Abstract
Introduction
Only a limited number of meta-analyses providing risk curve functions of dose–response relationships between various smoking-related variables and cancer-specific risk are available.
Methods and analysis
To identify all relevant original publications on the issue, we will conduct a series of comprehensive systematic reviews based on three subsequent literature searches: (1) an umbrella review, to identify meta-analyses, pooled analyses and systematic reviews published before 28 April 2017 on the association between cigarette smoking and the risk of 28 (namely all) malignant neoplasms; (2) for each cancer site, an updated review of original publications on the association between cigarette smoking and cancer risk, starting from the last available comprehensive review identified through the umbrella review; and (3) a review of all original articles on the association between cigarette smoking and site-specific cancer risk included in the publications identified through the umbrella review and the updated reviews. The primary outcomes of interest will be (1) the excess incidence/mortality of various cancers for smokers compared with never smokers; and (2) the dose–response curves describing the association between smoking intensity, duration and time since stopping and incidence/mortality for various cancers. For each cancer site, we will perform a meta-analysis by pooling study-specific estimates for smoking status. We will also estimate the dose–response curves for other smoking-related variables through random-effects meta-regression models based on a non-linear dose–response relationship framework.
Ethics and dissemination
Ethics approval is not required for this study. Main results will be published in peer-reviewed journals and will also be included in a publicly available website. We will provide therefore the most complete and updated estimates on the association between various measures of cigarette smoking and site-specific cancer risk. This will allow us to obtain precise estimates on the cancer burden attributable to cigarette smoking.
Trial registration number
This protocol was registered in the International Prospective Register of Systematic Reviews (CRD42017063991).
Keywords: cancer, risk, cigarette smoking, dose-response relationship, systematic review, meta-analysis
Strengths and limitations of this study.
This study represents the most complete and updated review on the association between cigarette smoking and site-specific cancer risk.
We will not conduct a systematic review of the entire scientific literature on the issue, but we will rather use an original and innovative approach combining an umbrella review and traditional systematic reviews.
We will carry out dose–response analyses using two-steps random-effects meta-regression models in order to examine potential non-linear relationships between smoking-related variables and the risk of cancer.
We will not systematically consider the assignment of a quality score to all original publications for each cancer site.
Introduction
In 1950, Ernst Wynder and Evards Graham1 and Richard Doll and Bradford Hill2 first reported an association between tobacco smoking and lung cancer risk. Over the subsequent 65 years, thousands of studies systematically confirmed this association and found that tobacco smoking also increases the risk of several other neoplasms.3 In 2004, tobacco smoking has been classified as carcinogenic to humans (group 1) by the International Agency for Research on Cancer (IARC),3 which provided evidence on the causal relationship between cigarette smoking and cancer of the lung, oral cavity, nasal cavity and paranasal sinuses, nasopharynx, oropharynx, hypopharynx, larynx, oesophagus, stomach, pancreas, liver, kidney, ureter, urinary bladder, cervix and myeloid leukaemia.3 The results on the association between (cigarette) smoking and other cancer sites, including cancers of the breast and endometrium, remain conflicting.3
Since the late 1980s, publications reporting results from systematic reviews and meta-analyses, the large majority of which were unnecessary and misleading, rapidly increased.4 Although the effects of cigarette smoking on cancer incidence and/or mortality have been largely investigated, only a limited number of systematic reviews/meta-analyses are available on the quantification of the dose–response relationship between selected smoking-related variables, including smoking intensity, duration, pack-years and time since quitting, and the risk of cancer. More importantly, just a few meta-analyses, if any, provided the cancer-specific risk curve functions of the dose–response relationships. Dose–response data, however, are crucial to provide reliable and accurate estimates of the cancer burden due to smoking, both at individual level—absolute cancer incidence/mortality obtained through lung cancer risk assessment models and risk charts5 6; and at population level—smoking-attributable deaths.7 8 Currently, the methods developed to quantify cancer burden due to smoking use cancer-specific estimates of relative risks (RR) according to tobacco smoking derived from a few large cohorts, mainly from the USA.9 The use of these cohorts to derive RRs may lead to validity problems when applying these estimates to other populations with different smoking patterns.7 10 Furthermore, these RRs are often estimated after allowance for a limited number of sociodemographic characteristics, excluding potentially important confounding variables, such as alcohol drinking.
Using an original and innovative approach, which combines an umbrella review11 and traditional systematic reviews, we aim at providing a comprehensive and updated picture of the association between various smoking-related variables and the risk of all cancers. We will be able to estimate the most robust cancer-specific RRs, obtained from the existing scientific literature, possibly derived after adjustment for relevant covariates. Moreover, for each cancer site, we will be able to provide the risk curves that best describe the dose–response effect of smoking intensity, smoking duration, pack-years and time since stopping smoking on cancer risk.
Methods
The present cancer-specific systematic reviews/meta-analyses will be based on the following three subsequent literature searches on the association between cigarette smoking and cancer risk:
umbrella review: a systematic review to identify published meta-analyses, pooled analyses and systematic reviews providing data on the association between cigarette smoking and cancer risk;
update of available cancer-specific reviews: for each cancer site, the conduction of a systematic review of studies providing original data (including pooled analyses on individual participants’ data) on the association between cigarette smoking and cancer-specific risk, starting from the last available comprehensive review publication identified through the umbrella review (point 1);
review of all original publications: a review of all original articles on the association between cigarette smoking and site-specific cancer risk included in the cancer-specific review publications identified through the umbrella review (point 1) or identified through the update of the available reviews (point 2).
The design of the present systematic reviews was developed following the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines12 and its extension for protocols (PRISMA-P).13 14 This protocol was registered in the International Prospective Register of Systematic Reviews on 4 May 2017 (registration number: CRD42017063991).
Umbrella review
Search strategy
We will conduct a systematic literature search to identify all published meta-analyses, pooled analyses and systematic reviews providing data on the association between cigarette smoking and the risk of various cancers. Literature search strategy will include combinations of medical subjects headings (MeSH) and text words related to cancer and tobacco or smoking, and will be restricted to the following publication types: meta-analyses, pooled analyses and systematic reviews. No restriction on cancer site or on publication date will be applied. The following databases will be used: MEDLINE, Embase, Institute for Scientific Information (ISI) Web of Science (WoS) and Cochrane Database of Systematic Reviews (CDSR). The search strings to be used in various databases are reported in (online supplementary appendix 1). To ensure literature saturation, reference lists of selected relevant publications identified through the search will also be checked. Besides the publications found through the databases searches, we will also consider the reviews of the literature on tobacco smoking provided within the IARC monographs volume 833 and volume 100E15 and the Surgeon General Report,16 three reports of known importance providing data on the association between tobacco smoking and various cancer sites.
bmjopen-2017-018930supp001.pdf (86KB, pdf)
Eligibility criteria
Study design
We will include meta-analyses, pooled analyses and systematic reviews of observational studies providing measures of RRs between cigarette smoking and cancer risk. Original observational studies (eg, case–control, cohort or cross-sectional studies) will be excluded. Reports, letters to the editor, book chapters, conference proceedings, dissertations and theses will not be considered.
Conditions
We will consider publications providing data on the following 28 (namely all) malignant neoplasms: cancer of lip, oral cavity and pharynx (International Classification of Diseases; ICD-10: C00-C14), nasopharynx (C11), oesophagus (C15), stomach (C16), colon (C18), rectum and anus (C19-C21), liver (C22), gall bladder (C23-C24), pancreas (C25), larynx (C32), lung trachea and bronchus (C33-C34), bone (C40-C41), melanoma of skin (C43), mesothelioma (C45), breast (C50), cervix uteri (C53), corpus uteri (C54), ovary (C56), prostate (C61), testis (C62), kidney (C64), bladder (C67), brain, central nervous system (C70-C72), thyroid (C73), Hodgkin’s lymphoma (C81), non-Hodgkin’s lymphoma (C82-C86, C96), multiple myeloma (C88-C90) and leukaemia (C91-C95). We will also consider review publications on groups of cancers (eg, head and neck, upper aerodigestive tract or intestinal cancers). Studies specifically based on benign neoplasms, such as colorectal polyps, acoustic neuroma and meningioma, and neuroendocrine tumours, will be excluded.
Participants
We will include review publications providing data on humans, in the general population. We will therefore exclude review publications based on patients with cancer or other diseases (ie, reporting data on the effect of smoking on the prognosis of the disease), or on subgroups of the population with selected lifestyle habits or other characteristics (eg, populations limited to alcohol drinkers or tobacco smokers). No restriction will be applied according to age of participants at cancer incidence or mortality given that practically all studies providing data on the association between cigarette smoking and cancer risk are based on adults only.
Exposures
We will include all review publications providing data on the use of cigarettes in the general population. Publications focused on the use of tobacco products other than cigarettes (eg, pipe, smokeless tobacco, cigar, water pipe, electronic cigarettes) or on the exposure to secondhand smoke will be excluded.
Outcomes
The primary outcomes of interest will be (1) the excess incidence and/or mortality of various cancers in current/former/ever smokers compared with never smokers; and (2) the dose–response curves describing the association between cigarette smoking duration, intensity and time since stopping and incidence and/or mortality for various cancers.
Languages
We will include only articles published in English language.
Study selection
All the review publications found in various electronic databases through the above-mentioned search strategy will be uploaded in an EndNote library (EN1), and duplicate records will be deleted. Titles and/or abstracts of the meta-analyses, pooled analyses and systematic reviews will be screened independently by two reviewers (AL, GP) to exclude publications that will not meet the eligibility criteria. The full text of the remaining review publications will be retrieved and independently assessed for eligibility by the two reviewers. Discrepancies between the two reviewers will be discussed and solved. In case of disagreement, a third reviewer (SG) will help to find a final decision. Other available reports will also be integrated in the same EN1 library.
Quality assessment
Assessment of the quality of various review publications is out of the scope of the present systematic review. Thus, no quality score will be assigned to the publications. No review publication will be excluded a priori for weakness of design or data quality.
Data extraction and management
A standardised form in Microsoft (MS) Excel will be used to extract data from each identified review publication. Relevant information will include first author, year of publication, type of study (ie, meta-analysis, pooled analysis or systematic review), cancer site(s) and/or subsite(s), endpoint (ie, incidence and/or mortality), and other information about the methodology of studies included in the reviews (eg, study design, country(ies) of study conduction, number of studies considered in and overall population size). Data will be extracted from each included meta-analysis, pooled analysis or systematic review by one reviewer and verified by a second reviewer. Any disagreement between the two reviewers will be solved by consensus. Otherwise, a third reviewer will help to find a final decision. After data extraction, publications will be grouped according to the considered cancer site.
Preliminary umbrella review
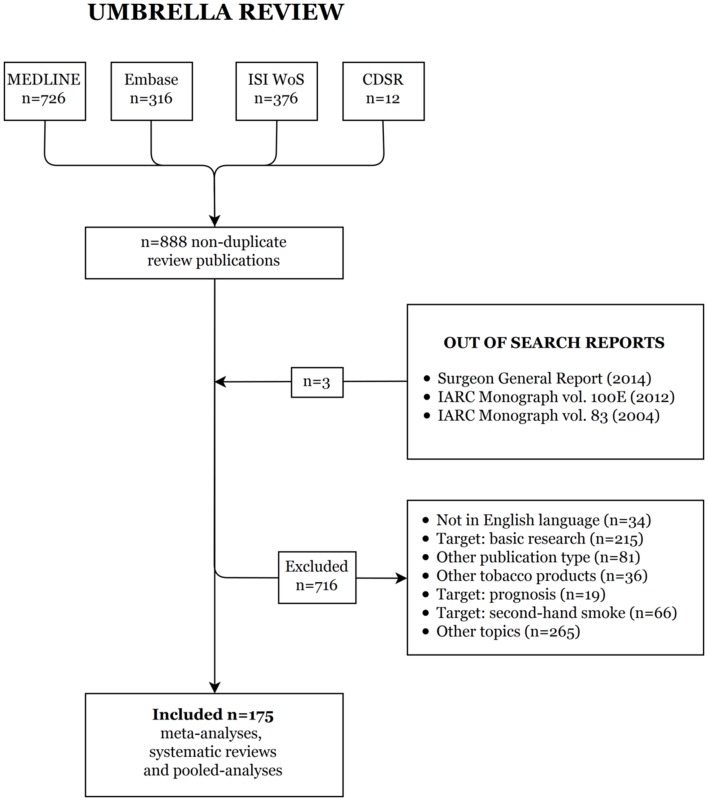
In April 2017, we already conducted a preliminary umbrella review through which we identified the meta-analyses, pooled analyses and systematic reviews on the association between smoking and cancer risk published before 28 April 2017. Within the comprehensive literature search, we found a total of 1430 publications from the four considered databases (726 from MEDLINE, 316 from Embase, 376 from ISI WoS and 12 from CDSR; figure 1). After the exclusion of duplicates (n=542) and ineligible papers (n=716), and after the inclusion of three important reports,3 15 16 we obtained a total of 175 relevant publications (ie, 107 meta-analyses, 52 pooled analyses and 16 systematic reviews) on the association between smoking and risk of various neoplasms.
Figure 1.
Flow chart for the selection of papers on tobacco smoking and cancer risk (published before 28 April 2017) in the umbrella review. CDSR, Cochrane Database of Systematic Reviews; IARC, International Agency for Research on Cancer; ISI WoS, Institute for Scientific Information Web of Science.
Update of the available cancer-specific reviews
Search strategy
For each of the 28 cancer sites previously described in the umbrella review, we will identify the last available comprehensive systematic review or meta-analysis on the association with cigarette smoking. We will then update the identified cancer-specific review through the conduction of a systematic literature search on all observational studies (eg, case–control, cohort and nested case–control studies) providing original data on the association between cigarette smoking and site-specific cancer risk, and published after the year of publication of the most recent article included in the last comprehensive review. Only studies published in English language will be considered.
The literature search strategy will be conducted in Medline and Embase, and will include combinations of MeSH terms and text words related to site-specific cancer and tobacco or smoking (see online supplementary appendix 2).
Eligibility criteria
Study design
We will include original observational studies (eg, case–control, cohort, nested case–control studies or pooled analysis of individual participant data) providing measures of RRs of the association between cigarette smoking and site-specific cancer risk. Reports, book chapters, conference proceedings, dissertations and theses will not be considered. We will exclude case–control studies using patients with cancer or other chronic diseases as comparison group.
Comparator
Never smokers are our comparators. We will in fact consider, as the measure of association, the RRs of smoking-related variables compared with never smokers and we will therefore exclude the RRs considering non-smokers (never and former smokers combined) as reference category.
Eligibility criteria for conditions, participants, exposures and outcomes are those reported also for the umbrella review.
Study selection
For each cancer site, we will upload all the original publications found using the above-mentioned search strategy in cancer-specific EndNote libraries (EN2_1-EN2_28), and we will delete the identified duplicates. Titles and/or abstracts of original articles will be screened independently by two reviewers (AL, GP) to exclude publications that do not meet the inclusion criteria outlined above. The full text of the remaining original publications will be retrieved and independently assessed for eligibility by the two reviewers. Discrepancies on the assessment between the two reviewers will be discussed and solved. In case of disagreement, a third reviewer (SG) will help to find a final decision.
Review of all original publications
For each cancer site, we will upload in an EndNote library (EN3_1-EN3_28) all the original publications obtained from the 28 cancer-specific reviews identified in the umbrella review (point 1). In the same EndNote libraries, we will add the original publications obtained from the corresponding updates of the reviews (point 2), and duplicate publications will be deleted. The full text of all the retained original publications will be retrieved. Non-English reports, unpublished studies, conference proceedings, dissertations and theses will be excluded.
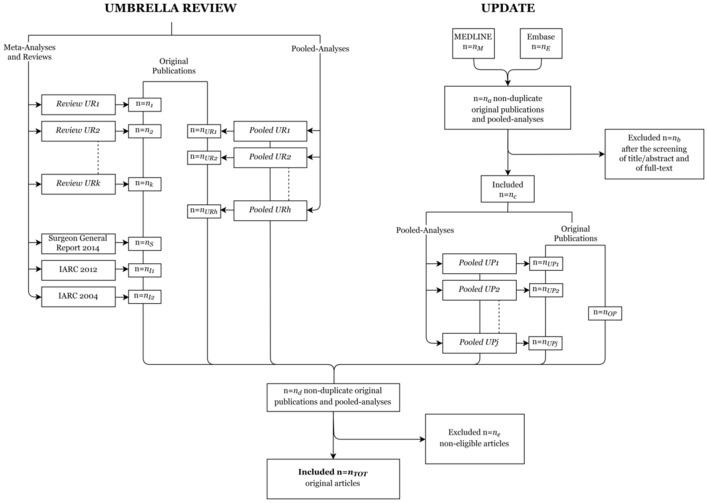
Figure 2 shows the flow chart we will consider for each of the 28 cancer-specific reviews. For each cancer site, original publications from both the umbrella review and the update of the reviews will be considered.
Figure 2.
Flow chart for each of the 28 cancer-specific reviews on tobacco smoking. IARC, International Agency for Research on Cancer.
Quality assessment
Although quality assessment of original publications is out of the scope of the present systematic review, we do not exclude the possibility, at least for selected neoplasms, to assign a quality score to the original publications in order to conduct sensitivity analyses excluding the publications with a relatively low quality. In this case, the quality (risk of bias) for each observational study will be assessed using the Newcastle-Ottawa Scale for observational studies.17
Data extraction and management
For each cancer site, two standardised forms in MS Excel will be used to collect relevant information on the study design and the risk estimates from the original publications. A first form will be used to extract data related to the study design, including first author, year of publication, journal, country, study name, period and design of study, outcome and sample size. In the second form we will collect the exposure categories (ie, smoking status, intensity, duration, pack-years, age at starting, time since stopping) and corresponding RR estimates (or other estimates, such as ORs and HRs) and 95% confidence intervals (CI). The number of cases in each exposure category and covariates used in the model will be also collected. When the results of the same study have been published in more than one original publication, only data from the most recent and/or complete article will be retained and reported in the second Excel form.
Data analysis
For each cancer site, we will pool all the RRs or other risk estimates (eg, HRs and ORs) in order to obtain the association between smoking status (separately for current, former and ever compared with never smokers) and the risk of cancer. Because cancer is a relatively rare outcome, we assume that ORs, risk ratios and rate ratios are all comparable estimates of the RR. Heterogeneity between studies will be assessed using the Cochran Q test and the I2 statistics, that is, the proportion of total variation contributed by between-study heterogeneity.18 As we anticipated between-study heterogeneity, we will present pooled RRs from random-effects models using the DerSimonian and Laird moment estimator of the between-study variance component.19 However, if no heterogeneity between study is detected, pooled estimates from the random-effects model will correspond to those deriving from the fixed-effect model.
When RRs are not reported for ever smokers, but only separately for current and former smokers, we will use the method for pooling non-independent estimates described by Hamling et al20 to obtain RRs for ever smokers besides those for current and former compared with never smokers. This method uses the number of subjects exposed to different levels of smoking and non-exposed subjects, and the corresponding risk estimates and 95% CIs, to derive a set of pseudo-numbers of cases and controls/subjects at risk, by taking into account the correlation between the original estimates due to the common reference group. The obtained pseudo-numbers will be used to compute the new adjusted RRs and 95% CIs. This methodology will also be used to convert RR estimates when the reference category considered in the study is not represented by never smokers.
For smoking intensity, duration and time since stopping smoking, we will compute pooled RRs according to various categories of the considered smoking-related variables (eg, low, intermediate, high cigarette consumption). Moreover, we will carry out dose–response analyses using two-steps random-effects meta-regression models in order to examine potential non-linear relationships between those variables and the risk of cancer. In particular, we will consider a method providing the best fitting two-term fractional polynomial model,21 and a method modelling the considered smoking-related variables using restricted cubic splines.22
If the necessary data are available, we will consider to further conduct separate analyses by sex, study period, geographical area and income (defined on the basis of per capita gross domestic product).
Analyses will be conducted using SAS V.9.4 software and R V.3.3.0 software (R Development Core Team, 2008), in particular meta and dosresmeta packages.
Ethics and dissemination
Ethics
This review does not require approval from an ethics committee since no individual-level patients’ data will be collected.
Implications and dissemination
Through these systematic reviews and meta-analyses, we will provide the most complete and updated estimates on the association between cigarette smoking and site-specific cancer risk. These estimates will be used to quantify the cancer burden due to cigarette smoking at both individual and population levels. This information is essential to guide policy decisions to control tobacco smoking and improve cancer prevention.
Given the relevance and originality of this project, we plan to publish results from the meta-analyses in peer-reviewed journals, considering either single cancer sites or various apparatus or tracts. A final publication will provide the summary results for all cancers. We will also include the main results of our systematic reviews and meta-analyses in a publicly available website. Readers will have the possibility to contact us to communicate possible lacks or updates.
Supplementary Material
Footnotes
Contributors: SG and AL had the original idea of the work. AL, GP and SG structured the study protocol, designed the search strategies and conducted the umbrella review. MR, VB and CB provided statistical and epidemiological supervision. All authors critically revised the draft of the manuscript and approved its final version.
Funding: This work was partially supported by the Italian League Against Cancer (Milan). AL was supported by a fellowship from the Italian Association for Cancer Research (AIRC). MR was supported by a fellowship from the Italian Foundation for Cancer Research (FIRC).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc 1950;143:329–36. [DOI] [PubMed] [Google Scholar]
- 2.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J 1950;2:739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC. Tobacco Smoke and Involuntary Smoking IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 83. Lyon, France: International Agency for Research on Cancer, 2004. [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis JP. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q 2016;94:485–514. 10.1111/1468-0009.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitz MR, Hong WK, Amos CI, et al. . A risk model for prediction of lung cancer. J Natl Cancer Inst 2007;99:715–26. 10.1093/jnci/djk153 [DOI] [PubMed] [Google Scholar]
- 6.Tammemägi MC, Katki HA, Hocking WG, et al. . Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728–36. 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Ríos M, Montes A. Methodologies used to estimate tobacco-attributable mortality: a review. BMC Public Health 2008;8:22 10.1186/1471-2458-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tachfouti N, Raherison C, Obtel M, et al. . Mortality attributable to tobacco: review of different methods. Arch Public Health 2014;72:22 10.1186/2049-3258-72-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACS. Cancer Prevention Study II (CPS II). Atlanta, Georgia: American Cancer Society, 2016. http://www.cancer.org/research/researchtopreventcancer/currentcancerpreventionstudies/cancer-prevention-study. [Google Scholar]
- 10.Gallus S, Muttarak R, Martínez-Sánchez JM, et al. . Smoking prevalence and smoking attributable mortality in Italy, 2010. Prev Med 2011;52:434–8. 10.1016/j.ypmed.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009;181:488–93. 10.1503/cmaj.081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Shamseer L, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamseer L, Moher D, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 15.IARC. A review of human carcinogens - Personal habits and indoor combustions IARC monograph on the evaluation of carcinogenic risks to humans volume 100E. Lyon, France: International Agency for Research on Cancer, 2012. http://monographs.iarc.fr/ENG/Monographs/vol100E/mono100E.pdf [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. The Health Consequences of Smoking - 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa: The Ottawa Hospital Research Institute, 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20.Hamling J, Lee P, Weitkunat R, et al. . Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 21.Rota M, Bellocco R, Scotti L, et al. . Random-effects meta-regression models for studying nonlinear dose-response relationship, with an application to alcohol and esophageal squamous cell carcinoma. Stat Med 2010;29:2679–87. 10.1002/sim.4041 [DOI] [PubMed] [Google Scholar]
- 22.Orsini N, Li R, Wolk A, et al. . Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018930supp001.pdf (86KB, pdf)