Abstract
A 70-year-old Indian woman, who had undergone primary bilateral total knee arthroplasty (TKA) for rheumatoid arthritis 10 months prior, presented with 10 days history of pain, swelling and erythema over both knees with pus discharging from the right knee. She had type 2 diabetes mellitus and was on long-term steroid, leflunomide and antitumour necrosis factor therapy for rheumatoid arthritis. Her clinical and laboratory features were suggestive of a haematogenous periprosthetic joint infection (PJI). The final diagnosis of bilateral Salmonella typhi PJI was made based on culture reports. Considering her underlying immunosuppression, a bilateral two-stage revision TKA was done with complete remission of symptoms and good functional recovery at last follow-up after 18 months. S. typhi infection of prosthetic joint has not been reported in the literature. Patients presenting with gastrointestinal complaints and PJI should alert the clinician to the possibility of infection with such atypical organisms endemic to the region.
Keywords: bone and joint infections, foodborne infections, biological agents, rheumatoid arthritis, orthopaedic and trauma surgery
Background
The incidence of periprosthetic joint infection (PJI) is around 0.3%–1.7% following a primary total hip arthroplasty (THA) and 0.8%–2% after a primary total knee arthroplasty (TKA).1 2 Staphylococcus species (Staphylococcus aureus and coagulase-negative species like S. epidermidis and S. saprophyticus) account for more than 50% of the cases. PJI due to Gram-negative bacilli account for less than 6% of cases,2 Escherichia coli and Pseudomonas being the most common organisms identified. Salmonella, a Gram-negative bacilli, belonging to the genus Enterobacteriaceae is a rare cause of PJI (42 cases with 44 joints reported to date) and is mostly seen in patients with underlying immunosuppression.3 Salmonella PJI (S-PJI) of the hip is commoner (32 cases reported) than that of the knee (11 cases with 12 knees reported to date) and is almost always unilateral. Non-typhoid Salmonella (NTS) strains are the causative organism in 98% (43/44) of these cases. Salmonella enteritidis and S. typhimurium, both belonging to the NTS group account for 61% (27/44) of S-PJIs reported in literature. Despite its endemicity affecting a large subgroup of the world population, Salmonella typhi, the causative organism for typhoid fever, is an extremely rare cause of PJI. In fact, to our knowledge, no case of S. typhi PJI (St-PJI) has been reported in the literature. We report a case of St-PJI involving bilateral knees 10 months after bilateral TKA in a 70-year-old Indian woman with rheumatoid arthritis and type 2 diabetes mellitus. Unique characteristics of Salmonella and its implications in deciding the mode of treatment of such cases are discussed.
Case presentation
A 70-year-old Indian woman presented with sudden onset of pain, swelling and restricted movements over both the knees for the past 10 days. She had undergone bilateral single-stage cemented TKA 10 months prior to this and her postoperative period was uneventful. She developed intermittent episodes of fever with chills, abdominal distension and loose stools over the previous 1 month and had taken a 5-day course of norfloxacin–tinidazole 2 weeks prior to the onset of joint symptoms. She had rheumatoid arthritis diagnosed for the past 15 years which had been treated with 5 mg prednisolone and 10 mg leflunomide once a day along with 40 mg adalimubab injection once a week for the past year. Other significant medical history includes type 2 diabetes mellitus controlled with oral hypoglycaemics (latest haemoglobin A1c 5.8), hypothyroidism since 10 years (on daily 100 μg thyroxine) and recently diagnosed hypertension treated with diltiazem (calcium channel blocker) 90 mg daily.
Physical examination revealed local warmth, tenderness and erythema over both the knees with active pus discharge and erythema was noticed over the proximal third of the right knee incision site. The range of motion was severely restricted to 30°–40° in both knees. She was febrile with a temperature of 37.5°C. The patient was hospitalised.
Investigations
Fresh radiographs of both the knees showed radiolucent lines under the tibial base plate on both the sides (figure 1). Haematological examination revealed a haemoglobin of 11.1 g/dL, white cell count (WCC) of 15.8×109/L with 76% neutrophils and 16% lymphocytes, low total serum protein 5.5 g/dL (reference value: 6.4–8.2 g/dL) and albumin 2.9 g/dL (reference value: 3.4–5 g/dL), erythrocyte sedimentation rate (ESR) 85 mm/hour and C reactive protein (CRP) 96 mg/L (normal <6 mg/L).
Figure 1.
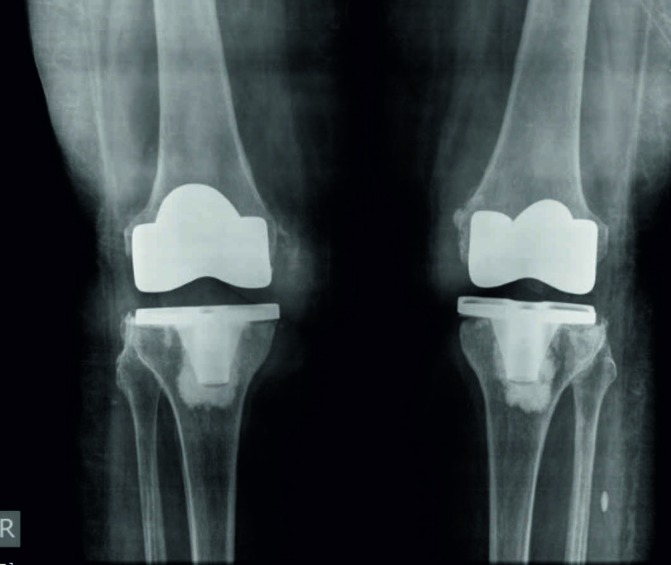
Bilateral peri-prosthetic joint infection following total knee arthroplasty due to Salmonella typhi—radiographs showing relatively well-fixed components with radiolucent lines under the tibial base plate.
On aspiration of both the knee joints, a seropurulent slightly reddish fluid was obtained from both the knees with a WCC of 13.2×109/L with 94% neutrophils on the right and 11.5×109/L with 90% neutrophils on the left. Gram staining revealed Gram-negative bacilli which after 2 days of culture was reported to be S. typhi, which was sensitive to cephalosporins, trimethoprim–sulfamethoxazole, chloramphenicol and fluoroquinolones. Stool and blood cultures were negative. Ultrasound examination of the abdomen was unremarkable with no hepatosplenomegaly and normal gallbladder.
Treatment
A two-staged revision with aggressive local debridement and removal of the previous implant was done. The right knee was operated first followed by the left knee after 2 days. The prosthesis was found to be well-fixed with no evidence of loosening on both the sides. An antibiotic-impregnated static cemented spacer (containing gentamicin) was used for both the sides and was kept in situ for 5 weeks. Five intraoperative samples were collected from each side including the joint fluid and biopsy samples from synovial tissue, granulation tissue under the femoral and tibial components and deep tibial intramedullary tissue. S. typhi was detected from three samples from the right and two from the left. The patient was kept on injectable third-generation cephalosporins (injection ceftriaxone 2 g for every 24 hours and oral ciprofloxacin (500 mg for every 12 hours) for 4 weeks. The surgical wounds were healthy with no evidence of local infection. Her ESR and CRP showed a decreasing trend and were 9 mm/hour and 2 mg/L at 5 weeks, respectively. The second stage of revision was carried out using a condylar constrained prosthesis (Legacy Constrained Condylar Knee LCCK; NexGen, Zimmer) 5 weeks after the first stage of revision (figure 2). Intraoperative frozen tissue biopsy revealed neutrophil count <5 cells/high power field. Culture from tissue samples obtained during the second stage did not detect any organism. Oral ciprofloxacin was continued for 6 weeks postoperatively.
Figure 2.
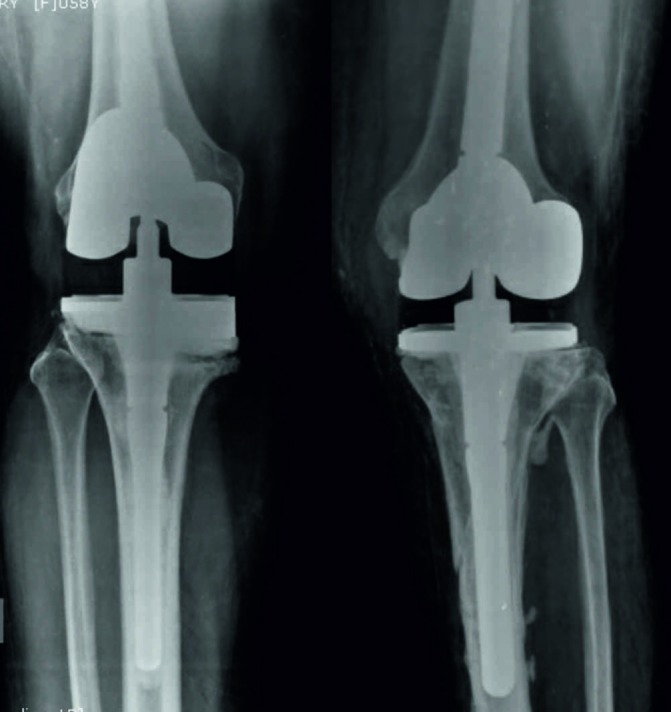
Postoperative radiographs of both knees following two-stage revision total knee arthroplasty.
Outcome and follow-up
The patient recovered well from the surgery and was able to ambulate using a walking stick after 3 months postoperatively. At her last follow-up at 18 months, there was no local warmth over the knees and the swelling and erythema had subsided completely. She was capable of independent ambulation and stair-climbing with a flexion range of 0°–100° on both knees. The patient died at 20 months after the second-stage revision due to an unrelated cardiovascular event.
Discussion
Salmonella infection of the prosthetic joints is a rare entity. The genus is classified as typhoid Salmonella (causative agents of enteric fever, includes S. enterica serovar Typhi and S. enterica serovar Paratyphi A, B, C) and non-typhoid Salmonella (NTS, which includes the rest of the strains).4
S-PJI is a rare entity. Literature review has revealed 32 cases of S-PJI following THA and 11 cases (with 12 knees) following TKA.5 6 NTS are the most common and almost exclusive causative organisms. Seventeen (11 hip and 6 knees) out of a total of 44 S-PJIs have been caused by the NTS strain of S. enterica serovar enteritidis while S. typhimurium was isolated from 6 (five hip and one knee) of the 44 cases. To the best of our knowledge, no case of S. typhi has been reported in the literature to date. S-PJI usually affects a single joint and bilateral involvement is extremely rare with only one case of unilateral hip and knee affection5 and one case of bilateral knee7 involvement reported in literature. The higher frequency of NTS strains causing PJI may be due to its global distribution and the fact that bacteraemia and extraintestinal manifestations are more common with NTS (seen in 5% of infected cases).4 8
A few important characteristic features of the bacteria deserve mention in order to understand its pathogenicity to guide further management. Salmonella is a Gram-negative enteroinvasive bacilli and a facultative anaerobe capable of intracellular survival within macrophages. The ability of the bacteria to invade non-phagocytic cells (including the intestinal epithelium) enables it to cause haematogenous seeding of distant organs.4 Involvement of the musculoskeletal system in salmonellosis is seen in the form of reactive arthritis (ReA, most common joint manifestation), abscess formation, osteomyelitis and Salmonella septic arthritis (SSA) of the native or prosthetic joint.4 An important property of Salmonella is the ability to form biofilm which helps in survival in the outside environment by adhering to plants, abiotic surfaces such as metal (prosthesis), plastics or glass and other food products.9 Chronic carrier state is said to be 1%–4% and one reason is due to the ability to survive by biofilm formation.4 The biofilm forms are more resistant to antibiotic therapy in vivo though they may demonstrate susceptibility in the laboratory. Hence, retention of implant may lead to persistence of infection in S-PJIs. Addition of biofilm-active drugs such as fluoroquinolones10 may be necessary. Widmer et al11 have reported a case where a PJI of hip due to Salmonella dublin failed prolonged treatment with oral co-trimoxazole and was eventually cured after addition of oral ciprofloxacin though recurrence was seen after 9 years.
S. typhi infection is a notifiable disease and humans are the only reservoir. Case reports of S. typhi infection of native joints causing SSA, usually in young children and immunocompromised adults,12–14 have been well reported.
The patient in our report belonged to India which is endemic for S. typhi. The incidence of S. typhi is estimated to be approximately 100 cases for 100 000 inhabitants per year in Southcentral and Southeast Asia.4 15 In a recent systematic review, the pooled estimate of typhoid burden in India was found to be 377 (178–801) per 100 000 person-years for typhoid.16 The incidence is significantly lower in the developed countries with a reported incidence of 0.31 cases per 100 000 in the year 2014 in the European Union countries, and predominantly acquired during travel to endemic countries particularly South Asia.17
As in the rest of the world, the numbers of TKA surgeries are increasing exponentially in India. PJI is the second most common cause of revision as reported in the Indian Joint Registry accounting for 26% of the cases of revision.18 This case study demonstrates the importance of recognising the fact that as the number of total knee replacements increase in the Indian subcontinent, investigations for causative organisms for PJI should include the effort to look for atypical tropical organisms endemic to this region, such as S. typhi. It is important to recognise that a part of the population undergoing a joint replacement in this community may be carriers for and prone to infection with the said organism. Also, with a rise in arthroplasty cases in the region, the population at risk for haematogenous PJI with S. typhi is also subject to increase, a possibility that should be kept in mind. Hence, preceding episodes of diarrhoea with fever and abdominal symptoms in the setting of immunosuppression should alert the clinician about the possibility of PJI with enteric bacteria such as S. typhi as one of the uncommon and rare causative organism. Other differential diagnoses include PJI due to Campylobacter, Clostridium difficile and Yersinia enterocolitica which may present with a similar picture.6
It is important to differentiate between ReA and SSA as the management differs—symptomatic treatment with anti-inflammatory medications and rest for ReA while aggressive approach is necessary in SSA or S-PJI. Oral and long-term antibiotics have less role in treating the former condition19 and association with HLA-B27 is seen in about 40%–90% of cases in some reports.20 21 The incidence of ReA following Salmonella gastroenteritis is reported to be between 1.2% and 7.3%,20 22 most commonly due to S. typhimurium (60%) and S. enteritidis (25%),20 is due to an immunogenic phenomenon and may present with slight arthralgia to severe disabling disease. The diagnosis is by the detection of specific antibodies from joint fluid using agglutination tests (Widal test), enzyme immunoassay, immunofluorescence or immunoblotting techniques.
SSA and S-PJI is most commonly seen in patients with history of immunosuppression due to underlying disease (diabetes mellitus, HIV) or following immunosuppressive treatment (eg, for rheumatoid arthritis, renal transplant recipients, connective tissue or haematological disorders, etc).5 Patients on anti-tumour necrosis factor (TNF) alpha therapy, such as infliximab,23 etanercept24 and certolizumab,25 are particularly susceptible due to reduction in the ability to kill the intracellular pathogens. The patient in our case report had been on adalimubab (Humira), a chimeric monoclonal anti-TNF antibody. A high degree of suspicion and the possibility of extraintestinal salmonellosis should be kept in mind while dealing with patients with PJI receiving anti-TNF therapy.
There is no consensus regarding the ideal treatment modality in case of S-PJI. Such cases are rare and varied outcomes following treatment have been reported. Cohen et al26 have reported better outcomes with Salmonella septic arthritis than with other Gram-negative organisms. Among the 11 cases (12 knees) of S-PJI involving TKA (table 1), three were treated with open debridement and antibiotic therapy with implant retention, another three underwent exchange of the polyethylene component, one with arthroscopic debridement with implant retention, one treated with only long-term antibiotics, two underwent a two-stage revision surgery and one case following failure of antibiotic therapy underwent prosthesis removal and subsequent arthrodesis. Failure was seen in the case treated with only antibiotics27 and chronic antibiotic suppression was required in the case reported by Kobayashi et al7 with bilateral knee prosthesis involvement treated with debridement and poly-exchange.
Table 1.
Reported cases of Salmonella PJI involving the knee in the literature
| Study | Age/sex | Underlying diseases | Age of prosthesis at presentation | Species of Salmonella detected | Treatment | Duration of follow-up | Outcome |
| Rae et al27 (1977) | 67/F | RA | 5 years | S. typhimurium | Chloramphenicol and amoxycillin with retention of prosthesis, followed by chronic antibiotic suppression with amoxycillin | NS | Required chronic antibiotic suppression |
| Boland et al29 (1999) | 51/F | RA on methotrexate and azathioprine | NS | S. enteritidis | Multiple closed drainage with needle aspiration and intravenous ceftriaxone for 4 weeks | 1 year | Uneventful follow-up |
| Madan et al30 (2001) | 75/F | RA on long-term oral steroids | 8 years | S. enteritidis | Ciprofloxacin for 6 weeks—flaring up of symptoms after 15 months with detection of Salmonella—ciprofloxacin continued for 3 months | NS | Asymptomatic until last follow-up* |
| Day et al28 (2002) | 55/M | OA, type 2 DM | 12 days | S. enteritidis | Open debridement, poly-exchange and retention of prosthesis followed by 6 weeks of ceftriaxone therapy | 6 years | No recurrence |
| Musante et al31 (2004) | 63/F | OA, history of gout | 8 weeks | S. typhimurium | Open irrigation and debridement with poly-exchange followed by 6 weeks of ceftazidime | 15 months | Cured |
| Miron et al32 (2006) | 75/M | NS | NS | S. enteritidis | Open debridement, prosthesis retention followed by 3 weeks of intravenous ceftriaxone and 3 months of oral ciprofloxacin | NS | Cured |
| Kobayashi et al7 (2008) | 71/F | RA on steroids, methotrexate, HCQ, azathioprine | Bilateral knee involvement Right—11 years, Left—6 years | S. enteritidis | Open debridement and poly-exchange with long-term antibiotic suppression with continuing oral ciprofloxacin until last follow-up | 30 months | Persistent warmth over joints, no other evidence of infection |
| Kenichi et al24 (2009) | 61/M | RA on long-term steroids, methotrexate and etanercept | 5 weeks | S. enteritidis | Arthroscopic debridement, implant retention followed by intravenous meropenem and oral levofloxacin for 2 weeks and oral minocycline for 3 months | 3 years | Cured, etanercept resumed 12 months after surgery |
| Carlile et al33 (2010) | 71/M | NS, revision surgery done for PJI after 3 years (no organism isolated), pyoderma gangrenous on long-term steroids | 2 years after revision surgery | S. choleraesuis | Two-stage revision surgery followed by intravenous cefotaxime for 1 week and oral ciprofloxacin for 3 weeks | 1 year | Cured |
| De la Torre et al5 (2012) | 72/M | RA, on prednisone and methotrexate | 10 months | S. enteritidis | Two-stage revision at first. Recurrence of infection after 9 months with simultaneous hip prosthetic joint involvement. Two-stage hip and knee revision carried out. |
3 ½ years | Cured |
| Gupta et al6 (2014) | RA, bladder cancer | 3 years | S. enteritidis | Initially aspiration followed by oral TMP-SMX for 12 weeks—failed treatment in 3 months—open debridement, prosthesis removal and arthrodesis | 15 years | Cured |
DM, diabetes mellitus; F, female; HCQ, hydroxycloroquine; M, male; NS, not specified; OA, osteoarthritis; PJI, prosthetic joint infection; RA, rheumatoid arthritis; TMP-SMX, trimethoprim–sulfamethoxazole.
Earlier, Day et al28 have reported that 9 out of 12 patients with S-PJI involving the hip (10 cases) and knee (2 cases) required removal of at least one or more components of the prosthesis to achieve favourable outcomes. In an extensive literature review of S-PJI, de la Torre et al5 have shown that the outcome following removal of part of prosthesis or complete replacement was uniformly favourable. They reported a case of recurrent knee PJI following two-stage revision with concomitant hip PJI with S. enteritidis. They advocated that an aggressive approach and two-stage revision should be undertaken especially in patients with compromised immune status. Gupta et al6 identified six patients with S-PJI from a 44-year study period of which five were following THA and one following TKA. They noted that all four patients treated without removal of implant failed treatment within a median time of 2.5 months (range 2–11) following commencement of treatment. They concluded that prosthesis removal was associated with higher rates of cure and implant retention and debridement alone had higher failure rates.
Among all the 42 cases (44 joints) of S-PJI reported to date, a documented two-stage revision was carried out in only nine cases. Of this, only one patient undergoing two-stage revision had recurrence of symptoms.5 A two-stage revision using antibiotic spacer yielded good result bilaterally in our case with no recurrence of symptoms and good functional outcome.
Learning points.
The possibility of Salmonella typhi prosthetic joint infection (St-PJI) should be kept in mind and investigated for in patients belonging to endemic regions or with history of recent travel to endemic countries. The likelihood increases in those with history of immunosuppression and/or with preceding gastrointestinal symptoms.
The ideal treatment in cases of Salmonella PJI should be individualised based on the duration of symptoms, age of the patient and systemic comorbidities and also if there is associated loosening of the implant. Open or arthroscopic debridement is usually not sufficient in these cases with higher rates of recurrences.
In patients with lesser duration of symptoms (<3–4 weeks), open debridement with exchange of polyethylene component can be attempted. The risk of persistence of infection and the need for prolonged antibiotic therapy should be explained to the patient.
The susceptibility of Salmonella species to antibiotics should be identified since strains with reduced susceptibility to first-line drugs and multidrug resistance is increasingly prevalent in the developing countries.
Considering the virulence of Salmonella and its ability to form biofilms with reports of late recurrences occurring upto 9 years postrevision,11 a two-stage revision should be considered as the gold standard of treatment and should be attempted as the definitive modality of treatment.
Footnotes
Contributors: All authors have made significant contributions in the conceptualisation, data collection, conducting a thorough review of literature, drafting and proof-reading of the article.The individual contributions of the authors are as mentioned below: AR: planning, conceptualisation, preparing the manuscript, proof-reading and approval of final version. IP: detailed literature review, writing the manuscript and data collection. AG: drafting the article, proof-reading, analysis of literature.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pulido L, Ghanem E, Joshi A, et al. . Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710–5. 10.1007/s11999-008-0209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 2009;361:787-94 10.1056/NEJMcp0905029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandiford JA, Higgins GA, Blair W. Remote salmonellosis: surgical masquerader. Am Surg 1982;48:54–8. [PubMed] [Google Scholar]
- 4.Sánchez-Vargas FM, Abu-El-Haija MA, Gómez-Duarte OG. Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Dis 2011;9:263–77. 10.1016/j.tmaid.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 5.de la Torre B, Tena D, Arias M, et al. . Recurrent prosthetic joint infection due to Salmonella enteritidis: case report and literature review. Eur J Orthop Surg Traumatol 2012;22:89–97. 10.1007/s00590-012-0955-6 [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Berbari EF, Osmon DR, et al. . Prosthetic joint infection due to Salmonella species: a case series. BMC Infect Dis 2014;14:633 10.1186/s12879-014-0633-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi H, Hall GS, Tuohy MJ, et al. . Bilateral periprosthetic joint infection caused by Salmonella enterica serotype Enteritidis, and identification of Salmonella sp using molecular techniques. Int J Infect Dis 2009;13:e463–e466. 10.1016/j.ijid.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 8.Khan MI, Ochiai RL, von Seidlein L, et al. . Non-typhoidal Salmonella rates in febrile children at sites in five Asian countries. Trop Med Int Health 2010;15:960–3. 10.1111/j.1365-3156.2010.02553.x [DOI] [PubMed] [Google Scholar]
- 9.Peng D. Dhanasekaran D, Biofilm Formation of Salmonella, Microbial Biofilms - Importanceand Applications, 2016. https://www.intechopen.com/books/microbial-biofilms-importance-and-applications/biofilm-formation-of-salmonella.
- 10.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645–54. 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 11.Widmer AF, Colombo VE, Gächter A, et al. . Salmonella infection in total hip replacement: tests to predict the outcome of antimicrobial therapy. Scand J Infect Dis 1990;22:611–8. 10.3109/00365549009027105 [DOI] [PubMed] [Google Scholar]
- 12.Chiu S, Chiu CH, Lin TY, et al. . Septic arthritis of the hip caused by Salmonella typhi. Ann Trop Paediatr 2001;21:88–90. 10.1080/02724930020028993 [DOI] [PubMed] [Google Scholar]
- 13.Yang WC, Huang YC, Tsai MH, et al. . Salmonella septic arthritis involving multiple joints in a girl with acute lymphoblastic leukemia at diagnosis. Pediatr Neonatol 2009;50:33–5. 10.1016/S1875-9572(09)60027-9 [DOI] [PubMed] [Google Scholar]
- 14.Pocock JM, Khun PA, Moore CE, et al. . Septic arthritis of the hip in a Cambodian child caused by multidrug-resistant Salmonella enterica serovar Typhi with intermediate susceptibility to ciprofloxacin treated with ceftriaxone and azithromycin. Paediatr Int Child Health 2014;34:227–9. 10.1179/2046905514Y.0000000123 [DOI] [PubMed] [Google Scholar]
- 15.Crump JA, Luby SP. and Mintz E.D.: The global burden of typhoid fever. Bull World Health Organ 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 16.John J, Van Aart CJ, Grassly NC. The Burden of Typhoid and Paratyphoid in India: Systematic Review and Meta-analysis. PLoS Negl Trop Dis 2016;10:e0004616 10.1371/journal.pntd.0004616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control. Annual Epidemiological Report 2016–Typhoid and paratyphoid fever. Stockholm: ECDC, 2016. [Google Scholar]
- 18.Pachore JA, Vaidya SV, Thakkar CJ, et al. . ISHKS joint registry: A preliminary report. Indian J Orthop 2013;47:505–9. 10.4103/0019-5413.118208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieper J, Fendler C, Laitko S, et al. . No benefit of long-term ciprofloxacin treatment in patients with reactive arthritis and undifferentiated oligoarthritis: a three-month, multicenter, double-blind, randomized, placebo-controlled study. Arthritis Rheum 1999;42:1386–96. [DOI] [PubMed] [Google Scholar]
- 20.Tuompo R, Hannu T, Mattila L, et al. . Reactive arthritis following Salmonella infection: a population-based study. Scand J Rheumatol 2013;42:196–202. 10.3109/03009742.2012.739201 [DOI] [PubMed] [Google Scholar]
- 21.Leirisalo-Repo M, Helenius P, Hannu T, et al. . Long-term prognosis of reactive salmonella arthritis. Ann Rheum Dis 1997;56:516–20. 10.1136/ard.56.9.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mäki-Ikola O, Granfors K. Salmonella-triggered reactive arthritis. Scand J Rheumatol 1992;21:265–70. 10.3109/03009749209099240 [DOI] [PubMed] [Google Scholar]
- 23.Katsarolis I, Tsiodras S, Panagopoulos P, et al. . Septic arthritis due to Salmonella enteritidis associated with infliximab use. Scand J Infect Dis 2005;37:304–6. 10.1080/00365540410021171-1 [DOI] [PubMed] [Google Scholar]
- 24.Oe K, Wada T, Ohno H, et al. . Salmonella septic arthritis following total knee arthroplasty for rheumatoid arthritis in a patient receiving etanercept. J Orthop Sci 2011;16:258–62. 10.1007/s00776-011-0023-9 [DOI] [PubMed] [Google Scholar]
- 25.Nandagudi AC, Kelly S. Ultrasound Detection of Salmonella Septic Arthritis in a Rheumatoid Arthritis Patient on Anti-TNF Treatment. J Investig Med High Impact Case Rep 2014;2:232470961453279 10.1177/2324709614532799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of salmonella infections. Medicine 1987;66:349–88. 10.1097/00005792-198709000-00003 [DOI] [PubMed] [Google Scholar]
- 27.Rae S, Webley M, Snaith ML. Salmonella typhimurium arthritis in rheumatoid disease. Rheumatol Rehabil 1977;16:150–1. 10.1093/rheumatology/16.3.150 [DOI] [PubMed] [Google Scholar]
- 28.Day LJ, Qayyum QJ, Kauffman CA. Salmonella prosthetic joint septic arthritis. Clin Microbiol Infect 2002;8:427–30. 10.1046/j.1469-0691.2002.00466.x [DOI] [PubMed] [Google Scholar]
- 29.Boland F, Kaushik P, Udo EE, et al. . Salmonella Septic Arthritis Complicating Rheumatoid Arthritis in a Patient with Total Knee Replacement. Medical Principles and Practice 1999;8:245–50. 10.1159/000026100 [DOI] [Google Scholar]
- 30.Madan S, Abbas D, Jowett RL, et al. . Salmonella enteritidis infection in total knee replacement. Rheumatology 2001;40:112–3. 10.1093/rheumatology/40.1.112 [DOI] [PubMed] [Google Scholar]
- 31.Musante DB, Ogden WS. Salmonella infection in joint arthroplasty. Orthopedics 2004;27:770–2. [DOI] [PubMed] [Google Scholar]
- 32.Miron D, Zuker M, Lev-El A. [Salmonella prosthetic knee septic arthritis successful retention of the prosthesis with prolonged suppressive therapy]. Harefuah 2006;145:261. [PubMed] [Google Scholar]
- 33.Carlile GS, Elvy J, Toms AD. Salmonella infection of a total knee replacement. Knee 2010;17:356–8. 10.1016/j.knee.2009.10.003 [DOI] [PubMed] [Google Scholar]


