Abstract
Objectives
Investigate the association of heat exposure on years of life lost (YLL) from non-communicable diseases (NCD) in Nouna, Burkina Faso, between 2000 and 2010.
Design
Daily time series regression analysis using distributed lag non-linear models, assuming a quasi-Poisson distribution of YLL.
Setting
Nouna Health and Demographic Surveillance System, Kossi Province, Rural Burkina Faso.
Participants
18 367 NCD-YLL corresponding to 790 NCD deaths recorded in the Nouna Health and Demographic Surveillance Site register over 11 years.
Main outcome measure
Excess mean daily NCD-YLL were generated from the relative risk of maximum daily temperature on NCD-YLL, including effects delayed up to 14 days.
Results
Daily average NCD-YLL were 4.6, 2.4 and 2.1 person-years for all ages, men and women, respectively. Moderate 4-day cumulative rise in maximum temperature from 36.4°C (50th percentile) to 41.4°C (90th percentile) resulted in 4.44 (95% CI 0.24 to 12.28) excess daily NCD-YLL for all ages, rising to 7.39 (95% CI 0.32 to 24.62) at extreme temperature (42.8°C; 99th percentile). The strongest health effects manifested on the day of heat exposure (lag 0), where 0.81 (95% CI 0.13 to 1.59) excess mean NCD-YLL occurred daily at 41.7°C compared with 36.4°C, diminishing in statistical significance after 4 days. At lag 0, daily excess mean NCD-YLL were higher for men, 0.58 (95% CI 0.11 to 1.15) compared with women, 0.15 (95% CI −0.25 to 9.63) at 41.7°C vs 36.4°C.
Conclusion
Premature death from NCD was elevated significantly with moderate and extreme heat exposure. These findings have important implications for developing adaptation and mitigation strategies to reduce ambient heat exposure and preventive measures for limiting NCD in Africa.
Keywords: heat, years of life lost, non-communicable disease, Sub-Saharan africa, time series
Strengths and limitations of this study.
This study investigated the relationship between two defining public health issues affecting Sub-Saharan Africa: increasing ambient heat from climate change and the rising prevalence of non-communicable disease.
Eleven years of high-quality health and demographic data from rural Africa was exploited for analysis.
Only premature death was quantified as the outcome because long-term morbidity data were unavailable.
Temperature data from a weather station located 53 km from the study location were used as a proxy for individual level temperature exposure.
Introduction
As the global average temperature rises, epidemiological evidence on the temperature–health association in neglected African populations is needed to develop appropriate interventions. Surface temperature over West Africa and the Sahel increased by 0.5°C–0.8°C between 1970 and 2010 and at a faster pace in the most recent 20 years.1 Analysis of longitudinal data from 12 Health and Demographic Surveillance Sites (HDSS), which include the Nouna HDSS in Burkina Faso, forecasts that the mean temperature in Africa will exceed the 1900–2000 decadal average by 2100 under all climate change scenarios.2 In a study applying six climate model-future scenarios across six HDSS sites, the most conservative combination, rapid economic growth and balanced energy sources, resulted in a 0.5°C–1°C temperature increase by 2100, whereas most combinations projected a 2°C–3°C temperature rise in the same period.2 Prolonged exposure to high ambient temperature in the subsistence farming community of Nouna, and low adaptive capacity makes this community particularly vulnerable to the effects of temperature increase.
Non-communicable disease (NCD) causes substantial economic drain to society by adversely affecting four pillars of economic growth: labour supply, productivity, investments and education. Projections from 2006 indicated that if no action was taken to reduce the risk of NCD in 23 low-income and middle-income countries, US$83 billion would be lost over the subsequent decade to the impact of heart disease, stroke and diabetes.3 As life expectancy increases in Burkina Faso, people will have more time to develop chronic and degenerative disorders; NCD will therefore contribute increasingly to population mortality. In 2014, NCD accounted for 32% of all deaths in Burkina Faso. The main contributors were cardiovascular disease, cancer, chronic respiratory disease and diabetes.4 In 2011, Friel et al presented a review exposing the link between climate change and a wide range of NCD, and argued that more frequent and intense heat extremes could exacerbate cardiovascular and respiratory health outcomes.5
Previous studies have explored the impact of extreme events such as heatwaves or cold waves6 7 on health, which are anticipated to increase in frequency and magnitude with climate change.8 A recent multicity study, however, found that milder non-optimal temperature rather than extreme temperature was responsible for most of the temperature-related mortality burden (defined as below the 2.5th percentile and above the 97.5th percentile).9 Unfortunately, no African studies were included by Gasparrini et al.9 Heat (and cold) waves are defined by magnitude and duration; for example, 2 or more consecutive days exceeding the 98th–99th (or 1st–2nd) percentiles of the temperature range. Excess risks are a comparison of heatwave periods with non-heatwave periods in previous years. Our study investigates the health risks of moderate to extreme heat, where extreme temperature is defined as below the 5th percentile and above the 95th percentile of maximum temperature.
Epidemiological studies on the temperature–health association in African populations have primarily measured daily deaths as the outcome.10 11 Rather than the number of deaths, we used years of life lost (YLL), a global burden of disease (GBD) outcome metric for ascertaining premature death. YLL are an aggregate of life expectancy and death counts that gives the absolute value of YLL from a certain exposure, rather than a relative risk (RR). In the only previous study set in Africa investigating the temperature–YLL association, Egondi et al found no heat effects on all-cause YLL in the East African highlands of Nairobi, Kenya. A reduction in temperature (21°C compared with 26°C), however, resulted in 27.4 excess all-cause YLL per day (95% CI 2.7 to 52.0).12 The current article addresses Africa’s dual challenge of coping with rising temperatures from climate change and increasing prevalence of NCD. The association between temperature and other health outcomes in Nouna, including infectious disease, will be the subject of future work. The paucity of population-based studies set in Africa focused on the impact of temperature on NCD health outcomes suggests further studies are required. Our study addresses this research gap by investigating the impact of 11 years of heat exposure on YLL from NCD in the Nouna HDSS.
Methods
Data collection
Health outcomes data were obtained from the HDSS, Centre de Recherche en Santé de Nouna, Burkina Faso.13 All registered deaths between 1 January 2000 and 31 December 2010 were included. Vital statistics for each resident included a unique identifying number (ID), date of birth, date of immigration into the HDSS, date of death, date of emigration from the HDSS and gender. Raw mortality data comprised a unique ID number for each death event, date of birth, date of death, sex, cause of death coded as an ICD-10 (International Classification of Diseases) code and an accompanying cause of death in French. Cause of death was established by verbal autopsy.14 Age of death was calculated as the difference between the date of death and birth. We applied the GBD cause-specific categories and ICD-10 codes to define NCD as an aggregate of: malignant neoplasms (C00–C97), other neoplasms (D00–D48), diabetes mellitus (E10–E14), endocrine disorders (D55–D64; minus-D64.9, D65–D89, E03–E07, E15–16, E20–E34, E65–E88), neuropsychiatric conditions (F01–F99, G06–G98), sense organ diseases (H00–H61, H68–H93), cardiovascular diseases (I00–I99), respiratory diseases (J30–J98), digestive diseases (K00–K92), genitourinary diseases (N00–N64, N75–N98), skin diseases (L00–L98), musculoskeletal diseases (M00–M99) and congenital anomalies (Q00–Q99).15
Computation of daily YLL
Different resolutions of life tables can be used to calculate YLL, that is, global, country-level or local life expectancy depending on the purpose of the study. In 1990, the GBD approach calculated YLL relative to the life expectancy of Japanese men and women, the highest for any societal group.16 Weights for age and time preference can additionally be applied to reduce the contribution of death before adulthood.17 For the GBD 2010 study, a reference standard of 86 years at birth was used for both men and women and YLL were calculated using a life table based on the lowest observed mortality in each age group in countries with more than 5 million inhabitants.18 This study used local rather than global life tables, as done in similar studies,19–21 to present realistic potential losses or gains in life years for the Nouna population grounded in real data (rather than modelled data), which is more meaningful for local decision-makers. The cause of death and demographic data from the Nouna HDSS were used to build life tables for the Nouna population. The use of global life expectancy would likely produce very large YLL for populations with low life expectancy such as in Burkina Faso. Furthermore, global life expectancy is likely to be more useful when comparing YLL between two countries, which was not the aim of this study.
We used the Nouna HDSS vital events and mortality data from 2000 to 2010 to produce age-specific death rates. We generated gender-specific life tables to account for varying life expectancies between men and women (details in online supplementary tables 1 and 2). Mean additional survival time, averaged between 2000 and 2010, was calculated for each age band to account for the changing population profile over this time. Abridged life tables were created in 5-year increments, producing stable life expectancy estimates for a relatively small population (approximately 90 000 inhabitants in 201210). The 0–1 and 1–5 age groups were, however, separated. Combining these ages would mask the lower remaining life expectancy for the 0–1 age group relative to the 1–5 age group, a consequence of high infant mortality. For each NCD death, YLL were calculated by matching age and sex with the relevant life table. Daily YLL were an aggregate of individual YLL on the respective day calculated as:
bmjopen-2017-018068supp001.pdf (990.6KB, pdf)
(A) individual YLL at time of death,
| (1) |
(B) total daily YLL,
| (2) |
where:
i is the ith individual,
LEremaining is the conditional life expectancy,
Agedeath is the age at death,
n is the number of deaths occurring on a given day.
We stratified NCD-YLL by sex to assess if gender differences existed.
Temperature data
Because temperature data for Nouna were not sufficiently complete for analysis, we obtained hourly mean (t-mean), maximum temperature (t-max) and minimum temperature (t-min) data from the National Climatic Data Centre for the Dédougou weather station (12.4° N, 3.4° W) from 1 January 2000 to 31 December 2010 (4071 days). Pearson’s correlation analysis was performed to compare maximum temperature between a local Nouna weather station (coordinates 12.7° N, 3.9° W) and the Dédougou weather station (located 53 km from Nouna). Over the study period of 4071 days, 2432 days (59%) of maximum temperature from Nouna were available for comparison. The very strong correlation coefficient of 0.93 (95% CI 0.92 to 0.94), p<2.2e−16, indicated there was little variability between the two sites, validating our use of Dédougou maximum temperature for Nouna. Hourly Dédougou data were averaged to give a daily temperature. The raw time series consisted of 25% missing t-mean, 14% t-max and 17% t-min. We created an imputation algorithm by averaging 15 consecutive days of temperature either side of a missing temperature value to create a 30-day moving average. The Time Indexes and Time Indexed Series (tis) package V.1.30 was applied in R software to impute missing temperature values.
Statistical modelling
We applied time series quasi-Poisson regression analysis using a distributed lag non-linear model (DLNM) to investigate the association between maximum daily temperature and NCD-YLL.
A natural cubic spline with 8 df per year was applied to control for season and long-term time trends. A heaping effect was found in the raw data (see online supplementary tables 3–5 and supplementary figures 1 and 2), where deaths of an unknown date were assigned to the ninth day of the corresponding month. An indicator variable was added to mark and control for heaping of deaths and day of the week. Exploratory analyses are found in online supplementary figures 3–5. The DLNM captured the immediate and delayed effects of temperature (lags) on health, known as the lag–response association as single lag days, or as it cumulates over time. The exposure–response curve was modelled with a natural cubic spline with knots placed at the 10th, 50th and 90th percentiles. The lag–response was modelled with a natural cubic spline of 2 df, resulting in default knot placement equally along a logarithmic scale. The model equation was:
| (3) |
where:
is the daily YLL,
is the y intercept,
is the smooth function of time with specified df
) is the cross-basis function of t-max and the associated lag dimension with vardf and lagdf df, respectively. DOW accounts for day of the week and HP for the heaping effect.
From the RR, absolute values of excess mean daily NCD-YLL were calculated as:
| (4) |
All effect estimates were presented against the median t-max of 36.4°C either as overall 4-day and 14-day cumulative RRs (and corresponding excess mean daily NCD-YLL), or single-day lags extending to 14 days.
Several sensitivity analyses were conducted to test the robustness of altering model choices, including: specifying alternative knot positions for exposure–response at the 10th, 75th and 90th, and 10th, 25th, 75th and 90th percentiles, extending df for the lag–response between 2 and 6 df, manipulating control for season and time trend ranging from 5 to 10 df, logarithm transformation of YLL and applying a Gaussian distribution, and extending the lag period to 28 days to assess if temperature exposure triggered NCD deaths on a longer time scale. Quasi-Akaike information criteria (QAIC) values were calculated to guide model selection. All statistical analyses were conducted using R software V.3.2.2. DLNMs were fitted using the DLNM package V.2.2.3.
Results
The 790 NCD deaths correspond to 18 367 YLL over the study period. Cardiovascular diseases were the largest contributor to NCD-YLL, accounting for 9095 or 50% of all NCD-YLL. Digestive disorders, malignant neoplasms, and genitourinary and neuropsychiatric conditions also contributed substantially towards NCD-YLL. Interestingly, endocrine disorders (including diabetes mellitus) formed a very small proportion (1%) of all NCD-YLL (table 1). Table 2 shows that maximum mortality peaked at five deaths per day, corresponding to 154 daily NCD-YLL. Daily mean NCD-YLL were 4.6, 2.4 and 2.1 person-years for all ages, men and women, respectively. Maximum daily temperature was 36.4°C at the 50th percentile, peaking at 43.9°C in the study period.
Table 1.
Cause-specific NCD outcomes with corresponding deaths and years of life lost. NCD accounted for 12% of total deaths and 7% of total YLL in Nouna between 2000 and 2010
| Disease | Death count | Death (%) | YLL count | YLL (%) |
| Cardiovascular diseases | 461 | 58 | 9095 | 50 |
| Digestive diseases | 137 | 17 | 3614 | 20 |
| Malignant neoplasms | 81 | 10 | 1720 | 9 |
| Genitourinary diseases | 38 | 5 | 1602 | 9 |
| Neuropsychiatric conditions | 37 | 5 | 1289 | 7 |
| Congenital anomalies | 8 | 1 | 481 | 3 |
| Respiratory diseases | 15 | 2 | 321 | 2 |
| Diabetes mellitus | 11 | 1 | 167 | 1 |
| Other endocrine disorders | 1 | 0 | 22 | 0 |
| Musculoskeletal diseases | 1 | 0 | 57 | 0 |
| Total | 790 | 100 | 18 367 | 100 |
NCD, non-communicable disease; YLL, years of life lost.
Table 2.
Summary statistics of daily NCD deaths, NCD years of life lost and temperature in Nouna, Burkina Faso, between 2000 and 2010
| Daily NCD and temperature descriptive statistics 2000–2010 | ||||||
| Minimum | 25% | 50% | Mean | 75% | Maximum | |
| Daily number of NCD deaths | ||||||
| Total | 0 | 0 | 0 | 0.2 | 0 | 5 |
| Male | 0 | 0 | 0 | 0.1 | 0 | 4 |
| Female | 0 | 0 | 0 | 0.1 | 0 | 3 |
| >65 years | 0 | 0 | 0 | 0.1 | 0 | 3 |
| Daily NCD years of life lost | ||||||
| Total | 0 | 0 | 0 | 4.6 | 0 | 154 |
| Male | 0 | 0 | 0 | 2.4 | 0 | 118.9 |
| Female | 0 | 0 | 0 | 2.1 | 0 | 127.5 |
| >65 years | 0 | 0 | 0 | 0.9 | 0 | 39.2 |
| Temperature (°C) | ||||||
| Daily minimum | 3.3 | 21.1 | 22.8 | 23.1 | 25 | 32.8 |
| Daily average | 17.2 | 27.5 | 29.2 | 29.6 | 31.7 | 37.8 |
| Daily maximum | 22.8 | 33.3 | 36.4 | 36.1 | 38.9 | 43.9 |
NCD, non-communicable disease.
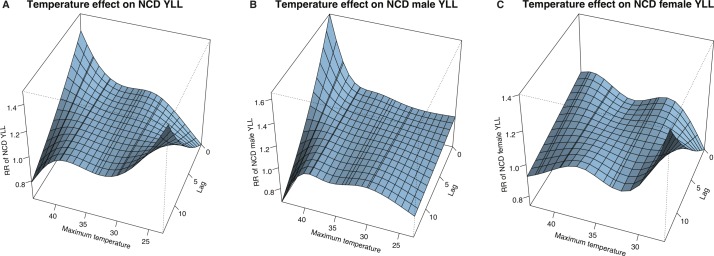
Figure 1 shows 3D graphs of the RR of NCD-YLL at a range of maximum temperature and lag values, centred at the reference temperature of 36.4°C (all RRs and excess mean daily NCD-YLL in the Results section are given as a comparison to this reference temperature). All-age (panel A) and male (panel B) plots showed a strong surge in the RR with high temperature close to the time of heat exposure. Men presented no noticeable effect with colder temperature. In contrast, women (panel C) and the all-age group showed more prominent health effects with cooler temperatures, which increased at longer lags. The lag structure of 0–4 days was used to identify immediate health effects,22 which were expanded to 14 days to verify if the effects persisted or were concentrated in earlier days. Single-day lagged effects from 0 to 14 days were also considered to identify mortality displacement trends with longer lags. The main results were the 4-day and 14-day cumulative (table 3 and figure 2) and single-day lagged RR of NCD-YLL (table 4 and figure 3), from which daily excess mean NCD-YLL were calculated (tables 5 and 6).
Figure 1.
Association of RR of NCD-YLL to maximum temperature and lag days, with reference to 36.4°C for (A) all ages, (B) men and (C) women. NCD, non-communicable disease; RR, relative risk; YLL, years of life lost.
Table 3.
Cumulative relative risk (and 95% confidence bounds) of maximum temperature on non-communicable disease years of life lost in Nouna stratified across lag 0–4 and lag 0–14 days and gender between 2000 and 2010. Relative risks are presented for: heat effects as 38.9°C, 41.1°C, 41.7°C and 42.8°C with reference to 36.4°C; cold effects as 27.8°C, 30°C, 31.1°C and 33.3°C with reference to 36.4°C. Results controlled for long-term trends, season, day of the week and heaping effect
| 27.8°C (1st percentile) |
30°C (5th percentile) |
31.1°C (10th percentile) |
33.3°C (25th percentile) |
38.9°C (75th percentile) |
41.4°C (90th percentile) |
41.7°C (95th percentile) |
42.8°C (99th percentile) |
|
| 34 days below 27.8°C | 174 days below 30°C | 325 days below 31.3°C | 912 days below 33.3°C | 883 days above 38.9°C | 300 days above 41.4°C | 189 days above 41.7°C | 28 days above 42.8°C | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Lag structure 0–4 | ||||||||
| All ages | 0.54 (0.22 to 1.36) | 0.82 (0.56 to 1.21) | 0.93 (0.72 to 1.21) | 1.00 (0.99 to 1.01) | 1.29 (0.81 to 2.04) | 1.96 (1.05 to 3.67) | 2.08 (1.08 to 4.01) | 2.61 (1.07 to 6.35) |
| Male | 0.53 (0.15 to 1.86) | 0.77 (0.46 to 1.3) | 0.88 (0.62 to 1.25) | 1.00 (0.99 to 1.01) | 1.36 (0.74 to 2.49) | 2.56 (1.14 to 5.75) | 2.86 (1.23 to 6.69) | 4.60 (1.45 to 14.64) |
| Female | 0.38 (0.10 to 1.41) | 0.81 (0.47 to 1.4) | 0.99 (0.68 to 1.43) | 1.00 (0.99 to 1.01) | 1.05 (0.54 to 2.04) | 1.20 (0.49 to 2.98) | 1.19 (0.46 to 3.05) | 1.06 (0.30 to 3.79) |
| Lag structure 0–14 | ||||||||
| All ages | 0.89 (0.22 to 3.67) | 0.94 (0.53 to 1.65) | 0.96 (0.64 to 1.42) | 1.00 (0.99 to 1.01) | 1.65 (0.82 to 3.35) | 1.98 (0.71 to 5.54) | 1.98 (0.66 to 5.91) | 1.88 (0.41 to 8.75) |
| Male | 0.71 (0.11 to 4.63) | 0.91 (0.43 to 1.94) | 0.97 (0.57 to 1.66) | 1.00 (0.99 to 1.01) | 1.57 (0.62 to 3.97) | 2.11 (0.57 to 7.85) | 2.14 (0.53 to 8.70) | 2.16 (0.29 to 16.35) |
| Female | 1.24 (0.16 to 9.27) | 1.09 (0.49 to 2.46) | 1.05 (0.59 to 1.84) | 1.00 (0.98 to 1.02) | 1.42 (0.50 to 3.99) | 1.28 (0.28 to 5.97) | 1.21 (0.24 to 6.17) | 0.92 (0.10 to 8.30) |
RR, relative risk.
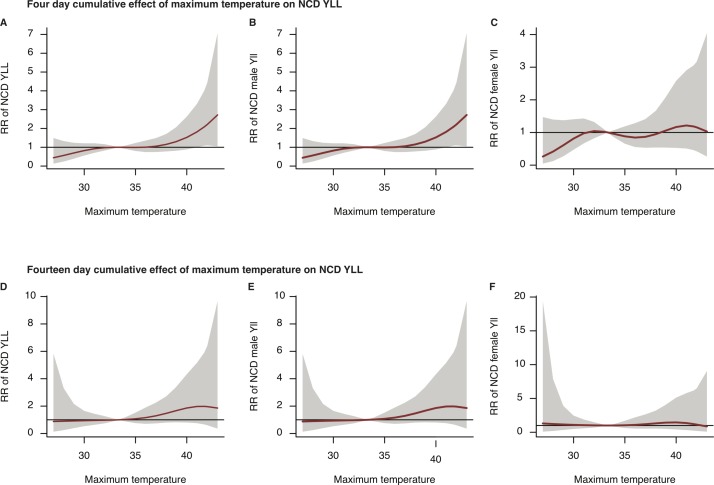
Figure 2.
Plots of 4-day (A: all ages, B: male, C: female) and 14-day (D: all ages, E: male, F: female) cumulative RR of NCD-YLL against maximum temperature (solid line) with 95% confidence bounds (grey area) for all ages, men and women in Nouna, Burkina Faso, between 2000 and 2010. The reference temperature is 36.4°C. Note: To improve legibility of the curves, the scales on the y-axis differ. NCD, non-communicable disease; RR, relative risk; YLL, years of life lost.
Table 4.
Relative risk (and 95% confidence bounds) of maximum temperature on non-communicable disease years of life lost in Nouna stratified by individual lag days for all ages, men and women between 2000 and 2010. Relative risks are presented for heat effects as 41.7°C (95th percentile) with reference to 36.4°C, cold effects as 30°C (5th percentile) with reference to 36.4°C. Results controlled for long-term trends, season, day of the week and heaping effect
| All ages | Male | Female | ||||
| 30°C (5th percentile) | 41.7°C (95th percentile) | 30°C (5th percentile) | 41.7°C (95th percentile) | 30°C (5th percentile) | 41.7°C (95th percentile) | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | ||||
| lag 0 | 0.96 (0.89 to 1.04) | 1.18 (1.03 to 1.35) | 0.96 (0.87 to 1.07) | 1.24 (1.04 to 1.48) | 0.96 (0.86 to 1.08) | 1.07 (0.88 to 1.30) |
| lag 1 | 0.97 (0.90 to 1.04) | 1.16 (1.03 to 1.31) | 0.97 (0.88 to 1.06) | 1.21 (1.04 to 1.42) | 0.97 (0.88 to 1.07) | 1.06 (0.89 to 1.27) |
| lag 2 | 0.97 (0.91 to 1.04) | 1.14 (1.02 to 1.27) | 0.97 (0.9 to 1.05) | 1.19 (1.03 to 1.36) | 0.98 (0.89 to 1.07) | 1.05 (0.90 to 1.24) |
| lag 3 | 0.98 (0.92 to 1.03) | 1.12 (1.01 to 1.23) | 0.98 (0.91 to 1.05) | 1.16 (1.02 to 1.31) | 0.98 (0.91 to 1.06) | 1.05 (0.91 to 1.21) |
| lag 4 | 0.98 (0.94 to 1.03) | 1.10 (1.01 to 1.20) | 0.98 (0.92 to 1.05) | 1.13 (1.01 to 1.27) | 0.99 (0.92 to 1.06) | 1.04 (0.91 to 1.18) |
| lag 5 | 0.99 (0.94 to 1.03) | 1.08 (1.00 to 1.17) | 0.99 (0.93 to 1.04) | 1.10 (1.00 to 1.22) | 0.99 (0.93 to 1.06) | 1.03 (0.91 to 1.16) |
| lag 6 | 0.99 (0.95 to 1.03) | 1.06 (0.99 to 1.15) | 0.99 (0.94 to 1.04) | 1.08 (0.98 to 1.19) | 1.00 (0.94 to 1.06) | 1.02 (0.91 to 1.14) |
| lag 7 | 1.00 (0.96 to 1.03) | 1.05 (0.97 to 1.13) | 0.99 (0.95 to 1.05) | 1.05 (0.96 to 1.16) | 1.01 (0.95 to 1.06) | 1.01 (0.91 to 1.13) |
| lag 8 | 1.00 (0.96 to 1.04) | 1.03 (0.96 to 1.11) | 1.00 (0.95 to 1.05) | 1.03 (0.93 to 1.13) | 1.01 (0.96 to 1.07) | 1. 00 (0.90 to 1.12) |
| lag 9 | 1.00 (0.96 to 1.05) | 1.01 (0.94 to 1.10) | 1.00 (0.95 to 1.06) | 1.00 (0.91 to 1.11) | 1.02 (0.96 to 1.08) | 1. 00 (0.89 to 1.12) |
| lag 10 | 1.01 (0.96 to 1.06) | 1.00 (0.91 to 1.09) | 1.01 (0.95 to 1.07) | 0.98 (0.88 to 1.09) | 1.02 (0.96 to 1.09) | 0.99 (0.87 to 1.12) |
| lag 11 | 1.01 (0.96 to 1.07) | 0.98 (0.89 to 1.08) | 1.01 (0.94 to 1.08) | 0.96 (0.85 to 1.08) | 1.03 (0.96 to 1.11) | 0.98 (0.85 to 1.13) |
| lag 12 | 1.02 (0.96 to 1.08) | 0.96 (0.86 to 1.07) | 1.02 (0.94 to 1.10) | 0.93 (0.81 to 1.07) | 1.04 (0.96 to 1.12) | 0.97 (0.83 to 1.14) |
| lag 13 | 1.02 (0.96 to 1.09) | 0.95 (0.84 to 1.07) | 1.02 (0.93 to 1.12) | 0.91 (0.78 to 1.06) | 1.04 (0.95 to 1.14) | 0.97 (0.81 to 1.15) |
| lag 14 | 1.03 (0.96 to 1.11) | 0.93 (0.82 to 1.06) | 1.03 (0.93 to 1.13) | 0.89 (0.75 to 1.06) | 1.05 (0.95 to 1.16) | 0.96 (0.79 to 1.16) |
RR, relative risk.
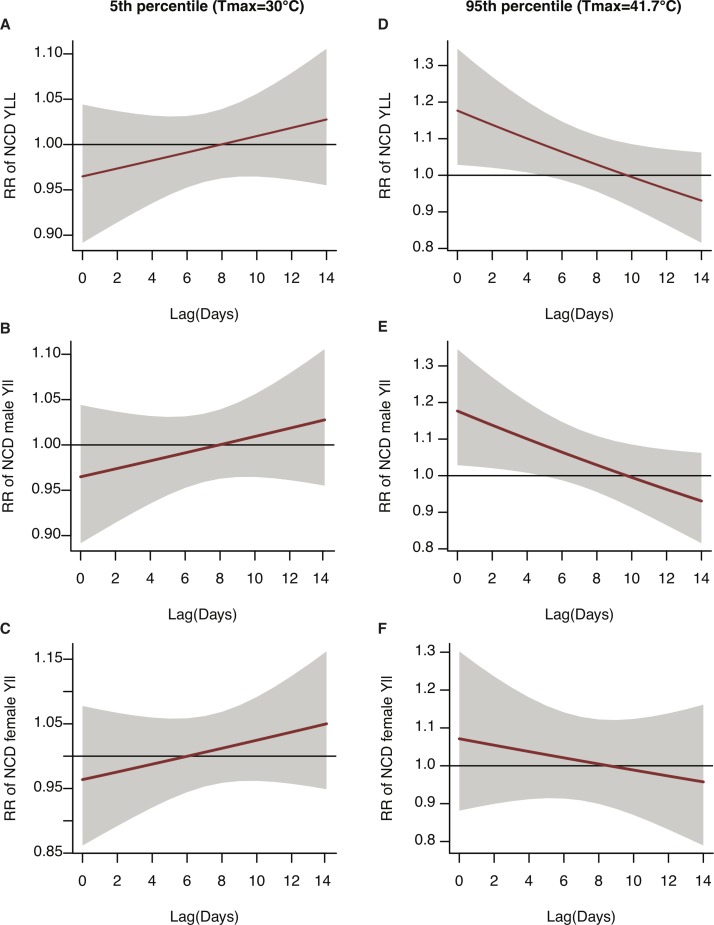
Figure 3.
Delayed effects of maximum temperature on the RR of NCD-YLL (solid line) and 95% confidence bounds (grey area) for all ages, men and women in Nouna, Burkina Faso, by lag 0–14 days. Plots (A) all ages, (B) male and (C) female represent cold effects at 30°C (5th percentile), and plots (D) all ages, (E) male and (F) female represent heat effects at 41.7°C (95th percentile) of maximum temperature. The reference temperature is 36.4°C. Note: To improve legibility of the curves, the scales on the y-axis differ. NCD, non-communicable disease; RR, relative risk; YLL, years of life lost.
Table 5.
Cumulative excess average daily NCD-YLL (and 95% confidence bounds) stratified across lag 0–4 and lag 0–14 days and gender between 2000 and 2010. Relative risks were used to calculate excess average daily NCD-YLL as follows: (Average daily NCD-YLL of all ages, male or female × relative risk) − Average daily NCD-YLL. NCD-YLL are presented for: heat effects as 38.9°C, 41.1°C, 41.7°C and 42.8°C with reference to 36.4°C; cold effects as 27.8°C, 30°C, 31.1°C and 33.3°C with reference to 36.4°C. Results controlled for long-term trends, season, day of the week and heaping effect
| 27.8°C (1st percentile) |
30°C (5th percentile) |
31.1°C (10th percentile) |
33.3°C (25th percentile) |
38.9°C (75th percentile) |
41.4°C (90th percentile) |
41.7°C (95th percentile) |
42.8°C (99th percentile) |
|
| 34 days below 27.8°C | 174 days below 30°C | 325 days below 31.3°C | 912 days below 33.3°C | 883 days above 38.9°C | 300 days above 41.4°C | 189 days above 41.7°C | 28 days above 42.8°C | |
| YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | |
| Lag structure 0–4 | ||||||||
| All ages | −2.11 (−3.61 to 1.64) | −0.81 (−2.02 to 0.97) | −0.31 (−1.30 to 0.97) | 0.00 (−0.03 to 0.03) | 1.33 (−0.86 to 4.80) | 4.44 (0.24 to 12.28) | 4.98 (0.38 to 13.83) | 7.39 (0.32 to 24.62) |
| Male | −1.13 (−2.04 to 2.06) | −0.55 (−1.30 to 0.72) | −0.29 (−0.92 to 0.59) | 0.00 (−0.03 to 0.02) | 0.86 (−0.62 to 3.57) | 3.73 (0.33 to 11.39) | 4.48 (0.54 to 13.65) | 8.65 (1.07 to 32.73) |
| Female | −1.30 (−1.88 to 0.86) | −0.39 (−1.11 to 0.85) | −0.03 (−0.66 to 0.90) | 0.01 (−0.01 to 0.03) | 0.10 (−0.97 to 2.19) | 0.43 (−1.08 to 4.16) | 0.39 (−1.13 to 4.32) | 0.12 (−1.48 to 5.86) |
| Lag structure 0–14 | ||||||||
| All ages | −0.48 (−3.6 to 12.27) | −0.28 (−2.15 to 3.01) | −0.20 (−1.64 to 1.95) | −0.01 (−0.06 to 0.04) | 3.01 (−0.84 to 10.82) | 4.53 (−1.33 to 20.89) | 4.5 (−1.55 to 22.57) | 4.07 (−2.73 to 35.66) |
| Male | −0.70 (−2.14 to 8.71) | −0.21 (−1.37 to 2.25) | −0.06 (−1.03 to 1.58) | 0.00 (−0.04 to 0.04) | 1.38 (−0.90 to 7.12) | 2.67 (−1.03 to 16.44) | 2.74 (−1.14 to 18.48) | 2.78 (−1.72 to 36.83) |
| Female | 0.50 (−1.75 to 17.37) | 0.20 (−1.08 to 3.07) | 0.09 (−0.86 to 1.77) | 0.00 (−0.03 to 0.03) | 0.87 (−1.05 to 6.28) | 0.59 (−1.52 to 10.44) | 0.44 (−1.60 to 10.86) | −0.18 (−1.89 to 15.32) |
NCD, non-communicable disease; YLL, years of life lost.
Table 6.
Excess average daily NCD-YLL (and 95% confidence bounds) in Nouna stratified by individual lag days for all ages, men and women between 2000 and 2010. Relative risks were used to calculate excess average daily NCD-YLL as follows: (Average daily NCD-YLL of all ages, male or female × relative risk) − Average daily NCD-YLL. NCD-YLL are presented for heat effects as 41.7°C (95th percentile) with reference to 36.4°C, cold effects as 30°C (5th percentile) with reference to 36.4°C. Results controlled for long-term trends, season, day of the week and heaping effect
| All ages | Male | Female | ||||
| 30°C (5th percentile) | 41.7°C (95th percentile) | 30°C (5th percentile) | 41.7°C (95th percentile) | 30°C (5th percentile) | 41.7°C (95th percentile) | |
| 174 days below 30°C | 189 days above 41.7°C | 174 days below 30°C | 189 days above 41.7°C | 174 days below 30°C | 189 days above 41.7°C | |
| YLL (95% CI) | YLL (95% CI) | YLL (95% CI) | ||||
| lag 0 | −0.16 (−0.50 to 0.20) | 0.81 (0.13 to 1.59) | −0.09 (−0.31 to 0.16) | 0.58 (0.11 to 1.15) | −0.08 (−0.29 to 0.16) | 0.15 (−0.25 to 0.63) |
| lag 1 | −0.14 (−0.45 to 0.19) | 0.72 (0.12 to 1.41) | −0.08 (−0.28 to 0.15) | 0.51 (0.09 to 1.01) | −0.06 (−0.26 to 0.15) | 0.13 (−0.23 to 0.56) |
| lag 2 | −0.12 (−0.40 to 0.17) | 0.63 (0.09 to 1.24) | −0.07 (−0.25 to 0.13) | 0.44 (0.07 to 0.87) | −0.05 (−0.23 to 0.14) | 0.11 (−0.21 to 0.50) |
| lag 3 | −0.10 (−0.35 to 0.16) | 0.55 (0.07 to 1.08) | −0.06 (−0.22 to 0.12) | 0.38 (0.05 to 0.75) | −0.04 (−0.20 to 0.13) | 0.10 (−0.20 to 0.43) |
| lag 4 | −0.08 (−0.30 to 0.15) | 0.46 (0.04 to 0.93) | −0.05 (−0.19 to 0.11) | 0.31 (0.02 to 0.64) | −0.03 (−0.17 to 0.13) | 0.08 (−0.19 to 0.38) |
| lag 5 | −0.06 (−0.25 to 0.14) | 0.38 (0.00 to 0.79) | −0.04 (−0.17 to 0.10) | 0.25 (−0.01 to 0.54) | −0.01 (−0.14 to 0.12) | 0.06 (−0.18 to 0.33) |
| lag 6 | −0.04 (−0.22 to 0.14) | 0.30 (−0.06 to 0.68) | −0.03 (−0.15 to 0.10) | 0.19 (−0.05 to 0.45) | 0.00 (−0.12 to 0.12) | 0.04 (−0.18 to 0.30) |
| lag 7 | −0.02 (−0.19 to 0.16) | 0.21 (−0.12 to 0.58) | −0.01 (−0.13 to 0.11) | 0.12 (−0.10 to 0.37) | 0.01 (−0.10 to 0.13) | 0.03 (−0.19 to 0.27) |
| lag 8 | 0.00 (−0.17 to 0.18) | 0.13 (−0.20 to 0.50) | 0.00 (−0.12 to 0.12) | 0.07 (−0.16 to 0.31) | 0.03 (−0.09 to 0.14) | 0.01 (−0.21 to 0.26) |
| lag 9 | 0.02 (−0.16 to 0.21) | 0.06 (−0.30 to 0.44) | 0.01 (−0.12 to 0.14) | 0.01 (−0.22 to 0.26) | 0.04 (−0.08 to 0.17) | −0.01 (−0.24 to 0.25) |
| lag 10 | 0.04 (−0.16 to 0.26) | −0.02 (−0.40 to 0.39) | 0.02 (−0.13 to 0.17) | −0.05 (−0.30 to 0.23) | 0.05 (−0.08 to 0.19) | −0.02 (−0.27 to 0.26) |
| lag 11 | 0.06 (−0.17 to 0.31) | −0.10 (−0.51 to 0.36) | 0.03 (−0.14 to 0.20) | −0.10 (−0.37 to 0.2) | 0.07 (−0.08 to 0.22) | −0.04 (-0.31 to 0.27) |
| lag 12 | 0.08 (−0.18 to 0.36) | −0.17 (−0.62 to 0.33) | 0.04 (−0.15 to 0.24) | −0.16 (−0.45 to 0.17) | 0.08 (−0.09 to 0.26) | −0.06 (−0.35 to 0.29) |
| lag 13 | 0.11 (−0.19 to 0.42) | −0.25 (−0.74 to 0.31) | 0.05 (−0.16 to 0.28) | −0.21 (−0.52 to 0.15) | 0.09 (−0.10 to 0.30) | −0.07 (−0.40 to 0.31) |
| lag 14 | 0.13 (−0.21 to 0.49) | −0.32 (−0.85 to 0.29) | 0.06 (−0.17 to 0.32) | −0.26 (−0.60 to 0.13) | 0.11 (−0.11 to 0.34) | −0.09 (−0.44 to 0.34) |
NCD, non-communicable disease; YLL, years of life lost.
Heat effects on NCD-YLL were felt strongly in Nouna above the 50th percentile. Over 4 cumulative days, exposure to moderate temperature (90th percentile at 41.3°C) was associated with a statistically significant increase in excess mean daily NCD-YLL by 4.44 (95% CI 0.24 to 12.28) for all ages, 3.73 (95% CI 0.33 to 11.39) for men, but remained statistically insignificant for women, 0.43 (95% CI –1.08 to 4.16). In comparison to the 90th percentile, excess mean daily NCD-YLL increased slightly at 95th percentile (41.7°C) for all ages and men, but not women (table 4). Extreme heat exposure (99th percentile) over 4 days increased excess daily mean NCD-YLL for all ages to 7.39 (95% CI 0.32 to 24.62) and 8.65 (95% CI 1.07 to 32.73) for men in contrast to the minimal increase for women; 0.12 (95% CI –1.48 to 5.86). Extending the cumulative effect to 14 days also resulted in elevated excess daily mean NCD-YLL, but wider 95% confidence bounds rendered the effect estimates for all three groups statistically insignificant. Detailed plots of cumulative effects are found in online supplementary figures 8–10.
Across 14 individual lag days (figure 3), the largest heat effects were felt immediately (at lag 0); excess daily mean NCD-YLL were 0.81 (95% CI 0.13 to 1.59) for all ages, 0.58 (95% CI 0.11 to 1.15) for men, and 0.15 (95% CI –0.25 to 0.63) for women at 41.7°C (table 6). Heat effects tapered after lag 0, but remained statistically significant to lag 4 at 41.7°C for all ages and men. For the 95th percentile, a gradual reduction in excess daily mean NCD-YLL (statistically insignificant) was observed up to 8–10 lag days for all ages, men and women, with no subsequent increase. Detailed risk estimates for individual lag days are found in online supplementary tables 6–8.
A reduction in temperature to 30°C (figure 3) resulted in a slightly protective effect at shorter lags (0–5), but after 14 days the excess daily NCD-YLL were slightly elevated for all subgroups: 0.13 (95% CI −0.21 to 0.49) for all ages; 0.06 (95% CI −0.17 to 0.32) for men; and 0.11 (95% CI −0.11 to 0.34) for women (table 6). Women were the only group to present a statistically significant increase in mean daily NCD-YLL with extreme cold (first percentile) at lags 13 and 14 (see online supplementary table 8b).
Excess mean daily NCD-YLL were elevated with heat exposure for the 65+ age group; however, the low sample size produced very large confidence bounds (ie, 0.14 (95% CI −0.89 to 86.35)) at 38.9°C vs 36.4°C.
Several sensitivity analyses were conducted to validate model selection, including generating QAIC, where lower QAIC indicate better model fit (see online supplementary tables 9 and 10). Because increasing the df produces lower QAIC values, we used prior examples to achieve a balance in controlling for season and long-term trend to 8 df per year.9 Applying 7 df per year, as used in other studies, did not greatly affect the risk estimates.21 23 The natural cubic spline produced lower QAIC in comparison to the more flexible cubic B-spline. Varying knot position and numbers for the exposure–response relationship also did not vary effect estimates. Using 3 df for the lag–response relationship produced the classic reversed J curve expected for heat effects; however, 2 df generated lower QAIC indicating better model fit. There was no evidence of autocorrelation (see online supplementary figures 6 and 7).
Discussion
A central finding of this study was that excess premature deaths from NCD increased with moderate and extreme heat in rural Sub-Saharan Africa. The magnitude of health effects worsened with heat intensity. The largest increase in excess premature mortality from NCD occurred rapidly on the day of heat exposure (lag 0), and diminished in statistical significance after 4 days. The effects of heat on NCD-YLL were greater in men in comparison to women.
In Nairobi, Kenya, increase in temperature over 14 days from 26°C to 30°C resulted in 3.3 (95% CI −19.7 to 26.4) YLL per day, but from all causes.12 Similarly, a change in temperature from the 50th to 75th percentile (36.4°C–38.9°C) in Nouna resulted in 3.01 (95% CI −0.84 to 10.82) excess daily NCD-YLL over 14 days. Unlike Nouna, the temperature in Nairobi does not typically exceed 40°C. As the only existing African study presenting outcomes as YLL, the comparison presented here indicated ~3 daily YLL in Nouna and Nairobi with a similar temperature increase. Unfortunately, a direct contrast of results between these two African studies is limited because YLL in Nouna were from NCD only, but from all causes of death in Nairobi.
In Australia and China, heat exposure increased the YLL from cardiovascular disease. A total of 45 years were lost daily from cardiovascular disease (95% CI 22 to 67 years) in Brisbane, Australia, at a mean temperature of 32°C vs 24°C.19 In Guangzhou, China (lags 0–14), a change in mean temperature from the 75th percentile (28°C) to the 99th percentile (32°C) resulted in 4.81 (95% CI −2.25 to 11.88) daily YLL from cardiovascular disease.21 Cardiovascular disease contributed to 50% of YLL in Nouna. Although subgroup analysis of NCD was limited by sample size in Nouna, the magnitude of effects was closer to Guangzhou than Brisbane; 4.07 (95% CI −2.73 to 35.66) and 7.39 (95% CI 0.32 to 24.62) mean daily YLL were found from all NCDs at lags 0–14 and lags 0–4, respectively, at the 50th vs 99th percentile. Heat can exacerbate cardiovascular strain through increased cardiac output, blood viscosity and coagulation, attenuated vasoconstriction and cerebral perfusion pressure.24 Our findings agree with those from Guangzhou and Brisbane,19 21 where heat effects occurred rapidly at lag 0, lasting a maximum of 4 days. In contrast to Brisbane, Nouna and Guangzhou exhibited fewer YLL for a similar age and temperature shift. All sites used regional or local life tables to calculate YLL rather than global life tables, so the elevated YLL in Brisbane are unlikely to be attributable to lower life expectancy in Nouna compared with Brisbane. Unlike Brisbane, the predominant cause of death in Nouna is still infectious disease; most days in the Nouna time series exhibited no YLL from NCD. Temperature-related premature death from NCD could increase in Nouna as the epidemiological transition progresses, increasing the proportion of deaths attributable to NCD in the future.
Daily respiratory YLL increased by 2.81 (95% CI −1.54 to 7.16) in Guangzhou at 28°C versus 32°C21 where infectious and chronic respiratory deaths were grouped together as ICD-10 J00–99. In the current analysis, however, chronic respiratory YLL (ICD-10 J30–98) only contributed to 2% of total NCD-YLL in Nouna. The separation of chronic and acute respiratory outcomes may be relevant for comparing findings from different studies and understanding the causal mechanisms. Digestive, renal and neuropsychiatric causes contributed substantially to overall NCD-YLL in Nouna. Heat is known to trigger renal25 and mental health-related deaths26; however, the link to chronic digestive causes requires further investigation.
Although excess NCD-YLL for women were elevated with heat exposure, male NCD-YLL were affected by a greater magnitude at lags 0–2. One explanation is that men working outdoors might have higher exposure to ambient heat. Occupational stress has been associated with excess risk of NCD morbidity including psychological distress27 and kidney disease.28 These results are somewhat unexpected considering that women in Nouna are exposed to extra heat from cooking and carrying wood/water for 2–3 hours daily. Women might die prematurely from other causes such as childbirth, leaving men to be more affected by diseases associated with longevity such as cardiovascular disease; however, further investigation of gender differences is warranted.
Contrary to findings across 14 European cities,29 we found no evidence of harvesting effects with heat; gradual reduction in YLL across lag days ensuing the initial surge was not associated with significant subsequent negative associations or a rise again in risk estimates for any subgroups.22 The public health relevance of our findings is therefore enhanced, as premature NCD mortality is not merely the advancement of death in frail individuals with pre-existing chronic conditions.
This study has several strengths. An 11-year time series of reliable, high-quality data from a rural African setting was used to quantify the burden of temperature on NCD-YLL. Variables such as the date, age and cause of death were subject to quality checks and continuous improvements at INDEPTH (International Network for the Demographic Evaluation of Populations and Their Health) sites including Nouna. These processes enabled one of the best quality and most extensive longitudinal health data sets in Africa and Asia to be used for this study. The DLNM accounted for non-linearity and lagged effects. In place of RRs which would have been obtained had only death counts been used, combining life expectancy and death counts gave an absolute value for YLL from NCD, which is relevant for policymaking.30 Despite the low number of NCD deaths, significant effects of heat on premature mortality were detected, indicating that the effects were strong. The results in the final model were robust and withstood variations of model parameters. Rather than focusing only on anomalous weather events such as heatwaves, one of the longest time series available in rural Africa was exploited to highlight that excess premature deaths from NCD occur during extreme heat and with moderate heat.
Some limitations are also noted. Caution should be exercised in generalising these findings to all rural African settings. Temperature data were obtained from the nearest location with a similar temperature profile to Nouna. Air pollution data were unavailable to assess potential confounding effects of the exposure–response relationship. The lower resolution and distribution of weather data in Burkina Faso compared with Organisation for Economic Co-operation and Development countries can make it challenging to obtain suitable weather data in Burkina Faso. Public health scientists ought to address this challenge by extending research beyond where the data are best, to where problems are the greatest and research/solutions most needed. It is likely that cancer or mental disorders were under-reported as sophisticated questionnaires and tests are needed to establish these causes. In 2004, the WHO estimated that NCD accounted for 20% of the burden of disease in Burkina Faso as a percentage of total disability-adjusted life years (DALY), which captures both premature death and life lived with disease.31 We found that only 7% of the burden from premature deaths or YLL in Nouna were from NCD. Although the YLL component of DALYs in the WHO estimate was obtained by multiplying the number of deaths at each age by the global standard life expectancy for each age (rather than the regional life expectancy for each age), the sole use of premature death is likely to have missed substantial burden from life lived with disease. Causal studies on the temperature–NCD association would benefit from using DALYs or quality-adjusted life years as the outcome measure, considering a large proportion of the burden of NCD comes from life lived with disease. The YLL life table approach does not differentiate health and sociodemographic risk profiles for each individual. Unfortunately, the sample size was insufficient to further stratify NCD by age (ie, elderly) or subgroups such as cardiovascular causes. The use of longer time series in the future with larger sample sizes is likely to enable such breakdowns by cause or age, reducing the uncertainty from wide confidence bounds, and supporting better quantification of heat impacts on NCD-YLL.
Conclusion
In rural Sub-Saharan Africa, where NCDs are not the main cause of premature death, we found that moderate and extreme heat exposure significantly increases excess daily premature mortality from NCD. As NCD prevalence increases in Africa due to demographic, dietary and lifestyle changes, climate change will increasingly contribute as a risk factor towards the burden of deaths from NCD. Subsistence farming communities in Africa, such as Nouna, would therefore benefit from the development of early preventive measures to curb heat-associated NCD deaths.
Supplementary Material
Footnotes
Contributors: AB and RS developed the research idea with input from JR. Data were provided by AS. AB, MOS, JR and RS developed the modelling strategy. AB conducted the analysis, which was verified by MOS and JR. AB wrote the manuscript. All authors contributed to revision of the manuscript.
Funding: AB was funded by the Klaus-Tschira Stiftung gGmbH (00.128.2008).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There is no additional unpublished work.
References
- 1. Niang I, Ruppel OC, Abdrabo MA, et al. Africa. In: climate change 2014: impacts, adaptation, and vulnerability-part B: regional aspects-contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. NY, USA: Cambridge University Press, 2014. [Google Scholar]
- 2. Hondula DM, Rocklöv J, Sankoh OA. Past, present, and future climate at select INDEPTH member Health and Demographic Surveillance Systems in Africa and Asia. Glob Health Action 2012;5:19083–6. 10.3402/gha.v5i0.19083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abegunde DO, Mathers CD, Adam T, et al. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007;370:1929–38. 10.1016/S0140-6736(07)61696-1 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization Burkina Faso. Non-communicable Diseases (NCD) country profiles. 2014. http://www.who.int/nmh/countries/bfa_en.pdf (accessed 12 May 2016).
- 5. Friel S, Bowen K, Campbell-Lendrum D, et al. Climate change, noncommunicable diseases, and development: the relationships and common policy opportunities. Annu Rev Public Health 2011;32:133–47. 10.1146/annurev-publhealth-071910-140612 [DOI] [PubMed] [Google Scholar]
- 6. D’Ippoliti D, Michelozzi P, Marino C, et al. The impact of heat waves on mortality in 9 European cities: results from the EuroHEAT project. Environ Health 2010;9:37 10.1186/1476-069X-9-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fouillet A, Rey G, Laurent F, et al. Excess mortality related to the August 2003 heat wave in France. Int Arch Occup Environ Health 2006;80:16–24. 10.1007/s00420-006-0089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. IPCC. Summary for Policymakers Climate change 2013: the physical science basis. contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. United Kingdom and New York, NY, USA: Cambridge University Press, Cambridge. [Google Scholar]
- 9. Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 2015;386:369–75. 10.1016/S0140-6736(14)62114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diboulo E, Sié A, Rocklöv J, et al. Weather and mortality: a 10 year retrospective analysis of the Nouna Health and Demographic Surveillance System, Burkina Faso. Glob Health Action 2012;5:19078–8. 10.3402/gha.v5i0.19078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egondi T, Kyobutungi C, Kovats S, et al. Time-series analysis of weather and mortality patterns in Nairobi’s informal settlements. Glob Health Action 2012;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Egondi T, Kyobutungi C, Rocklöv J. Temperature variation and heat wave and cold spell impacts on years of life lost among the urban poor population of Nairobi, Kenya. Int J Environ Res Public Health 2015;12:2735–48. 10.3390/ijerph120302735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sié A, Louis VR, Gbangou A, et al. The Health and Demographic Surveillance System (HDSS) in Nouna, Burkina Faso, 1993-2007. Glob Health Action 2010;3 10.3402/gha.v3i0.5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramroth H, Lorenz E, Rankin JC, et al. Cause of death distribution with InterVA and physician coding in a rural area of Burkina Faso. Trop Med Int Health 2012;17:904–13. 10.1111/j.1365-3156.2012.02998.x [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. The global burden of disease. Geneva 2008. updated 2004. [Google Scholar]
- 16. Murray CJ, Lopez AD, The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Geneva: World Health Organization, 1996. [Google Scholar]
- 17. : Pruss-Ustun A, Mathers C, Corvolan C, Introduction and methods: assessing the environmental burden of disease at national and local levels. Geneva, 2003. [Google Scholar]
- 18. Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet 2012;380:2063–6. 10.1016/S0140-6736(12)61899-6 [DOI] [PubMed] [Google Scholar]
- 19. Huang C, Barnett AG, Wang X, et al. Effects of extreme temperatures on years of life lost for cardiovascular deaths: a time series study in Brisbane, Australia. Circ Cardiovasc Qual Outcomes 2012;5:609–14. 10.1161/CIRCOUTCOMES.112.965707 [DOI] [PubMed] [Google Scholar]
- 20. Huang C, Barnett AG, Wang X, et al. The impact of temperature on years of life lost in Brisbane, Australia. Nat Clim Chang 2012;2:265–70. 10.1038/nclimate1369 [DOI] [Google Scholar]
- 21. Yang J, Ou CQ, Guo Y, et al. The burden of ambient temperature on years of life lost in Guangzhou, China. Sci Rep 2015;5:12250 10.1038/srep12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basu R, Malig B. High ambient temperature and mortality in California: exploring the roles of age, disease, and mortality displacement. Environ Res 2011;111:1286–92. 10.1016/j.envres.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 23. Bhaskaran K, Gasparrini A, Hajat S, et al. Time series regression studies in environmental epidemiology. Int J Epidemiol 2013;42:1187–95. 10.1093/ije/dyt092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keatinge WR, Coleshaw SR, Easton JC, et al. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med 1986;81:795–800. 10.1016/0002-9343(86)90348-7 [DOI] [PubMed] [Google Scholar]
- 25. Semenza JC, McCullough JE, Flanders WD, et al. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 1999;16:269–77. 10.1016/S0749-3797(99)00025-2 [DOI] [PubMed] [Google Scholar]
- 26. Basu R, Samet JM. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect 2002;110:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tawatsupa B, Lim LL, Kjellstrom T, et al. The association between overall health, psychological distress, and occupational heat stress among a large national cohort of 40,913 Thai workers. Glob Health Action 2010;3:5034 10.3402/gha.v3i0.5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tawatsupa B, Lim LL, Kjellstrom T, et al. Association between occupational heat stress and kidney disease among 37,816 workers in the Thai Cohort Study (TCS). J Epidemiol 2012;22:251–60. 10.2188/jea.JE20110082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baccini M, Kosatsky T, Biggeri A. Impact of summer heat on urban population mortality in Europe during the 1990s: an evaluation of years of life lost adjusted for harvesting. PLoS One 2013;8:e69638 10.1371/journal.pone.0069638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013;369:448–57. 10.1056/NEJMra1201534 [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization Regional Office for Africa. Health situation analysis in the African region. Brazzaville 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018068supp001.pdf (990.6KB, pdf)