outcomes of interest included the LOS, length of intensive care unit (ICU) stay and total hospitalization cost. LOS was calculated from the admission day to the discharge day. Length of ICU stay referred to the duration of time spent in ICU after surgery.
Studies reporting any one of the above outcomes were included in this review. Sufficient demographics or statistics should be available for the estimation of relative risks (RRs) or weighted mean differences (WMDs).
Publications
In addition, only the most recent study was included if a series of studies was performed on overlapping patients. Only full-text papers published in peer-reviewed journals were included. No language restriction was imposed.
Data collection
Process
We designed a Microsoft Office Excel spreadsheet to extract relevant information from each included RCT. This process was performed by two researchers and cross-checked by another reviewer.
Data items
The following details were collected from each study:
“publication data” including authors, years, nations and languages;
“experimental data” including the RCT design (single center/multicenter), study period, surgical procedures, operative approaches (thoracotomy/VATS), ERAS elements and follow-ups;
“demographic data” including the total sample size, the number of patients enrolled in the ERAS group and the control group, gender, age, body mass index, forced expiratory volume in 1 second, American Society of Anesthesiologists scores, histologic subtypes and clinical stages; and
“outcome data” including the dichotomous statistics or demographics about the morbidity and mortality, and continuous data for the LOS, length of ICU stay and hospitalization cost.
Quality assessment
We computed the Jadad score of each included study according to the Jadad scale, as a valid and reliable three-question measure tool for assessing the methodological quality of RCTs.14 A scoring system with up to 5 points was utilized in the Jadad scale. It conferred up to 2 points to the randomization, 2 points to the blinding of studies and 1 point to the reasons of withdrawals. Finally, a Jadad score ≥3 points indicated a high-quality RCT.
Statistical analysis
All of the following statistical analyses were accomplished by STATA 12.0.
Summary measures
For primary outcomes, we calculated the RR with 95% CI to summarize the effects of ERAS programs on postoperative morbidity and mortality. With regard to secondary outcomes, the WMD with 95% CI served as the appropriate statistic to summarize the mean values with SDs for the LOS, length of ICU stay and hospitalization cost.
If the SD was not available for continuous data, we did not incorporate it in the quantitative synthesis because the extrapolation of SD was only applicable for studies with a large sample size and normal distribution of outcomes according to the guidelines of Cochrane Collaborations.15
Synthesis of results
Cochrane Q test and I2 statistic were used to quantify the heterogeneity level. A rough guide to the threshold for interpreting the I2 statistic was given by the Cochrane Collaborations, and I2 of 40%, 60% and 75% represented low, moderate and considerable variance, respectively.15
In our study, fine heterogeneity was defined by I2<40% and p>0.1, and a standard fixed-effect model test (Mantel–Haenszel method) was utilized for quantitative synthesis. Otherwise, a random-effect model test (DerSimonian–Laird method) was adopted when moderate-to-considerable heterogeneity was revealed by 40%≤I2<75% and p≤0.1.
However, if there was considerable heterogeneity between studies (I2≥75% and p≤0.1), a meta-analysis was canceled because it might be misleading to quote an average value for the intervention effects. Instead, a qualitative summary of evidence was performed.15
Additional analyses
We conducted a sensitivity analysis, in which the impact of each study on the overall estimates could be detected by omitting the individual study sequentially, to further examine the stability of pooled estimates. The strong robustness of our meta-analysis was confirmed if there was no substantial variation between the adjusted results and the primary results.15
To evaluate the efficacy of ERAS programs on postoperative morbidity in detail, we classified all enrolled patients into three subgroups according to their pulmonary, surgical and cardiovascular complications. A meta-analysis was then performed on each of these subgroups. Pulmonary, surgical and cardiovascular complications were judged according to the criteria used in recent large-registry trials based on the French Society of Thoracic and Cardiovascular Surgery database.5,16
Publication bias
Both Begg’s test and Egger’s test were used to detect any potential publication bias within the meta-analyses. The presence of bias was suggested by visual symmetry of Begg’s funnel plot, in which log (RRs) or log (WMDs) were plotted against their corresponding standard errors.17 In addition, its significance was also revealed if Egger’s p value was <0.05.
Results
Study selection
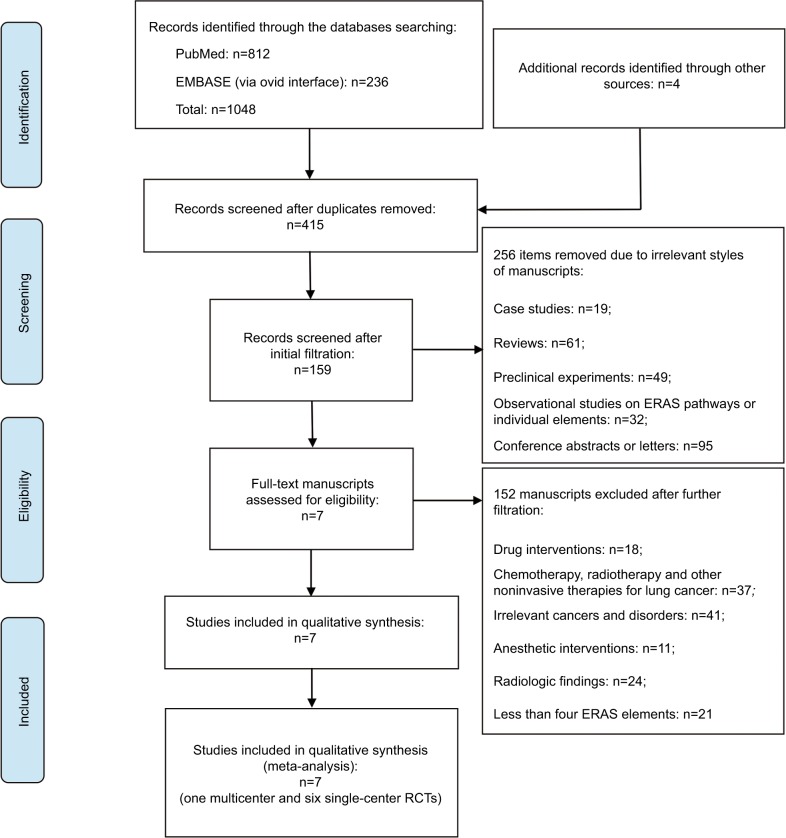
Our literature retrieval is presented as a PRISMA diagram (Figure 2).
Figure 2.
PRISMA flow diagram of literature retrieval.
Abbreviations: ERAS, enhanced recovery after surgery; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized controlled trial.
A total of 1048 publication items were primarily identified, including 812 PubMed citations and 236 EMBASE citations. In addition, a manual search of the reference lists also yielded four relevant studies. After excluding the duplicates, 415 items entered the initial filtration by screening on their titles and abstracts. Then, 256 of them were directly excluded from further filtration because of their irrelevant literature styles. By reading through 159 retrieved articles, we further excluded 152 articles addressing irrelevant topics and considered the remaining 7 studies for possible eligibility. Finally, these seven studies were judged to meet all the eligibility criteria and were included in our review.18–24
Study characteristics
Baseline characteristics and major perioperative outcomes in each RCT are summarized in Tables 1 and 2, respectively.
Table 1.
Baseline characteristics
| Reference | Language | Country | RCT design | Study period | Sample size
|
Mean age (years)
|
Gender (male ratio)
|
Mean BMI (kg/m2)
|
Mean FEV1 (L)
|
ASA score (I–II/III–IV)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | |||||
| Dong et al18 | English | China | Single center | 2012–2014 | 35 | 17 | 18 | 55.1 | 56.6 | 13 (76.5%) | 14 (77.8%) | 26.8 | 25.6 | 2.9 | 2.8 | 17/0 | 18/0 |
| Huang et al19 | English | China | Single center | 2015–2016 | 60 | 30 | 30 | 63.0 | 63.6 | 20 (66.7%) | 21 (70.0%) | NI | NI | 2.3 | 2.2 | 27/3 | 28/2 |
| Lai et al20 | Chinese | China | Single center | 2015 | 48 | 24 | 24 | 63.1 | 64.0 | 15 (62.5%) | 13 (54.2%) | NI | NI | 2.4 | 2.5 | NI | NI |
| Licker et al21 | English | Switzerland | Multicenter | 2011–2014 | 151 | 74 | 77 | 64.0 | 64.0 | 41 (55.4%) | 50 (64.9%) | 25.0 | 24.4 | NI | 52/22 | 49/28 | |
| Muehling et al22 | English | Germany | Single center | NI | 58 | 30 | 28 | 67.0 | 64.0 | 20 (66.7%) | 23 (82.1%) | NI | NI | 2.1 | 2.4 | 4/26 | 5/23 |
| Sokouti et al23 | English | Iran | Single center | 2010 | 60 | 30 | 30 | 49.7 | 40.5 | 26 (86.7%) | 19 (63.3%) | NI | NI | 2.7 | 2.3 | 12/18 | 27/3 |
| Zhao et al24 | Chinese | China | Single center | 2008–2009 | 74 | 38 | 36 | 53.2 | 55.3 | 24 (63.2%) | 25 (69.4%) | NI | NI | NI | NI | NI | NI |
| Reference | Diagnoses
|
Clinical stages (I–II/III–IV)
|
Surgical procedures
|
Operative approaches (open/VATS)
|
ERAS items | Follow-up | Jadad score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ||||
| Dong et al18 | AC=2, SCC=15 | AC=2, SCC=16 | NI | NI | PN=17 | PN=18 | 17/0 | 18/0 | 11 | 30 days | 5 |
| Huang et al19 | All NSCLCs | 26/4 | 28/2 | LB=30 | LB=30 | 13/17 | 11/19 | 5 | 30 days | 3 | |
| Lai et al20 | AC=11, SCC=11, others=2 | AC=10, SCC=12, others=2 | 21/3 | 21/3 | LB=11, ST/WR=12, others=1 | LB=15, ST/WR=8, others=1 | 7/17 | 9/15 | 5 | 30 days | 3 |
| Licker et al21 | All NSCLCs | 61/13 | 67/10 | LB=49, ST=12, PN/BL=13 | LB=46, ST=15, PN/BL=17 | 62/12 | 63/14 | 8 | 30 days | 4 | |
| Muehling et al22 | NSCLC=25, others=5 | NSCLC=19, others=9 | NI | NI | LB/BL=20, PN=3, WR/SR=7 | LB/BL=20, PN=1, WR/SR=7 | 30/0 | 28/0 | 8 | 30 days | 4 |
| Sokouti et al23 | Malignant with benign lesions | NI | NI | LB/BL=16, PN=4, WR/SR=10 | LB/BL=15, PN=3, WR/SR=12 | 30/0 | 30/0 | 5 | 30 days | 3 | |
| Zhao et al24 | AC=13, SCC=22, others=3 | AC=12, SCC=20, others=4 | 25/13 | 25/11 | LB=38 | LB=36 | 38/0 | 36/0 | 10 | 30 days | 3 |
Abbreviations: AC, adenocarcinoma; ASA, American Society of Anesthesiologists; BL, bilobectomy; BMI, body mass index; ERAS, enhanced recovery after surgery; FEV1, forced expiratory volume in 1 second; LB, lobectomy; NI, no information; NSCLC, non-small-cell lung cancer; PN, pneumonectomy; RCT, randomized controlled trial; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; SR, sleeve resection; ST, segmentectomy; VATS, video-assisted thoracoscopic surgery; WR, wedge resection.
Table 2.
Postoperative outcomes
| Reference | Overall morbidity
|
In-hospital mortality
|
Length of hospital stay (days)
|
Length of ICU stay (days)
|
Total cost (RMB, thousand Yuan)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERAS (%) | Control (%) | ERAS (%) | Control (%) | ERAS | Control | ERAS | Control | ERAS | Control | |
| Dong et al18 | 4 (23.5) | 6 (33.3) | 0 (0.0) | 0 (0.0) | 18.1±1.4 | 27.4±6.6 | NI | NI | 29.9±2.7 | 37.2±3.6 |
| Huang et al19 | 5 (13.3) | 12 (40.0) | 0 (0.0) | 1 (3.3) | 14.1±2.7 | 17.3±4.3 | NI | NI | NI | NI |
| Lai et al20 | 2 (8.3) | 5 (20.8) | 0 (0.0) | 0 (0.0) | 14.0±3.2 | 15.8±3.2 | NI | NI | 46.5±5.1 | 45.5±4.2 |
| Licker et al21 | 27 (36.5) | 39 (50.6) | 2 (2.7) | 2 (2.6) | 10 (IQR 8–12) | 9 (IQR 7–13) | 0.7±0.3 | 1.0±0.4 | NI | NI |
| Muehling et al22 | 8 (26.7) | 13 (46.4) | 1 (3.3) | 1 (3.6) | 11 (8–33) | 11 (7–34) | 1 (1–33) | 1 (1–12) | NI | NI |
| Sokouti et al23 | 5 (16.7) | 17 (56.7) | 0 (0.0) | 1 (3.3) | 8.0±1.3 | 14.0±1.2 | 2.0±0.2 | 3.0±0.6 | NI | NI |
| Zhao et al24 | 14 (36.8) | 20 (55.5) | 0 (0.0) | 0 (0.0) | 4.0±1.0 | 9.0±1.0 | NI | NI | 15.6±7.6 | 23.6±5.4 |
Notes: The length of hospital and ICU stay in Muehling et al22 are both given as medians with the corresponding ranges. The length of stay in Licker et al21 is presented as median with its IQR. Data for the length of stay, length of ICU stay and hospitalization costs in the other references are all presented as the mean values with SDs.
Abbreviations: ERAS, enhanced recovery after surgery; ICU, intensive care unit; IQR, interquartile range; NI, no information; RMB, Renminbi.
Study designs
The seven included RCTs were published between 2008 and 2017. Six of them were single-center RCTs18–20,22–24 and only one study was a multicenter RCT.21 There were five papers written in English language18,19,21–23 and two papers written in Chinese language.20,24 Their sample size ranged from 35 to 151.
Participants
This review comprised a total of 486 surgical patients who were consecutively enrolled from 2007 to 2016. Approximately half of these patients were from China (n=217; ratio=44.7%),18–20,24 followed by 209 patients from Europe (ratio=43.0%)21,22 and 60 patients from the Middle East (ratio=12.3%).23 Half of these patients were randomized to the ERAS group (n=243) and the other half to the control group (n=243).
Majority of the patients were diagnosed with primary NSCLCs (n=472; ratio=97.1%). With respect to operative modes, there were 326 patients who underwent lobectomy (ratio=67.1%), while pneumonectomy was performed on 78 patients (ratio=16.0%) and sublobar resections on 82 patients (ratio=16.9%). Most of the patients were operated by standard posterolateral thoracotomy (n=392; ratio=80.7%), and only 94 patients were operated by VATS procedures (ratio=19.3%). The comparisons of the above perioperative parameters and other available patient characteristics between the ERAS group and the control group are shown in Table 1.
Interventions
Complete details of ERAS interventions estimated in each RCT are summarized in Table 3. The number of ERAS elements utilized in all seven RCTs ranged from 5 to 11.18–24 They varied across these seven RCTs, but overlapped for some common components, as shown in Table 3.
Table 3.
ERAS protocols implemented in eligible RCTs
| ERAS elements | Eligible RCTs
|
||||||
|---|---|---|---|---|---|---|---|
| Dong et al18 | Huang et al19 | Lai et al20 | Licker et al21 | Muehling et al22 | Sokouti et al23 | Zhao et al24 | |
| Preoperative interventions | |||||||
| Patient education/counseling | ✓ | ✓ | ✓ | ✓ | |||
| Shortened fasting | ✓ | ✓ | ✓ | ||||
| Prophylactic antibiotics | ✓ | ✓ | |||||
| Respiratory drug intervention | ✓ | ||||||
| Intensive pulmonary physiologic therapy | ✓ | ✓ | ✓ | ✓ | |||
| Physical muscle exercise training | ✓ | ✓ | ✓ | ||||
| Cardiopulmonary exercise testing | ✓ | ✓ | |||||
| Optimized diets | ✓ | ✓ | |||||
| Intraoperative interventions | |||||||
| Epidural anesthesia/analgesia | ✓ | ✓ | ✓ | ||||
| Primary/modified fissureless surgical techniques | ✓ | ||||||
| Protective lung ventilation | ✓ | ||||||
| Single chest tube placement | |||||||
| Prevention of hypothermia | ✓ | ✓ | ✓ | ||||
| Postoperative interventions | |||||||
| Epidural analgesia/nonsteroidal analgesic painkillers | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Measures to promote bowel movements | ✓ | ||||||
| Optimized chest tube management | ✓ | ✓ | |||||
| Intravenous fluid restriction | ✓ | ✓ | ✓ | ✓ | |||
| Early removal of epidural catheter | |||||||
| Early removal of urinary catheter | ✓ | ✓ | |||||
| Early oral feeding | ✓ | ✓ | ✓ | ✓ | |||
| Early ambulation | ✓ | ✓ | ✓ | ✓ | |||
Abbreviation: ERAS, enhanced recovery after surgery; RCT, randomized controlled trial.
Outcome measures
The outcome data reported in each RCT are outlined in Table 2.
For the primary outcomes of interest, all seven RCTs compared the morbidity rates between the ERAS group and the control group.18–24 Effective in-hospital mortality rates were available in four included RCTs.19,21–23
For the secondary outcomes of interest, all seven RCTs focused on the LOS, while five of them reported the continuous data that could be incorporated in the quantitative synthesis.18–20,23,24 The length of ICU stay was also evaluated in three RCTs,21–23 but only two of them reported sufficient mean values with SDs.21,23 In addition, there were three RCTs that reported the continuous data for hospitalization cost, and they were all conducted among Chinese participants.18,20,24
Quality assessment
Complete records for the Jadad scale of each RCT are tabulated in Table 4. Finally, all seven RCTs got a Jadad score ≥3 points.18–24 Their mean Jadad score was 3.6 (range 3–5), suggesting that they were of fairly high quality.
Table 4.
Quality assessment of eligible RCTs
| Reference | Randomization | Double blinding | Withdrawals and dropout | Jadad score |
|---|---|---|---|---|
| Dong et al18 | 2 | 2 | 1 | 5 |
| Huang et al19 | 2 | 0 | 1 | 3 |
| Lai et al20 | 2 | 0 | 1 | 3 |
| Licker et al21 | 2 | 1 | 1 | 4 |
| Muehling et al22 | 2 | 1 | 1 | 4 |
| Sokouti et al23 | 1 | 1 | 1 | 3 |
| Zhao et al24 | 1 | 1 | 1 | 3 |
Abbreviation: RCT, randomized controlled trial.
Overall analysis
Overall morbidity
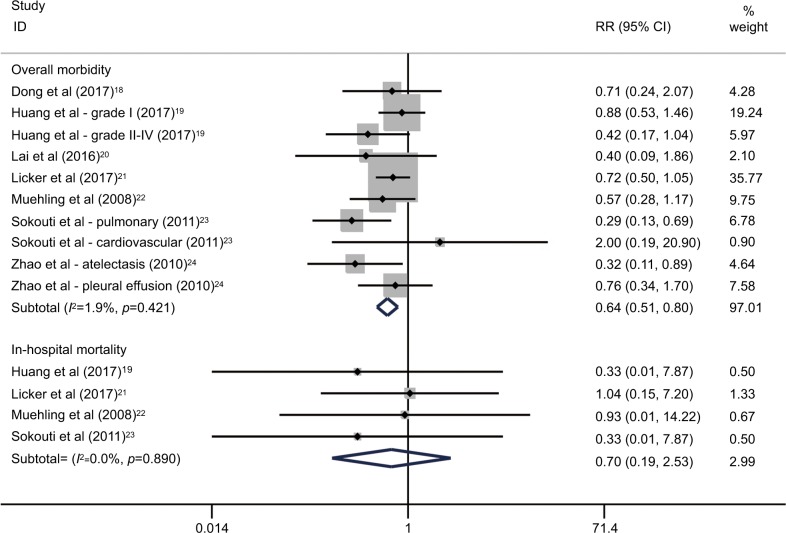
As shown in Table 2, the morbidity rates in the ERAS group and the control group ranged 8.3%–36.8% and 20.8%–56.7%, respectively. A fixed-effect model was used based on the low heterogeneity between studies (I2=1.9%; p=0.42). The pooled RR of all seven RCTs was 0.64, with its 95% CI ranging 0.51–0.80, revealing a significant decrease of morbidity rate in patients who received an ERAS program compared to that in patients who received conventional care (Table 5; Figure 3).18–24
Table 5.
Meta-analyses for effects of the ERAS programs on postoperative morbidity and mortality
| Outcomes | Number of studies | Sample size
|
Heterogeneity(I2, p) | Model | RR with 95% CI | p-Value | Publication bias
|
Conclusion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ERAS | Control | Begg (p) | Egger (p) | |||||||
| Overall analysis | |||||||||||
| Overall morbidity | 7 | 486 | 243 | 243 | I2=1.9%, p=0.42 | Fixed | 0.64 (0.51–0.80) | <0.001 | 0.59 | 0.31 | Significant |
| In-hospital mortality | 4 | 329 | 164 | 165 | I2=0.0%, p=0.89 | Fixed | 0.70 (0.19–2.53) | 0.58 | 0.17 | 0.13 | Not significant |
| Subgroup analyses | |||||||||||
| Pulmonary complications | 7 | 486 | 243 | 243 | I2=0.0%, p=0.59 | Fixed | 0.43 (0.31–0.60) | <0.001 | 1.0 | 0.46 | Significant |
| Surgical complications | 5 | 377 | 188 | 189 | I2=0.0%, p=0.74 | Fixed | 0.46 (0.25–0.83) | 0.010 | 0.81 | 0.23 | Significant |
| Cardiovascular complications | 4 | 304 | 151 | 153 | I2=0.0%, p=0.96 | Fixed | 1.46 (0.77–2.77) | 0.25 | 1.0 | 0.61 | Not significant |
Abbreviations: ERAS, enhanced recovery after surgery; RR, relative risk.
Figure 3.
Overall analyses for effects of the ERAS programs on postoperative morbidity and mortality in patients undergoing lung cancer surgery.
Abbreviations: ERAS, enhanced recovery after surgery; RR, relative risk.
In-hospital mortality
There were three deaths in the ERAS group and five deaths in the control group (Table 2). The pooled analysis of four available RCTs used a fixed-effect model (I2=0.0%; p=0.89) and showed no significant difference in the mortality rate between the ERAS group and the control group (RR=0.70; 95% CI=0.19–2.53; p=0.58), as shown in Table 5 and Figure 3.19,21–23
Length of hospital stay
With regard to the LOS, we tried to integrate the valid continuous data available from five RCTs, but found a significantly high heterogeneity between studies (I2=86.5%; p<0.001).18–20,23,24 Thus, we canceled meta-analysis of these RCTs, but summarized their results qualitatively.
Among the five RCTs, four indicated that the mean LOS was significantly shortened in the ERAS-treated patients (by 3.20–9.30 days), as shown in Table 2.18,19,23,24 Another RCT reported by Lai et al20 emphasized that the length of postoperative stay in ERAS group (6.17±2.91 days) was significantly shorter than that in control group (8.08±2.21 days; p=0.013), although no significant difference was observed in the LOS between these two groups (p=0.072), as shown in Table 2.
The remaining two RCTs enrolling 209 European patients reported the LOS as a median with its range.21,22 Both of them showed no difference in the median LOS between the ERAS group and the control group, as shown in Table 2.
Length of ICU stay
As for the length of ICU stay, we also qualitatively reviewed three available RCTs21–23 since a considerable heterogeneity was revealed (I2=94.7%; p<0.001).
Both the RCTs reporting the length of ICU stay as mean±SD21,23 suggested that the length of ICU stay was significantly shortened in the patients who received an ERAS program (by 0.33–1.0 days). However, in another RCT reported by Muehling et al,22 there was no difference in the median length of ICU stay between the ERAS group and the control group (Table 2).
Total hospitalization cost
As reported in three available RCTs,18,20,24 the mean hospitalization cost in ERAS group and control group ranged 15.60–46.46 and 23.60–45.53 thousand Yuan RMB, respectively. We gave up a meta-analysis of these studies because of a significantly high heterogeneity (I2=92.8%; p<0.001).
Two RCTs based on 109 enrolled patients showed significantly lower hospitalization costs in the ERAS group (differed 7.3–8.0 thousand Yuan RMB).18,24 However, there was no significant difference in hospitalization costs between these two groups in another one smaller RCT (Table 2).20
Subgroup analysis
To assess the effects of ERAS programs on postoperative morbidity in detail, we included all seven RCTs in the subgroup of pulmonary complications,18–24 five RCTs in the subgroup of surgical complications19–23 and four RCTs in the subgroup of cardiovascular complications.18,21–23
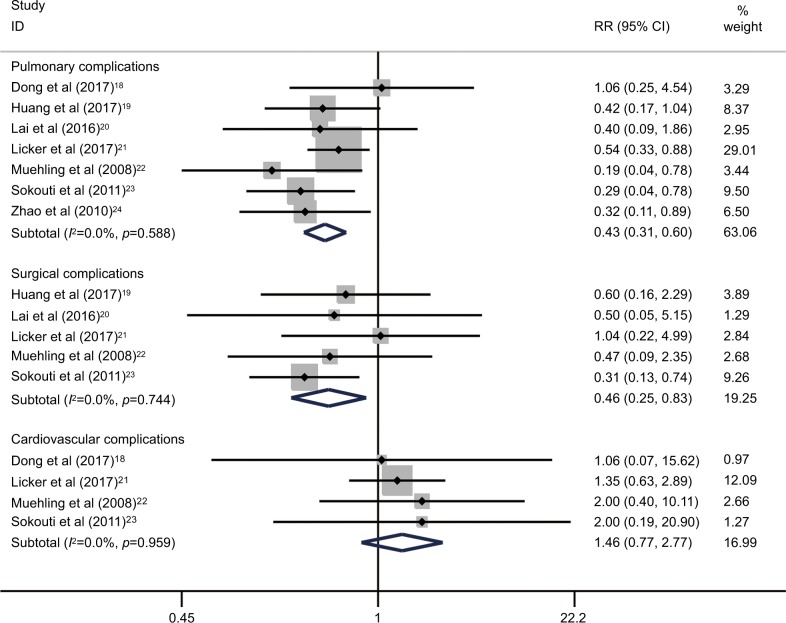
The syntheses of results in all subgroups are summarized in Table 5. As shown in Figure 4, the integrated estimates revealed that both pulmonary complications (RR=0.43; 95% CI=0.31–0.60; p<0.001; I2=0.0%, p=0.59) and surgical complications (RR=0.46; 95% CI=0.25–0.83; p=0.010; I2=0.0%, p=0.74) were significantly decreased in the patients who received an ERAS care. However, no significant improvement was found among the ERAS-treated patients in terms of cardiovascular complications (RR=1.46; 95% CI=0.77–2.77; p=0.25; I2=0.0%, p=0.96), as shown in Table 5 and Figure 4.
Figure 4.
Subgroup analyses for effects of the ERAS programs on pulmonary, surgical and cardiovascular complications following lung cancer surgery.
Abbreviations: ERAS, enhanced recovery after surgery; RR, relative risk.
Sensitivity analysis
By omitting the individual study sequentially, none of the pooled RRs based on the remaining studies in each group of meta-analysis was out of the estimated range, as shown in the Supplementary materials. No substantial variation was found between adjusted pooled estimates and primary pooled estimates. The strong robustness of our meta-analysis was thus confirmed.
Publication bias
As shown in Table 5 and the Supplementary materials, these was no significant evidence detected by either Begg’s test or Egger’s test for publication bias within the meta-analyses of postoperative morbidity and mortality. In addition, no publication bias was detected in each subgroup analysis.
Discussion
Summary of evidence
The concept of ERAS was formerly known as the “fast-track surgery”. Since it was firstly introduced by Kehlet and Mogensen9 in 1999, a package of ERAS protocols has been proposed by different institutions according to their medical care conditions. In recent years, the ERAS programs have been advocated and utilized in a wide range of surgical specialties, including the orthopedics, urologic and cardiothoracic surgery, although the majority of evidence regarding the benefits of ERAS originated from the colorectal surgery.7,11 Currently, there are up to ~20 elements of perioperative care recognized from the relevant investigations, although the number of these elements varies across different ERAS programs.25
The first consensus ERAS guideline, responding to the need in colorectal surgery, was proposed by the European Society of Clinical Nutrition and Metabolism in 2005.26 Since then, similar guidelines applied to other major procedures have been published for further optimizing the perioperative care of surgical patients.25 With such a background, the ERAS Society, an international nonprofit academic and multidisciplinary organization, was formed in London in 2010, with objectives to improve the perioperative care and enhance the implementation of best practices worldwide.35
However, there is no consensus guideline for thoracic surgery until now. As Nicholson et al12 suggested, a great majority of evidence regarding the application of ERAS in lung surgery was from observational studies, resulting in high bias risks caused from confounding factors. The latest systematic review reported by Fiore et al7 confirmed this viewpoint, since only five observational studies and one RCT were available. The authors concluded that the clinical significance of ERAS programs in lung resections remained controversial because of the scarcity of conclusive evidence.
Key results and interpretations
Given the above reviews, we considered that it might be necessary to make appropriate adjustments to the eligibility criteria. According to prior evidence-based reviews,12,27,28 we modified the following items of eligibility criteria and then utilized them in this systematic review.
First, only RCTs were included in order to guarantee strong evidence intensity and eliminate the methodological restrictions. Second, the target diseases were limited to operable lung cancers to avoid bias risks from different disease patterns. Third, an ERAS program that incorporated more than four care elements covering two or all of three phases of perioperative care was included, as suggested by Nicholson et al12 in their large-scale meta-analysis of 38 RCTs. Finally, we removed the language limitation and allowed non-English articles to be included, although they had to get a Jadad score ≥3 points.
To our knowledge, our study was the first systematic review with meta-analysis to explore the beneficial effects of ERAS programs for patients undergoing lung cancer surgery based on the outcome data from RCTs. This evidence-based review covered all prescribed contents for a standardized PRISMA report. On the basis of modified eligibility criteria, seven RCTs with a mean Jadad score of 3.6 were included in our review.18–24 When pooling the data regarding postoperative morbidity and mortality, low to moderate levels of heterogeneity were observed between studies, indicating the need for a meta-analysis that integrated the appropriate statistics to draw global conclusions.3,29
Therefore, by applying meta-analyses of these RCTs, we discovered that the overall morbidity rate in patients receiving an ERAS program was significantly lower than that in patients managed with conventional care. Besides, the rates of pulmonary and surgical complications were both significantly decreased in ERAS-treated patients. However, no significant effect of ERAS programs was observed on either in-hospital mortality or cardiovascular complications. Sensitivity analyses and publication bias tests were also performed to confirm the stability and accuracy of these pooled estimates. We speculated that the following three possible reasons might be considered when trying to explain this phenomenon.
First, preoperative short-term intensive pulmonary physiotherapy and aerobic training were used as ERAS interventions in three RCTs enrolling 53.3% of all enrolled patients (n=259), in order to improve the functional capacity and cardiopulmonary intolerance before surgery.19–21 Previous evidence had shown that moderate-to-intensive rehabilitation programs could effectively bring physical benefits to surgical patients and prevent the development of pulmonary complications following lung cancer surgery.30 However, their effects on postoperative cardiovascular complications were still controversial. Most of the current studies showed no significant decrease of cardiovascular events after pulmonary resections combined with chest physiotherapy, which was supported by our pooled estimates.21,30
Second, several perioperative care elements implanted in the ERAS programs had the potential to affect the incidence of complications induced by surgical procedures. For instance, poor pulmonary fitness in the high-risk patients, a principal risk factor for alveolar air leaks, could be significantly improved by intensive physiotherapy and physical exercise before surgery.30,31 Thus, patients receiving such interventions were expected to have a lower rate of prolonged air leak. Another example was the formation of bronchial fistula, which could be largely limited by using prophylactic antibiotics and sustainable corticosteroids, because the underlying inflammations might predispose to bronchial stump insufficiency.32 In addition, a “fissureless” technique dealing with the densely fused interlobar fissures intraoperatively in an ERAS program could also significantly decrease the incidences of prolonged air leak and pneumothorax.20 However, all of above care elements appeared not to affect the risk of cardiovascular morbidity directly.
Third, there were only eight deaths in our systematic review, with a mortality rate of 1.6%.19,21–23 Three of them underwent an ERAS program and the other five cases received a traditional pathway, indicating little difference in the in-hospital mortality between groups. We suspected that limited sample size might cause a large decline in the precision of effect size estimations and the analytical power. Thus, the safety of ERAS programs should be further verified by more large-scale multicenter RCTs in the future.
For the secondary outcomes of interest, we gave up summarizing the continuous data regarding the LOS, length of ICU stay and hospitalization cost by quantitative methods due to considerable heterogeneity between relevant studies (I2≥75%). This was not only an issue worth being discussed and interpreted, but also a major limitation that should be acknowledged in our study. First of all, the number of individual ERAS elements differed largely across the RCTs, resulting in a dramatically increased heterogeneity. Most of the ERAS programs were developed based upon the principles from similar works in colorectal and gastric surgical arenas.12,27,28 Without a contemporary consensus on the ERAS interventions for lung cancer surgery, there was a large variation, while many care elements overlapped among the current ERAS programs. On the other hand, the practical execution of some common ERAS interventions might not be standardized. The intensity of preoperative physiotherapy, the usage and dosage of drugs, surgical techniques and the time to remove various catheters were almost dependent on the clinicians’ experiences and institutional policies rather than the consensus ERAS guidelines. This might be another source of heterogeneity.
By applying a qualitative summary, we found that four eligible RCTs enrolling 47.1% of all enrolled patients (n=229) showed a significantly shortened LOS in the ERAS-treated patients.18,19,23,24 Another single-center RCT conducted by Lai et al20 based on 48 high-risk patients also reported that the length of postoperative stay of ERAS group was significantly shorter than that of control group (p=0.013). The remaining two RCTs, including the currently largest RCT (n=151) and the earliest RCT (in 2008), reported a similar LOS between the ERAS group and the control group.21,22 However, the length of ICU stay was significantly shortened in the ERAS-treated patients in the largest RCT (p<0.001).21 Similar results were also reported in a smaller RCT enrolling 60 patients (p=0.017).23
The cost-effectiveness analyses performed in the studies included also demonstrated an economic benefit of the ERAS programs for lung cancer surgery. Among three available RCTs, a significant reduction of hospitalization costs in the ERAS-treated patients was reported in two studies based on a total of 109 patients.18,24 On the contrary, only one smaller RCT (n=48) showed slightly higher hospitalization costs in the ERAS group than that in the control group.20 These three RCTs covering a cost-effectiveness analysis were all conducted among Chinese populations.18,20,24 The majority of current evidence supported that the implementation of an ERAS program for elective lung resections was significantly associated with lower hospitalization costs, which was in favor of the findings from a recent Canadian prospective study recruiting 133 patients.33 In that study, Paci et al33 found that a multidisciplinary ERAS program could contribute to improve the clinical outcomes and save the hospitalization and societal costs. However, in an earlier Chinese prospective study based on 142 high-risk patients, Gao et al34 found that the ERAS group patients had slightly higher hospitalization costs than those of the control group patients. Unfortunately, we were unable to further assess the influence of huge variations in socioeconomic levels and medical care between the eastern and western worlds.
Generalizability
Altogether, this study could be regarded as a substantial update to the recent review reported by Fiore et al.7 Our review optimized the eligibility criteria, and we performed a formal analysis of statistical heterogeneity based on more than six included studies.7,15 Our findings demonstrated the favorable effects of ERAS programs on preventing postoperative complications, shortening the LOS and ICU duration and saving the hospitalization costs based on outcome data from RCTs. These evidence-based results would help to popularize the ERAS protocols for lung cancer surgery and improve the perioperative outcomes.
Limitations
Several limitations must be taken into account regarding the interpretations in our review. First, the number of ERAS care elements varied across the seven included RCTs, resulting in an inherent heterogeneity when evaluating the primary and secondary outcomes. Second, the practical execution of ERAS elements might almost depend on the clinicians’ experiences. Third, our sample size of 486 participants was generally smaller than that in other meta-analyses, which might limit the analytical power. Fourth, some important outcomes, such as the quality of life and readmission rate, were not analyzed due to the lack of supporting data, resulting in a decrease in the integrity of this systematic review. Finally, approximately half of the enrolled patients were from China. Therefore, our findings should be judiciously considered in the clinical settings of other nations.
Conclusion
In conclusion, this systematic review of RCTs demonstrates that the implementation of an ERAS program in lung cancer surgery can significantly decrease the overall morbidity, pulmonary complications, surgical complications and hospitalization costs and shorten the LOS and length of ICU stay, without compromising patients’ safety. A worldwide consensus guideline is urgently required to standardize the ERAS protocols for elective lung resections in the future.
Acknowledgments
Special thanks to the Institution of Medical Statistics, West China School of Public Health, Sichuan University, Chengdu, China for its contributions to statistical analysis. This study was supported by the Foundation of Science and Technology support plan Department of Sichuan Province (2015SZ0158).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vázquez S, Casal J, Afonso Afonso FJ, et al. EGFR testing and clinical management of advanced NSCLC: a Galician Lung Cancer Group study (GGCP 048-10) Cancer Manag Res. 2016;8:11–20. doi: 10.2147/CMAR.S85173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi M, Rizvi SM, Belani CP. Afatinib for the treatment of metastatic non-small cell lung cancer. Cancer Manag Res. 2015;7:75–82. doi: 10.2147/CMAR.S51808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Wang Z, Huang J, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? Eur J Cardiothorac Surg. 2017;51(5):817–828. doi: 10.1093/ejcts/ezw386. [DOI] [PubMed] [Google Scholar]
- 4.Ghanem S, El Bitar S, Hossri S, Weerasinghe C, Atallah JP. What we know about surgical therapy in early-stage non-small-cell lung cancer: a guide for the medical oncologist. Cancer Manag Res. 2017;9:267–278. doi: 10.2147/CMAR.S139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas PA, Berbis J, Falcoz PE, et al. EPITHOR Group National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. 2014;45(4):652–659. doi: 10.1093/ejcts/ezt452. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Zhou K, Wang M, Lin R, Fan J, Che G. Degree of pulmonary fissure completeness can predict postoperative cardiopulmonary complications and length of hospital stay in patients undergoing video-assisted thoracoscopic lobectomy for early-stage lung cancer. Interact CardioVasc Thorac Surg. 2017 Aug 11; doi: 10.1093/icvts/ivx261. Epub. [DOI] [PubMed] [Google Scholar]
- 7.Fiore JF, Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg. 2016;151(3):708–15.e6. doi: 10.1016/j.jtcvs.2015.09.112. [DOI] [PubMed] [Google Scholar]
- 8.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86(2):227–230. doi: 10.1046/j.1365-2168.1999.01023.x. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248(2):189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 11.Schatz C. Enhanced recovery in a minimally invasive thoracic surgery program. AORN J. 2015;102(5):482–492. doi: 10.1016/j.aorn.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101(3):172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 16.Thomas PA, Berbis J, Baste JM, et al. EPITHOR group Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg. 2015;149(1):73–82. doi: 10.1016/j.jtcvs.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 18.Dong Q, Zhang K, Cao S, Cui J. Fast-track surgery versus conventional perioperative management of lung cancer-associated pneumonectomy: a randomized controlled clinical trial. World J Surg Oncol. 2017;15(1):20. doi: 10.1186/s12957-016-1072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Lai Y, Zhou X, et al. Short-term high-intensity rehabilitation in radically treated lung cancer: a three-armed randomized controlled trial. J Thorac Dis. 2017;9(7):1919–1929. doi: 10.21037/jtd.2017.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai Y, Su J, Yang M, Zhou K, Che G. Impact and effect of preoperative short-term pulmonary rehabilitation training on lung cancer patients with mild to moderate chronic obstructive pulmonary disease: a randomized trial. Chin J Lung Cancer. 2016;19(11):746–753. doi: 10.3779/j.issn.1009-3419.2016.11.05. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licker M, Karenovics W, Diaper J, et al. Short-term preoperative h-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12(2):323–333. doi: 10.1016/j.jtho.2016.09.125. [DOI] [PubMed] [Google Scholar]
- 22.Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg. 2008;34(1):174–180. doi: 10.1016/j.ejcts.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Sokouti M, Aghdam BA, Golzari SE, Moghadaszadeh M. A comparative study of postoperative pulmonary complications using fast track regimen and conservative analgesic treatment: a randomized clinical trial. Tanaffos. 2011;10(3):12–19. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao G, Huang Y, Chen X, et al. Research on fast track surgery application in lung cancer surgery. Chin J Lung Cancer. 2010;13(2):102–106. doi: 10.3779/j.issn.1009-3419.2010.02.04. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steenhagen E. Enhanced recovery after surgery: it’s time to change practice! Nutr Clin Pract. 2016;31(1):18–29. doi: 10.1177/0884533615622640. [DOI] [PubMed] [Google Scholar]
- 26.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Beamish AJ, Chan DS, Blake PA, Karran A, Lewis WG. Systematic review and meta-analysis of enhanced recovery programmes in gastric cancer surgery. Int J Surg. 2015;19:46–54. doi: 10.1016/j.ijsu.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 29.Zeng ZH, Chen JF, Li YX, Zhang R, Xiao LF, Meng XY. Induction regimens for transplant-eligible patients with newly diagnosed multiple myeloma: a network meta-analysis of randomized controlled trials. Cancer Manag Res. 2017;9:287–298. doi: 10.2147/CMAR.S138932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23(3):486–497. doi: 10.1093/icvts/ivw152. [DOI] [PubMed] [Google Scholar]
- 31.Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg. 2010;89(4):1327–1335. doi: 10.1016/j.athoracsur.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Li SJ, Zhou XD, Huang J, Liu J, Tian L, Che GW. A systematic review and meta-analysis-does chronic obstructive pulmonary disease predispose to bronchopleural fistula formation in patients undergoing lung cancer surgery? J Thorac Dis. 2016;8(7):1625–1638. doi: 10.21037/jtd.2016.05.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paci P, Madani A, Lee L, et al. Economic impact of an enhanced recovery pathway for lung resection. Ann Thorac Surg. 2017;104(3):950–957. doi: 10.1016/j.athoracsur.2017.05.085. [DOI] [PubMed] [Google Scholar]
- 34.Gao K, Yu PM, Su JH, et al. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: a study for 342 cases. Thorac Cancer. 2015;6(4):443–449. doi: 10.1111/1759-7714.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ERAS Society [homepage] [Accessed August 1, 2017]. www.erassociety.org.