Abstract
Objectives
With the present study, we aimed to investigate the association between menopausal hormone therapy (HT) and risk of colorectal cancer (CRC).
Setting
Cohort study based on the linkage of Norwegian population-based registries.
Participants
We selected 466822 Norwegian women, aged 55–79, alive and residing in Norway as of 1 January 2004, and we followed them from 2004 to 2008. Each woman contributed person-years at risk as non-user, current user and/or past HT user.
Outcome measures
The outcome of interest was adenocarcinoma of the colorectal tract, overall, by anatomic site and stage at diagnosis. Incidence rate ratios (RRs) with 95% CIs were estimated by Poisson regression and were used to evaluate the association between HT and CRC incidence.
Results
During the median follow-up of 4.8 years, 138 655 (30%) women received HT and 3799 (0.8%) incident CRCs occurred. Current, but not past, use of HT was associated with a lower risk of CRC (RR 0.88; 95% CI 0.80 to 0.98). RRs for localised, regionally advanced and metastatic CRC were 1.13 (95% CI 0.91 to 1.41), 0.81 (95% CI 0.70 to 0.94) and 0.79 (95% CI 0.62 to 1.00), respectively. RRs for current use of oestrogen therapy (ET) were 0.91 (95% CI 0.80 to 1.04) while RR for current use of combined oestrogen–progestin therapy (EPT) was 0.85 (95% CI 0.70 to 1.03), as compared with no use of HT. The same figures for ET and EPT in oral formulations were 0.83 (95% CI 0.68 to 1.03) and 0.86 (95% CI 0.71 to 1.05), respectively.
Conclusions
In our nationwide cohort study, HT use lowered the risk of CRC, specifically the most advanced CRC.
Keywords: menopausal hormone therapy, colorectal cancer, estrogens, progestins
Strengths and limitations of this study.
Our cohort study, based on a linkage between nationwide registries in Norway, provided strong evidence showing that use of hormone therapy (HT) is associated with a reduced risk of colorectal cancer (CRC).
HT had no impact on localised CRC but it protected against regionally advanced CRC and even more strongly against metastatic CRC. We therefore hypothesised that HT might play a key role in the inhibition of cancer progression.
For the first time, we showed that oestrogens—in oral formulations—were associated with a decreased risk of CRC in a dose–response fashion.
The main strength of our study is that the registry linkages ensured detailed information on exposure of HT, including type of HT, with no risk of self-selection of women to participate.
However, we did not have information on recognised risk factors for CRC (eg, family history of CRC, body mass index, physical activity, diet, alcohol use and smoking) or information on aspirin use, so we could not adjust our estimates for those factors.
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in females and the third in males worldwide, with estimated 1.4 million cases and 700 000 deaths occurring globally in 2012.1 The detection and removal of precancerous lesions through CRC screening and the intervention on modifiable risk factors for CRC, such as diet, alcohol consumption, physical activity and tobacco smoking, can reduce both CRC incidence and mortality.2 3 Currently, new preventive strategies are being explored through different medications, aspirin being the most promising.4 In addition to aspirin, menopausal hormone therapy (HT) has been suggested to reduce CRC risk. A 2012 meta-analysis of 4 clinical trials and 16 observational studies found that use of HT was associated with a 20%–30% lower risk of CRC.5 Moreover, a 2016 Danish nationwide cohort study involving 1 million women showed that the use of HT was associated with approximately a 15% reduction in CRC risk.6 Nevertheless, results from the Women’s Health Initiative (WHI) clinical trial were not supportive of the protective effect of HT on CRC. Among women with no uterus, there was no difference in the risk of CRC between women who took oestrogen therapy (ET) and those who took the placebo.7 Among women with an intact uterus, women who received combined oestrogen–progestin therapy (EPT) had a lower risk of CRC than women who took the placebo. However, the CRCs that occurred in the treatment group were more advanced at detection than those in the placebo group,8 suggesting that use of HT might simply delay CRC diagnosis.
Given these conflicting results, the association between use of menopausal HT and the risk of CRC remains controversial. With the present nationwide cohort study, based on the linkage of population-based registries, our aim was to supply new evidence on the association between HT and risk of CRC. We present results on the association between different types, routes of administration and doses of HT on the risk of CRC, overall, by anatomic site and stage at diagnosis.
Patients and methods
Cohort characteristics and definition of exposure to HT were described in detail elsewhere.9 Briefly, an 11-digit unique personal identification number allowed univocal linkage between different national Norwegian registries. We linked information about year and month of birth, immigration and emigration status, death, cause of death, education level and municipality of residence (Statistics Norway and the Population Registry), redeemed prescriptions (the Norwegian Prescription Database) and cancer cases (the Cancer Registry of Norway).
We included data from 466 822 women born in Norway between 1925 and 1949, alive and residing in Norway as of 1 January 2004 (aged 55–79 years), who did not have a CRC or any other cancer diagnosis before 1 January 2004. Women were followed until 31 December 2008.
Research reporting checklists
The present article follows the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for research reporting of observational studies.
Exposure to HT
We retrieved data on use of menopausal hormone therapy (Anatomical Therapeutic Chemical (ATC) group G03) in the period 2004–2008. We did not have any data on prescriptions before 2004. Duration of HT use was estimated for each different type of drug as number of total treatment days, calculated from the package size multiplied by the number of packages prescribed regarding the dosing intervals recommended. The estimated duration of HT use was extended by 4 months to account for prolonged HT use beyond the treatment days prescribed. If there were gaps of more than 4 months between HT exposures, women contributed person-years at risk as a previous user from the date that the estimated duration of HT use ended, until the next redeemed prescription date if any, or end of the study period. Women receiving prescriptions of sex hormones other than ET, EPT or tibolone, such as oral contraceptives and progestogen only, were censored at the date of prescription.
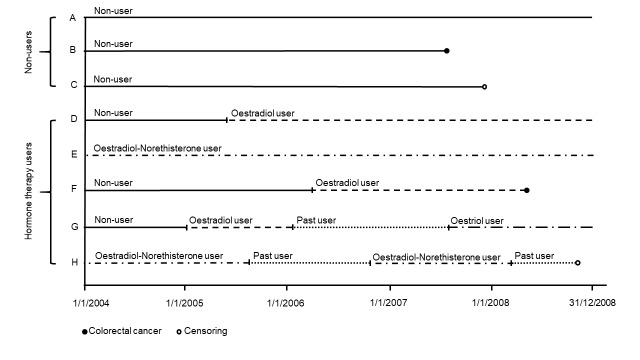
Women were included in the various types of HT preparation categories based on the specific product dispensed (figure 1). Women who switched from one type of HT to another (eg, from oestradiol to oestriol) contributed person-years at risk to the specific product dispensed. When studying the effect of the different hormone types on CRC incidence, women who redeemed at least two simultaneous prescriptions of different hormone types were classified in the ‘other’ category. The same approach was used when studying the route of administration. Women were classified as ET users if they redeemed only ET prescriptions, and EPT users if they redeemed only EPT prescriptions during the follow-up. All combined regimens of oestrogen–progestin available in Norway contain oestradiol and norethisterone acetate. Use of other progestin types, such as medroxyprogesterone acetate or dienogest, is almost non-existent in Norway.
Figure 1.
Follow-up of study participants.
All women in the study population contributed person-years at risk as a non-user, a current user and/or a past HT user (figure 1). Person-years at risk were calculated from start of the study period, 1 January 2004, until event, censoring or end of follow-up. Women contributed person-years at risk as current users according to the accumulated duration of treatment for the type of HT dispensed. If there were gaps of more than 4 months between prescriptions, women contributed person-years at risk as a past user from the date that the estimated duration of HT use ended, until the next redeemed prescription date, if any, or end of the study period. Non-users contributed person-years at risk from 1 January 2004 until the date of the first redeemed prescription, if any, event, censoring or end of follow-up.
Outcome
The outcome of interest was adenocarcinoma of the colorectal tract (topography codes C18–C20 according to the International Classification of Diseases, Tenth Revision, Clinical Modification). CRC with histology other than adenocarcinoma (ie, small cell carcinoma, squamous cell carcinoma, carcinoid, sarcoma, gastrointestinal stromal tumour and lymphoma) were not analysed as CRC cases and were censored at diagnosis.
Statistical analysis
Incidence rate ratios (RRs) with 95% CIs were estimated by Poisson regression. The number of incident CRCs was analysed as a log-linear function of exposure time, HT use, analysed as a time-dependent variable (figure 1) and adjusting covariates. Women were censored at death, emigration, any tumour diagnosis, prescription of sex hormones other than ET, EPT or tibolone or end of follow-up (31 December 2008), whichever came first. We adjusted HT estimates for age in years, number of births (nulliparous, 1, 2, 3 and ≥4), highest level of education (elementary, high school, university or higher, and missing) and marital status (not married, married or partnered, widowed, and divorced or separated) registered at the beginning of follow-up and use of antihypertensive drugs (ATC groups C02, C03, C07–C09), antidiabetic drugs (A10), statins (C10) and thyroid therapy (H03) registered anytime during follow-up. Time on study was used as timescale in the Poisson regression and split into 1-year time intervals assuming a constant risk of CRC within each interval. At the beginning of each interval, age of all women was updated. In each analysis, the reference group was non-users of HT. When analysing the association of HT with CRC stage at diagnosis, only CRCs at a specific stage were analysed as events, while CRCs at other stages were analysed as censoring events. When analysing the association of HT with cancer diagnosed in a specific site of the colorectal tract (eg, left colon), only cancer diagnosed in that specific site were analysed as events, while others were analysed as censoring events.
We evaluated the oestrogen and progestin dose–response effect by limiting analyses to current oral ET and oral EPT users and non-users. The dose of oestrogen and the dose of progestin were obtained from each prescription of oral ET and EPT. Doses of oestrogens and progestins in non-users were set to zero. The dose of oestrogen and the dose of progestins were entered simultaneously in the multivariable models as two continuous variables.
All tests were two sided with a 5% significance level. Statistical analyses were performed using SAS V.9.4 (SAS Institute) and R software (http://cran.r-project.org/).
Results
We followed 466 822 women born in Norway and with no previous history of cancer from 2004 to 2008. During the follow-up, which had a median duration of 4.8 years, 3 799 CRCs occurred. A total of 138 655 (30%) women used HT. Characteristics of the study population were not homogeneously distributed between HT users and non-users, and between ET users and EPT users (table 1). Notably, ET users were substantially older than EPT users (median age was 64.0 and 60.0 years, respectively; P<0.001).
Table 1.
Characteristics of the study population by HT use
| HT non-users, n (%) |
HT users,* n (%) |
P value | ET users,* n (%) |
EPT users,* n (%) |
P value | ||
| All women | 328 167 | 138 655 | 79 195 | 30 455 | |||
| Number of CRC | 3020 (0.92) | 779 (0.56) | 434 (0.55) | 202 (0.66) | |||
| Age† | Median (IQR) | 65.0 (59–72) | 62.0 (57–67) | <0.001 | 64.0 (58–70) | 60.0 (57–64) | <0.001 |
| Highest education† | Elementary school | 127 238 (38.8) | 42 317 (30.5) | <0.001 | 26 455 (33.4) | 8592 (28.2) | <0.001 |
| High school | 143 564 (43.7) | 68 401 (49.3) | 38 197 (48.2) | 15 684 (51.5) | |||
| University and higher | 40 899 (12.5) | 27 189 (19.6) | 14 094 (17.8) | 6013 (19.7) | |||
| Missing | 16 466 (5.0) | 748 (0.5) | 449 (0.6) | 166 (0.5) | |||
| Number of children† | 0 | 45 536 (13.9) | 10 857 (7.8) | 0.004 | 5984 (7.6) | 2715 (8.9) | <0.001 |
| 1 | 39 595 (12.1) | 15 761 (11.4) | 8731 (11.0) | 3685 (12.1) | |||
| 2 | 106 742 (32.5) | 55 416 (40.0) | 29 982 (37.9) | 12 795 (42.0) | |||
| 3 | 81 622 (24.9) | 37 495 (27.0) | 21 784 (27.5) | 8059 (26.5) | |||
| >3 | 54 672 (16.7) | 19 126 (13.8) | 12 714 (16.1) | 3201 (10.5) | |||
| Marital status† | Single | 27 218 (8.3) | 5129 (3.7) | <0.001 | 2770 (3.5) | 1427 (4.7) | <0.001 |
| Married/partnered | 154 016 (46.9) | 80 077 (57.8) | 44 774 (56.5) | 17 361 (57.0) | |||
| Widow | 103 202 (31.4) | 31 982 (23.1) | 21 460 (27.1) | 5400 (17.7) | |||
| Divorced/separated | 43 731 (13.3) | 21 467 (15.5) | 10 191 (12.9) | 6267 (20.6) | |||
| Antihypertensives* | User | 163 131 (49.7) | 69 572 (50.2) | 0.004 | 42 166 (53.2) | 13 688 (45.5) | <0.001 |
| Antidiabetics* | User | 23 988 (7.3) | 7748 (5.6) | <0.001 | 5207 (6.6) | 1274 (4.2) | <0.001 |
| Statins* | User | 100 863 (30.7) | 42 646 (30.8) | 0.886 | 27 821 (35.1) | 7036 (23.1) | <0.001 |
| Thyroid therapy* | User | 38 511 (11.7) | 20 948 (15.1) | <0.001 | 12 334 (15.6) | 4160 (13.7) | <0.001 |
*Prescribed anytime during the follow-up.
†Registered at baseline.
CRC, colorectal cancer; EPT, combined oestrogen–progestin therapy; ET, oestrogen therapy; HT, hormone therapy.
Current use of HT was associated with a decreased risk of CRC compared with non-use, with a RR of 0.88 (95% CI 0.80 to 0.98; table 2).
Table 2.
Use of HT and risk of colorectal cancer
| HT use | PY | CRC cases | RR (95% CI) | |
| Status | Non-use | 2126 753 | 3020 | Reference |
| Current use | 320 202 | 441 | 0.88 (0.80 to 0.98) | |
| Past use | 203 759 | 338 | 0.98 (0.87 to 1.09) | |
| Ever use | 523 961 | 779 | 0.92 (0.85 to 1.00) | |
| HT type | Non-use | 2126 753 | 3020 | Reference |
| ET* | 159 495 | 252 | 0.91 (0.80 to 1.04) | |
| ET (oestradiol)* | 118 910 | 159 | 0.87 (0.74 to 1.03) | |
| ET (oestriol)* | 40 585 | 93 | 0.98 (0.79 to 1.21) | |
| Tibolone* | 20 043 | 21 | 0.86 (0.56 to 1.32) | |
| EPT* | 91 654 | 106 | 0.85 (0.70 to 1.03) | |
| Other* | 49 010 | 62 | 0.86 (0.67 to 1.10) | |
| Route | Non-use | 2126 753 | 3020 | Reference |
| ET oral* | 57 031 | 94 | 0.83 (0.68 to 1.03) | |
| ET vaginal* | 89 719 | 134 | 0.92 (0.77 to 1.09) | |
| ET transdermal* | 7246 | 15 | 1.63 (0.98 to 2.71) | |
| EPT oral* | 90 126 | 106 | 0.86 (0.71 to 1.05) | |
| EPT transdermal* | 1163 | 0 | – | |
| Other | 74 917 | 92 | 0.86 (0.70 to 1.06) | |
| Oral dose | Oestrogen 1 mg/day* | 0.87 (0.73 to 1.04) | ||
| Unit increase | Progestin 10 mg/month* | 1.01 (0.86 to 1.19) |
Incidence RRs were adjusted for age, number of births, highest level of education, marital status, use of antihypertensives, antidiabetics, statins and thyroid therapy.
*Current use.
CRC, colorectal cancer; EPT, combined oestrogen–progestin therapy; ET, oestrogen therapy. The italic font denotes subtypes of ET; HT, hormonal therapy; PY, person-years; RR, rate ratio.
The same figure for past and ever use (current or past users) was 0.98 (95% CI 0.87 to 1.09) and 0.92 (95% CI 0.85 to 1.00). RRs for current use of ET and EPT versus non-use were 0.91 (95% CI 0.80 to 1.04) and 0.85 (95% CI 0.70 to 1.03), respectively.
From each prescription of oral ET and EPT, we retrieved the information on the administered dose of oestrogens and progestins. Mean oestrogen doses in oral ET and EPT treatments were 1.40 and 1.36 mg/day, respectively. Mean progestin dose in oral EPT users was 18.3 mg/month. We analysed the dose effect of oral oestrogen and progestin as continuous variables on CRC risk, and we found that oestrogens were associated with a decreased risk of CRC in a dose–response fashion, even if the result was not statistically significant (RR 0.87 for each additional mg/day; 95% CI 0.74 to 1.03; table 2) while progestins showed no effect. We then repeated the analysis to estimate the dose effect of oestrogens on CRC risk after censoring EPT users at time of a first use of EPT, to avoid a possible interference of progestins, and the RR estimate for each additional mg/day of oestrogens was 0.88 (95% CI 0.74 to 1.04).
In table 3 we reported the association between HT intake and CRC diagnosed at different stages: 698 localised, 2023 regionally advanced and 737 metastatic CRCs.
Table 3.
Use of HT and risk of CRC by stage
| HT use | PY | Localised CRC |
RR (95% CI) | Regionally advanced CRC | RR (95% CI) | Metastatic CRC |
RR (95% CI) | |
| Status | Non-use | 2126 753 | 548 | Reference | 1607 | Reference | 598 | Reference |
| Current use | 320 202 | 101 | 1.13 (0.91 to 1.41) | 216 | 0.81 (0.70 to 0.94) | 78 | 0.79 (0.62 to 1.00) | |
| Past use | 203 759 | 49 | 0.79 (0.59 to 1.06) | 200 | 1.08 (0.93 to 1.26) | 61 | 0.90 (0.69 to 1.18) | |
| Ever use | 523 961 | 150 | 0.99 (0.82 to 1.20) | 416 | 0.92 (0.83 to 1.03) | 139 | 0.83 (0.69 to 1.01) | |
| HT type | Non-use | 2126 753 | 548 | Reference | 1607 | Reference | 598 | Reference |
| ET* | 159 495 | 67 | 1.33 (1.03 to 1.72) | 125 | 0.84 (0.70 to 1.02) | 34 | 0.64 (0.45 to 0.91) | |
| ET (oestradiol)* | 118 910 | 44 | 1.36 (0.99 to 1.85) | 75 | 0.78 (0.62 to 0.98) | 22 | 0.60 (0.39 to 0.93) | |
| ET (oestriol)* | 40 585 | 23 | 1.27 (0.83 to 1.94) | 50 | 0.97 (0.73 to 1.30) | 12 | 0.73 (0.41 to 1.29) | |
| Tibolone* | 20 043 | 5 | 1.20 (0.50 to 2.90) | 12 | 0.93 (0.52 to 1.64) | 4 | 0.75 (0.28 to 2.01) | |
| EPT* | 91 654 | 17 | 0.79 (0.49 to 1.29) | 56 | 0.84 (0.64 to 1.10) | 24 | 0.91 (0.60 to 1.37) | |
| Other* | 49 010 | 12 | 0.94 (0.53 to 1.66) | 23 | 0.60 (0.40 to 0.90) | 16 | 1.09 (0.67 to 1.80) | |
| Route | Non-use | 2126 753 | 548 | Reference | 1607 | Reference | 598 | Reference |
| ET oral* | 57 031 | 24 | 1.15 (0.76 to 1.73) | 48 | 0.79 (0.59 to 1.06) | 13 | 0.63 (0.36 to 1.10) | |
| ET vaginal* | 89 719 | 36 | 1.36 (0.97 to 1.91) | 67 | 0.86 (0.68 to 1.10) | 17 | 0.59 (0.37 to 0.96) | |
| ET transdermal* | 7246 | 6 | 3.80 (1.70 to 8.50) | 7 | 1.44 (0.69 to 3.04) | 1 | 0.51 (0.07 to 3.65) | |
| EPT oral* | 90 126 | 17 | 0.81 (0.50 to 1.31) | 56 | 0.86 (0.65 to 1.12) | 24 | 0.86 (0.61 to 1.39) | |
| EPT transdermal* | 1163 | 0 | – | 0 | – | 0 | - | |
| Other* | 74 917 | 18 | 0.96 (0.60 to 1.54) | 38 | 0.67 (0.49 to 0.93) | 23 | 1.05 (0.69 to 1.60) | |
| Oral dose | Oestrogen 1 mg/day* | 1.13 (0.82 to 1.57) | 0.75 (0.57 to 0.98) | 0.97 (0.67 to 1.39) | ||||
| Unit increase | Progestin 10 mg/month* | 0.80 (0.57 to 1.13) | 1.11 (0.88 to 1.41) | 1.02 (0.74 to 1.42) | ||||
Incidence RRs were adjusted for age, number of births, highest level of education, marital status, use of antihypertensives, antidiabetics, statins and thyroid therapy.
*Current use.
CRC, colorectal cancer; EPT, combined oestrogen–progestin therapy; ET, oestrogen therapy. The italic font denotes subtypes of ET; HT, hormonal therapy; PY, person-years; RR, rate ratio.
Compared with non-use, current use of HT was associated with a decreased risk of regionally advanced (RR 0.81; 95% CI 0.70 to 0.94) and metastatic CRC (RR 0.79; 95% CI 0.62 to 1.00), but not of localised CRC (RR 1.13; 95% CI 0.91 to 1.41).
In online supplementary table 1 we reported the association between HT and risk of CRC diagnosed in different sites of the colorectal tract. RRs for the association of current use of HT with colon cancer, right colon cancer, left colon cancer and rectal cancer were 0.88 (95% CI 0.78 to 0.99), 0.89 (95% CI 0.77 to 1.04), 0.85 (95% CI 0.69 to 1.04) and 0.90 (95% CI 0.75 to 1.09), respectively.
bmjopen-2017-017639supp001.pdf (183.5KB, pdf)
We repeated the main analyses after censoring the CRC cases that occurred in the first year of follow-up (2004), and results were stronger than in the main analysis (online supplementary table 2). RRs for use of HT, ET, EPT, oral ET and oral EPT were 0.83 (95% CI 0.74 to 0.93), 0.86 (95% CI 0.75 to 1.00), 0.74 (95% CI 0.59 to 0.92), 0.72 (95% CI 0.57 to 0.92) and 0.75 (95% CI 0.60 to 0.94), respectively, compared with no use. Finally, oestrogens were significantly associated with a decreased risk of CRC in a dose–response fashion (RR 0.79 for each additional mg/day; 95% CI 0.64 to 0.96).
bmjopen-2017-017639supp002.pdf (21.8KB, pdf)
Discussion
In this Norwegian nationwide cohort study, we evaluated the effect of menopausal HT on CRC incidence. Our results suggest that the current use of HT is associated with a reduced risk of CRC, specifically the most advanced CRC. Current users of any HT had a 12% reduction of CRC, 19% reduction of regionally advanced CRC and 21% reduction of metastatic CRC. Furthermore, we found that, in current users, the risk of CRC decreased with increasing doses of oral oestrogens.
Colorectal polyps and tumours occur more frequently in men than in women, and many preclinical and clinical studies have provided evidence that female sex hormones, specifically oestrogen, might form the basis for the protective effect in women.10 Researchers have found that the oestrogen receptor beta (ERβ) regulates DNA repair, increases apoptosis and reduces cell proliferation, and that ERβ activation can consequently reduce tumour occurrence and inhibit progression.11–14 Consistent evidence showed an inverse relationship between ERβ expression in the colon and the presence and stage of colorectal polyps and tumours.15–19 The possible protective effect of HT was evaluated in many observational studies and two clinical trials, with conflicting results. Current use of ET was associated with a 30% decreased CRC risk in a meta-analysis published in 20125 and a 23% reduction of colon cancer and 17% reduction of rectal cancer in a recent nationwide registry-based study among 1 million Danish women.6 In contrast to those findings, a lack of association was reported in 136 000 postmenopausal women in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.20 The only placebo-controlled clinical trial on the subject, the WHI, included 10 739 women with hysterectomy, showed no difference in either the risk of CRC or the stage of disease at diagnosis between women who took oestrogen alone and those who took the placebo.7 The effect of EPT use on CRC risk is also controversial. In the 2012 meta-analysis,5 current use of EPT was associated with a significant 20% reduction of CRC, and in the Danish study,6 it was associated with a significant 12% reduction of colon cancer and 11% reduction of rectal cancer. In the EPIC cohort, a non-significant 6% risk reduction due to EPT use was reported.20 In the WHI, among the 16 608 postmenopausal women with intact uterus, authors reported that EPT was associated with a significant 28% reduction of CRC after 5.6 years of intervention (11.6 years of follow-up). However, EPT was associated with more advanced CRC, and the investigators concluded that their findings did not support a clinically meaningful benefit for EPT on CRC.8 They hypothesised a potential CRC diagnostic delay due to EPT-related conditions, such as vaginal bleeding. The discrepancies observed in the literature might be explained by several factors, including the different designs (clinical trials, case–control studies and cohort studies) and methods of HT exposure assessment (eg, self-reported vs registry-based) used in the different studies.5
Our study provides new evidence on the protective effect of HT use against CRC. For the first time, we also found that increasing doses of oral oestrogens, and not progestins, were associated with decreasing risk of CRC. Altogether these results might indicate that oestrogens reduce the risk of CRC, while progestins have no effect. In support of our findings, a recent study showed that the risk of CRC decreased with increasing levels of endogenous oestrogen while it did not depend on progesterone levels.21
Our results could be interpreted to support the hypothesis that HT inhibits cancer progression, rather than formation. In our study, use of HT had no impact on localised CRC (RR=1.13) but it protected against regionally advanced CRC (RR=0.81) and metastatic CRC (RR=0.79). Similarly, in the Iowa Women’s Health Study, the RR estimates for ever versus never use of HT by stage were 0.91 for localised, 0.78 for regional and 0.72 for distant disease.22 In the California Teachers Study, current HT use versus baseline non-use was associated with these RRs: 0.99 for localised, 0.68 for regional and 0.33 for distant disease.23 Results from the Danish study showed that HT had a stronger impact on metastatic rather than non-metastatic CRC,6 and other authors reported that HT users were significantly more likely to be diagnosed at an earlier disease stage as compared with HT non-users.24 25
In the 2012 meta-analysis5 and the 2016 Danish study,6 HT was associated with lower risk of colon cancer but less so with rectal cancer. In our study, we found similar estimate for colon and rectal cancer. Within the colon tract, we found similar estimates for left and right colon cancer. More studies are warranted to understand whether HT has different effects in CRC depending on the anatomical location.
Our study has several strengths. The registry linkages ensured detailed information on exposure of HT, including type of HT. There was no self-selection of women to participate, and the large size of the study population provided a large number of incident CRCs. However, our study has important limitations. First, we did not have information on recognised risk factors for CRC (eg, family history of CRC, body mass index, physical activity, diet, alcohol use and smoking) or information on aspirin use. Some authors showed no significant effect of those factors on the association between HT and CRC risk,23 24 26 but in the California Teachers Study, HT use was more strongly associated with CRC risk among women with a family history of CRC.23 In addition, our estimates could be affected by the healthy user bias: it is probable that HT users were more concerned about their health than non-users and, for example, underwent more bowel examinations or had a better lifestyle. This bias could have resulted in overestimation of the HT protective effect. In fact we found that HT users had a higher education level than non-users, and education is positively associated with general good health and use of medical services.27 However, the fact that HT had no effect on risk of early stage CRC and strong effect on risk of advanced stage CRC indicates no healthy user bias, as more health conscious women are likely to have CRC detected in earlier rather than later stages. Finally, given the relatively short follow-up of our study, we were not able to evaluate the influence of duration of HT use on CRC risk, as other authors did.6
In conclusion, we provided evidence that use of HT is associated with a reduced risk of CRC, in particular advanced CRC. The effect was similar for ET and EPT in women of age 55 years or older.
Supplementary Material
Acknowledgments
We would like to acknowledge Margrethe Meo for administrative assistance and Marta Román for data linkage and management.
Footnotes
Contributors: Study concept and design: EB, SS, NCS, SG-I, GU, SV, EW. Acquisition of data: SS, SG-I, SH, GU, SV. Analysis of data: EB, NCS, SS, SG-I. Statistical analysis of data: EB, NCS, VB. Interpretation of data: all. Drafting of the manuscript: EB. Critical revision of the manuscript for important intellectual content: SS, SG-I, SH, TdL, GU, SV, EW.
Competing interests: None declared.
Ethics approval: The study was approved by the regional ethics committee in the South East region of Norway, and concession to data linkage was granted by the Norwegian Data Protection Authority.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Authors are willing to share any data that are not published in the manuscript. Please contact the corresponding author.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467 10.1136/bmj.g2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43. 10.1053/j.gastro.2010.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016;16:173–86. 10.1038/nrc.2016.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin KJ, Cheung WY, Lai JY, et al. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer 2012;130:419–30. 10.1002/ijc.26026 [DOI] [PubMed] [Google Scholar]
- 6. Mørch LS, Lidegaard Ø, Keiding N, et al. The influence of hormone therapies on colon and rectal cancer. Eur J Epidemiol 2016;31:481–9. 10.1007/s10654-016-0116-z [DOI] [PubMed] [Google Scholar]
- 7. Lavasani S, Chlebowski RT, Prentice RL, et al. Estrogen and colorectal cancer incidence and mortality. Cancer 2015;121:3261–71. 10.1002/cncr.29464 [DOI] [PubMed] [Google Scholar]
- 8. Simon MS, Chlebowski RT, Wactawski-Wende J, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol 2012;30:3983–90. 10.1200/JCO.2012.42.7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Román M, Sakshaug S, Graff-Iversen S, et al. Postmenopausal hormone therapy and the risk of breast cancer in Norway. Int J Cancer 2016;138:584–93. 10.1002/ijc.29810 [DOI] [PubMed] [Google Scholar]
- 10. Williams C, DiLeo A, Niv Y, et al. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett 2016;372:48–56. 10.1016/j.canlet.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu Y, Waters CE, Lewis AE, et al. Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta. J Endocrinol 2002;174:369–77. 10.1677/joe.0.1740369 [DOI] [PubMed] [Google Scholar]
- 12. Hartman J, Edvardsson K, Lindberg K, et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res 2009;69:6100–6. 10.1158/0008-5472.CAN-09-0506 [DOI] [PubMed] [Google Scholar]
- 13. Martineti V, Picariello L, Tognarini I, et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr Relat Cancer 2005;12:455–69. 10.1677/erc.1.00861 [DOI] [PubMed] [Google Scholar]
- 14. Hsu HH, Cheng SF, Wu CC, et al. Apoptotic effects of over-expressed estrogen receptor-beta on LoVo colon cancer cell is mediated by p53 signalings in a ligand-dependent manner. Chin J Physiol 2006;49:110–6. [PubMed] [Google Scholar]
- 15. Konstantinopoulos PA, Kominea A, Vandoros G, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer 2003;39:1251–8. 10.1016/S0959-8049(03)00239-9 [DOI] [PubMed] [Google Scholar]
- 16. Barone M, Scavo MP, Papagni S, et al. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol 2010;45:1320–8. 10.3109/00365521.2010.487915 [DOI] [PubMed] [Google Scholar]
- 17. Di Leo A, Barone M, Maiorano E, et al. ER-beta expression in large bowel adenomas: implications in colon carcinogenesis. Dig Liver Dis 2008;40:260–6. 10.1016/j.dld.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 18. Jassam N, Bell SM, Speirs V, et al. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol Rep 2005;14:17–21. [PubMed] [Google Scholar]
- 19. Nüssler NC, Reinbacher K, Shanny N, et al. Sex-specific differences in the expression levels of estrogen receptor subtypes in colorectal cancer. Gend Med 2008;5:209–17. 10.1016/j.genm.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 20. Tsilidis KK, Allen NE, Key TJ, et al. Menopausal hormone therapy and risk of colorectal cancer in the European prospective investigation into cancer and nutrition. Int J Cancer 2011;128:1881–9. 10.1002/ijc.25504 [DOI] [PubMed] [Google Scholar]
- 21. Murphy N, Strickler HD, Stanczyk FZ, et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst 2015;107:djv210 10.1093/jnci/djv210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Limsui D, Vierkant RA, Tillmans LS, et al. Postmenopausal hormone therapy and colorectal cancer risk by molecularly defined subtypes among older women. Gut 2012;61:1299–305. 10.1136/gutjnl-2011-300719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delellis Henderson K, Duan L, Sullivan-Halley J, et al. Menopausal hormone therapy use and risk of invasive colon cancer: the California Teachers Study. Am J Epidemiol 2010;171:415–25. 10.1093/aje/kwp434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slattery ML, Anderson K, Samowitz W, et al. Hormone replacement therapy and improved survival among postmenopausal women diagnosed with colon cancer (USA). Cancer Causes Control 1999;10:467–73. 10.1023/A:1008974215622 [DOI] [PubMed] [Google Scholar]
- 25. Newcomb PA, Chia VM, Hampton JM, et al. Hormone therapy in relation to survival from large bowel cancer. Cancer Causes Control 2009;20:409–16. 10.1007/s10552-008-9255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kampman E, Potter JD, Slattery ML, et al. Hormone replacement therapy, reproductive history, and colon cancer: a multicenter, case-control study in the United States. Cancer Causes Control 1997;8:146–58. 10.1023/A:1018459911147 [DOI] [PubMed] [Google Scholar]
- 27. Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff 2002;21:60–76. 10.1377/hlthaff.21.2.60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-017639supp001.pdf (183.5KB, pdf)
bmjopen-2017-017639supp002.pdf (21.8KB, pdf)