Abstract
We present the case report of an 80-year-old woman with chronic kidney disease stage G5 admitted to the hospital with fluid overload and hyperkalaemia. Sodium polystyrene sulfonate (SPS, Kayexalate) in sorbitol suspension was given orally to treat her hyperkalaemia, which precipitated an episode of SPS in sorbitol-induced ischaemic colitis with the subsequent complication of vancomycin-resistant Enterococcus (VRE) bacteraemia. SPS (Kayexalate) in sorbitol suspension has been implicated in the development of intestinal necrosis. Sorbitol, which is added as a cathartic agent to decrease the chance of faecal impaction, may be primarily responsible through several proposed mechanisms. The gold standard of diagnosis is the presence of SPS crystals in the colon biopsy. On a MEDLINE search, no previous reports of a VRE bacteraemia as a complication of biopsy-confirmed SPS in sorbitol ischaemic colitis were found. To the best of our knowledge, ours would be the first such case ever reported.
Keywords: drugs: gastrointestinal system, contraindications and precautions, gi bleeding, endoscopy, infectious diseases
Background
Sodium polystyrene sulfonate (SPS, Kayexalate) in sorbitol has been implicated in the development of intestinal necrosis and bleeding. Sorbitol, which is added as a cathartic agent to decrease the chance of faecal impaction, may be primarily responsible.1 2 Incidence of SPS-mediated intestinal injury has been estimated to be between 0.27% and 1.8%. Mortality is approximately 36%.3 4
Enterococci are primarily opportunistic pathogens. The majority of vancomycin-resistant Enterococcus (VRE) colonisation involves the gastrointestinal (GI) tract, the urinary tract, the skin and the oral cavity.5 Risk factors for infection with VRE include length of hospital stay, diabetes, kidney disease and use of antibiotics (particularly cephalosporins and drugs with activity against anaerobic bacteria).5 6 Defects of epithelial barriers (mainly the intestinal mucosa) might rarely cause VRE bacteraemia due to bacterial translocation. Enterococcal bacteraemia has a high reported mortality of around 34%.7
We believe that this case is important taking into consideration that the considerably high mortality of both entities highlights the importance of educating our colleagues on safer therapeutic options when managing hyperkalaemia, such as the substitution of oral or rectal SPS for intravenous furosemide, whenever appropriate.
Additionally, no previous reports of a VRE bacteraemia as a complication of biopsy-confirmed SPS in sorbitol ischaemic colitis were found on our MEDLINE search. To the best of our knowledge, ours would be the first such case ever reported.
Case presentation
An 80-year-old woman presents to the emergency department with the complain of shortness of breath, decreased exercise tolerance, chest tightness and progressive bilateral lower extremity swelling onset 3 days before admission.
On physical examination, the patient was alert and oriented to self, place and time. Her lung examination revealed bilateral ronchi, no wheezing and no rales. She also had bilateral lower extremity pitting oedema.
Our patient had a medical history of hypertension, diabetes, chronic kidney disease (CKD) stage G5 with recurrent episodes of hyperkalaemia, coronary artery disease requiring two stents, chronic obstructive pulmonary disease global initiative for obstructive lung disease D and chronic anaemia.
On admission, her laboratory results showed a normal white cell count, a baseline normocytic anaemia with a haemoglobin of 8.2 g/dL, hyperkalaemia 6.2 mEq/L with a creatinine of 3.5 mg/dL (baseline of 3.0 mg/dL) and urea of 46 mg/dL. Brain natriuretic peptide was 529 pg/mL.
ECG showed new T wave inversions in the inferior and lateral leads. Troponin was twice negative.
Her hyperkalaemia was managed with intravenous furosemide, inhaled albuterol and intravenous dextrose with insulin. Her hyperkalaemia improved to 5.0 mEq/L.
Cardiology was consulted, but they determined that the patient’s symptoms were more likely to be related to fluid overload secondary to her CKD, rather than an episode of congestive heart failure, in the light of a recent echocardiogram showing a normal ejection fraction.
The patient was then admitted to the general medical floors (GMFs) for aggressive diuresis under the diagnostic impression of fluid overload and hyperkalaemia in the context of a patient with CKD stage G5. Nephrology was consulted to determine the need for haemodialysis, but it was determined that she did not require emergent haemodialysis at that time. On hospital day 2, recurrent hyperkalaemia at 6.6 mEq/L was noticed for which she received appropriate emergent medical management. In addition, two doses of 30 g of oral SPS in sorbitol (presentation as a premixed solution of Kayexalate 15 g/sorbitol 20 g/60 mL) were given to the patient over the course of the day. Of note, this dose is within the accepted daily dosage for SPS in sorbitol. Serum potassium was followed up a few hours later, and it had come back to normal 5.1 mEq/L.
On the morning of day 3, the patient complained of severe abdominal pain onset overnight, associated with several watery, non-bloody bowel movements. She was afebrile and had no nausea and no vomiting. Her abdominal examination was remarkable for hypoactive bowel sounds, diffuse, severe tenderness mainly in the lower quadrants, no guarding and no rebound.
On her laboratory results, she had an abrupt increase in her white cell count from 7400/µL the day before to 29 400/µL with a left shift. Serum creatinine increased to 3.9 mg/dL. Lactic acid was elevated at 3.8 mmol/L. An initial set of blood cultures were obtained, which were ultimately negative. Empiric intravenous piperacillin/tazobactam and metronidazole were started.
Given the clinical presentation and laboratory findings, our differential diagnosis included SPS in sorbitol-induced ischaemic colitis versus mesenteric ischaemia versus infectious colitis. The need for a CT angiogram of the abdomen to rule out mesenteric ischaemia was discussed with the patient, but after disclosing to her that intravenous contrast could further deteriorate her already poor kidney function and that after that she might have required haemodialysis, the patient decided against having the study done.
The alternative of a CT abdomen and pelvis with oral contrast was then offered to the patient, to which she agreed to. This study showed wall thickening and pneumatosis coli of the extending transverse colon with mild adjacent fat stranding (figure 1).
Figure 1.
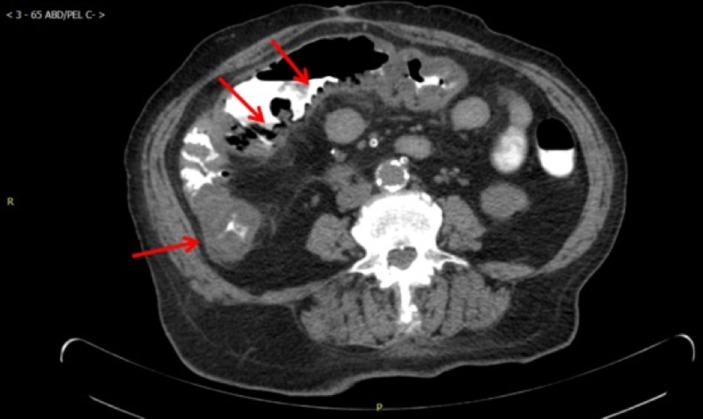
CT with oral contrast shows bowel wall thickening at ascending colon and proximal transverse colon. There are foci of air in the bowel wall concerning for pneumatosis coli. Finding is compatible with colitis. Evaluation is limited owing to lack of intravenous contrast.
General surgery was consulted. Emergent abdominal surgery was discussed. However, given the patient’s advanced age and multiple comorbidities, they determined her surgical prognosis to be extremely poor. It was decided that the procedure should only be considered as a last-resort measure if the patient’s condition worsened.
Gastroenterology was also consulted. They recommended against scoping the patient at that time. They also recommended to rule out infectious colitis.
The patient was transferred to the medical intensive care unit (ICU) to closely monitor her clinical status. Her lactic acid level improved with gentle intravenous hydration. The following day, her abdominal pain had improved with intravenous antibiotics and hydration. General surgery re-evaluated the patient and recommended no surgical intervention and to continue medical management.
On day 6, owing to resolution of her symptoms and tolerance to oral diet, patient was transferred back to the GMF. Infectious colitis was ruled out after the stool PCR for Clostridium difficile was negative, stool ova and parasites were negative and stool cultures for Shigella, Salmonella, Campylobacter, Escherichia coli, Cryptosporidium, Isospora and Cyclospora were all negative. Faecal Wright stain was negative for leucocytes.
The patient had a torpid hospital course with recurrent episodes of hyperkalaemia due to dietary indiscretions, as well as persistent leucocytosis (in a range between 15 000/µL and 23 000/µL) without fever in spite of therapy with intravenous piperacillin/tazobactam and metronidazole. Since there was uncertainty regarding the aetiology of her persistent leucocytosis in the absence of fever, intravenous antibiotics were discontinued after completing a 12-day course. Serum procalcitonin as well as repeat blood cultures were ordered on hospital day 15. A urinalysis and urine culture were also ordered at the same time.
On hospital day 16, owing to hyperkalaemia 6.9 with ECG changes, the patient was transferred from the GMF to the telemetry unit. Once there, she finally spiked a fever of 38.7 °C/101.7 °F overnight. Her hyperkalaemia was managed with diuretics. Serum procalcitonin came back elevated at 0.57 ng/mL (normal range of 0–0.08 ng/mL), and the repeat blood cultures were preliminarily reported as growing Gram-positive cocci in clusters and chains in the aerobic bottles. Renally dosed intravenous vancomycin was then started. Urinalysis and urine cultures came back negative. On day 17, a family meeting was held, after which the patient agreed to haemodialysis.
A tunnelled dialysis catheter was then placed by interventional radiology, and the patient was immediately taken for emergent haemodialysis, which corrected her serum potassium back to normal 5.3 mEq/L. After her dialysis, she was transferred back to the GMF. Final identification of the organism growing in the blood culture was made, turning out to be a vancomycin-resistant Enterococcus faecium. Antibiotic therapy was adjusted according to sensitivity.
A few hours after her return to the GMF, the patient had a new spontaneous episode of abdominal pain associated with four episodes of profuse, frank haematochezia, causing a drop in her postdialysis haemoglobin from 9.4 to 6.3 g/dL, requiring the emergent transfusion of 4 units of packed red cells.
She was once more transferred to the medical ICU on day 24, and gastroenterology was consulted again. They recommended to obtain a CT angiogram of the abdomen, to which the patient now agreed, as she had been already started on haemodialysis. Surgery was consulted again and recommended to stabilise the patient with intravenous fluids and transfusions as needed.
CT angiogram showed wall thickening of the transverse colon with mild adjacent fat stranding. The previously visualised pneumatosis coli was no longer present. Mucosal enhancement with multiple foci of contrast extravasation was present, which was compatible with active bleeding (figure 2). On addition, the study revealed a patent coeliac artery (figure 3). Patency was also demonstrated in the distal superior mesenteric artery (SMA) and inferior mesenteric artery (IMA) (figure 4).
Figure 2.
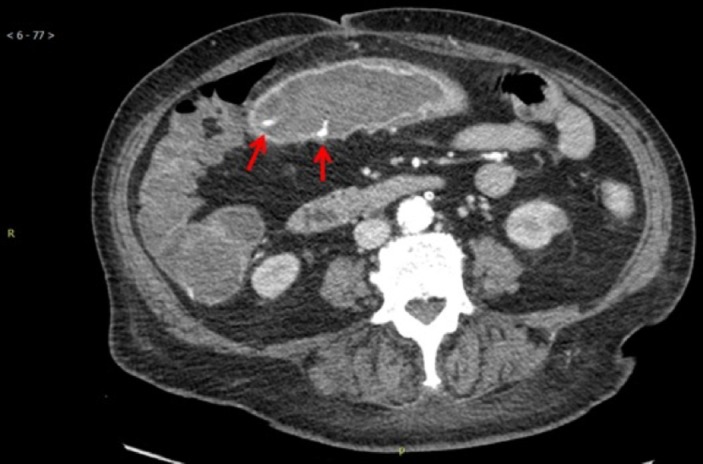
CT with intravenous contrast again shows bowel wall thickening at ascending and proximal transverse colon. There is mucosal enhancement and areas of contrast extravasation (arrows) compatible with reperfusion injury and active bleeding.
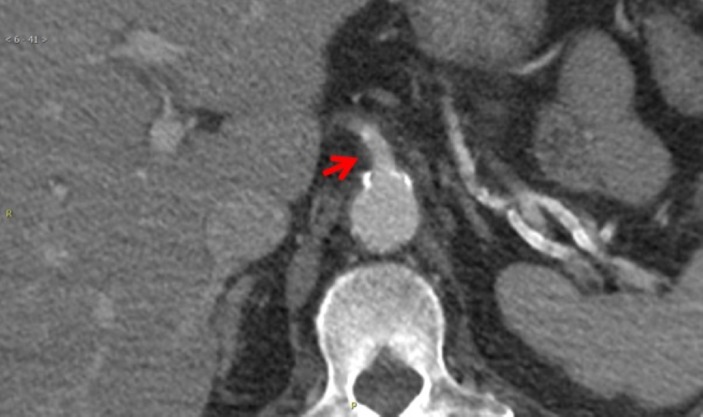
Figure 3.
CT with intravenous contrast shows patent coeliac artery.
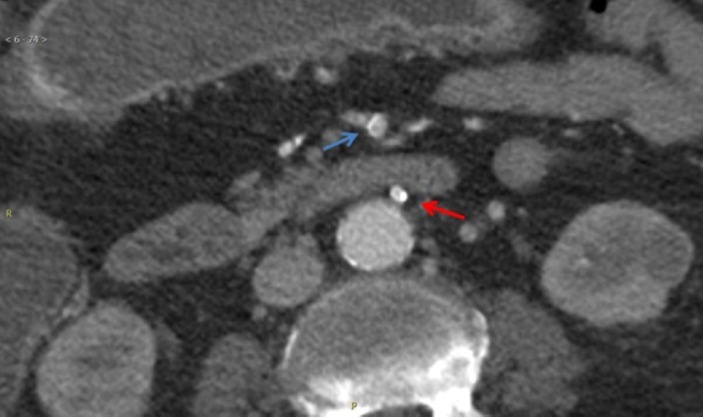
Figure 4.
CT with intravenous contrast shows patent distal superior mesenteric artery (blue arrow) and patent inferior mesenteric artery (red arrow).
After the angiogram was completed, an emergent, unprepped oesophagogastroduodenoscopy (OGD) and colonoscopy were done. The OGD was unremarkable, as no lesions were seen. The colonoscopy, though, showed signs of active bleeding in the descending and sigmoid colon, as well as the rectum. There was a lumen obstructing clot in the midtransverse colon (~60 cm). The adjacent mucosa looked denudated and unhealthy, with contact oozing and friability. Healing old ulcers were seen, and biopsies were taken. The study could not be completed as the scope could not be safely traversed past the large clot. Biopsy results showed basophilic crystalline material consistent with SPS crystals (figure 5).
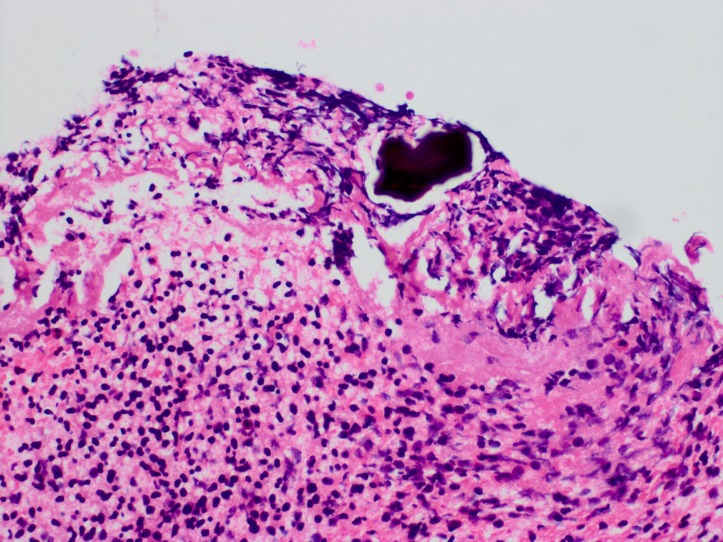
Figure 5.
Pathology slide of our patient’s colonic biopsy showing an amorphous basophilic crystalline material, morphologically compatible with sodium polystyrene sulfonate (Kayexalate) crystal.
Investigations
Mesenteric ischaemia was ruled out with the CT abdominal angiogram showing patency of the coeliac trunk, SMA and IMA.
Infectious colitis was ruled out with the negative stool ova and parasites, negative stool culture for Shigella, Salmonella, Campylobacter, E. coli, Cryptosporidium, Isospora and Cyclospora and negative stool PCR for C. difficile. The faecal Wright stain was also negative for leucocytes.
SPS in sorbitol-induced colitis was confirmed by the presence of SPS crystals in the colon biopsy.
Differential diagnosis
Our differential diagnosis included SPS in sorbitol-induced ischaemic colitis versus mesenteric ischaemia versus infectious colitis.
Outcome and follow-up
During the following 48 hours, the patient’s haematochezia resolved spontaneously.
She was restarted on diet, which she tolerated well and was transferred back to the GMF for completion of antibiotic therapy. Repeat blood cultures were obtained, which came back negative. Without further incident, the patient was discharged from the hospital on day 39.
Discussion
SPS (Kayexalate) is a cation-exchange resin used to treat hyperkalaemia. It can be administered orally or rectally. After entering the GI tract, sodium ions are exchanged for potassium ions, which are then excreted into the stools.1 Most potassium binding happens in the colon. SPS can cause faecal impaction, so a premixed solution of SPS in sorbitol is commonly used by many hospital pharmacies to prevent SPS-induced impaction and to hasten delivery of the resin to the colon when administered orally.2
The precise mechanism by which SPS in sorbitol causes intestinal injury still hasn’t been elucidated, but it is thought to be primarily mediated by the sorbitol component.1 Sorbitol, which is a hyperosmotic cathartic, may directly damage the intestinal mucosa, exacerbate inflammation due to increased prostaglandin production and cause vasospasm of the colonic blood vessels as a response to osmotic mucosal injury. Intestinal ischaemia, necrosis and bleeding are recognised risks of SPS in sorbitol.3 4
In 2006, the Food and Drug Administration allowed the premixed formulation of Kayexalate (SPS) in 33% sorbitol (which is the one available in our institution) to continue in the market, as previous reports of adverse GI events followed administration of the resin in 70% sorbitol.2 More recently, even the current formulation of SPS in 33% sorbitol at its recommended daily dose was also implicated in cases of intestinal necrosis. Alternate vehicles for SPS (syrup, lactulose, brownies, etc) have been proposed, but studies are lacking.3 Newer agents such as patiromer (Veltassa) should be considered as a safer alternative to Kayexalate when cation exchange resins are required.
Risk factors for developing SPS in sorbitol-induced ischaemic colitis include uraemia, hypovolaemia, hypotension (after haemodialysis or surgery), peripheral vascular disease, thrombocytopaenia and CKD with elevated renin, which might cause angiotensin-mediated vasoconstriction precipitating non-occlusive mesenteric ischaemia.8
Incidence of SPS-mediated intestinal injury has been estimated to be between 0.27% and 1.8%. Mortality is approximately 36%.3 Most patients who develop symptoms of intestinal injury after oral ingestion of SPS in sorbitol do so between 3 hours and 11 days of its use.2 The most common reported symptoms are abdominal pain, nausea, diarrhoea and haematochezia.9
In the case of our patient, she had CKD and uraemia as risk factors, and she developed symptoms of acute abdominal pain associated with watery, non-bloody diarrhoea a few hours after oral administration of SPS in sorbitol.
The endoscopic findings in cases of SPS in sorbitol-induced intestinal injury are consistent with those seen in ischaemic enterocolitis. The gold standard for the diagnosis of SPS in sorbitol ischaemic colitis is the presence of SPS crystals in the colonic mucosa.1 In the case of our patient, the colonoscopy was not done until after she developed massive haematochezia, almost 21 days after her initial symptoms. In spite of this delay, the biopsy results showed basophilic crystalline material morphologically compatible with SPS crystals as well as ischaemic bowel mucosa and purulent granulation tissue.
Causation was assessed with the Naranjo adverse drug reaction (ADR) probability scale (table 1).10 The calculated ADR score suggested that SPS in sorbitol was the probable cause for the patient’s GI symptoms.
Table 1.
The Naranjo ADR probability scale
| Yes | No | Do not know | SPS in sorbitol-induced ischaemic colitis score | |
| 1. Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | +1 |
| 2. Did the adverse event occur after the suspected drug was administered? | +2 | −1 | 0 | +2 |
| 3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| 4. Did the adverse reaction reappear when the drug was readministered? | +2 | −1 | 0 | 0 |
| 5. Are there alternative causes (other than the drug) that could have on their own caused the reaction? | −1 | +2 | 0 | +2 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| 7. Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | +1 |
| Total score | 7 | |||
ADR score: >9=definitive, 5–8=probable, 1–4=possible, 0=doubtful.
ADR, adverse drug reaction; SPS, sodium polystyrene sulfonate.
Enterococci are primarily opportunistic pathogens; risk factors for infection with VRE include length of hospital stay, diabetes, kidney disease and use of antibiotics (particularly cephalosporins and drugs with activity against anaerobic bacteria).5 6 The majority of VRE colonisation involves the GI tract, the urinary tract, the skin and the oral cavity.5 Of the risk factors for infection with VRE, our patient had diabetes, CKD and exposure to piperacillin/tazobactam and metronidazole during her hospital stay.
Enterococcal bacteraemia has a high reported mortality, at around 34%, but it is not clear if this high mortality is due to the infection or due to the underlying comorbidities that predispose to the development of the bacteraemia.7 One of the most common sources of nosocomial VRE bacteraemia tends to be central intravascular catheters.11 The urinary tract is also a potential source of bacteraemia, especially when a urinary catheter is present.7
Our patient had positive blood cultures before she had the dialysis catheter placed, never had the need for urinary catheterisation and the urinalysis and urine culture that were obtained at the same time that the second set of blood cultures showed no sign of infection. Thus, the only reasonable source of seeding for her bacteraemia was her colon, whose mucosal integrity had been compromised by the SPS-induced ischaemic colitis. To the best of our knowledge, there are no previously reported cases of vancomycin-resistant E. faecium bacteraemia as a consequence of SPS-induced ischaemic colitis.
Treatment options for VRE infections include linezolid, daptomycin, quinupristin–dalfopristin and tigecycline.5
Of note, the episode of massive haematochezia our patient suffered almost immediately after her first session of haemodialysis can be explained by the fact that her colonic mucosa had already been injured by the SPS in sorbitol-induced ischaemic colitis. On top of this, once patients reach end-stage renal disease requiring haemodialysis, changes in the volaemia during haemodialysis, hyper-reninaemia, increased prostaglandin production and focal colonic mesenteric vasospasm can precipitate intestinal necrosis.3 These facts together could explain the severity of the patient’s haematochezia.
Learning points.
Sodium polystyrene sulfonate (SPS, Kayexalate) in sorbitol is associated with life-threatening episodes of ischaemic colitis.
Physicians should consider safer alternatives to promote the excretion from the body of the excessive potassium associated with hyperkalaemia; for example, intravenous furosemide.
Hospital pharmacies should consider using an alternate premixed solution of Kayexalate, given the risks involved in the concomitant use of Kayexalate and sorbitol, or switch to a newer, safer agent such as patiromer (Veltassa).
SPS in sorbitol-induced ischaemic colitis increases the gut permeability, potentially causing bacterial translocation into the bloodstream.
Patients with end-stage renal disease undergoing haemodialysis after suffering an episode of ischaemic colitis of SPS in sorbitol can suffer episodes of haematochezia due to the haemodynamic, metabolic and inflammatory changes that occur during and after dialysis.
Footnotes
Contributors: RCC-R was in charge of performing the chart review, obtaining consent from the patient and doing the initial literature review on the topic. He was also responsible for writing the drafts for the case report and making any modifications required by the Institutional Review Board or the supervising attending physician. DA-A was in charge of additional literature review, on obtaining additional sources for medical records of the patient that could be relevant to our case report and of obtaining authorisation from the Radiology and Pathology Department to use the images presented in this case report. BBC provided additional interpretation of the images obtained during the patient’s hospital admission during the write-up of this case report. Additionally, he was directly involved in the patient’s clinical care as the radiologist in charge of notifying the multiple life-threatening findings to the primary team in an expedited fashion. AA was in charge of the overall execution of the case report, verifying that all steps required by our Institutional Review Board were fulfilled, as well as proofreading of the initial versions of the manuscript and approving the final version of the submitted manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chou YH, Wang HY, Hsieh MS. Colonic necrosis in a young patient receiving oral kayexalate in sorbitol: case report and literature review. Kaohsiung J Med Sci 2011;27:155–8. 10.1016/j.kjms.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 2.Sterns RH, Rojas M, Bernstein P, et al. . Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010;21:733–5. 10.1681/ASN.2010010079 [DOI] [PubMed] [Google Scholar]
- 3.McGowan CE, Saha S, Chu G, et al. . Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J 2009;102:493–7. 10.1097/SMJ.0b013e31819e8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Food and Drug Administration. Kayexalate SODIUM POLYSTYRENE SULFONATE, USP Cation-Exchange Resin, 2010https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/011287s023lbl.pdf (accessed 24 Jun 2017).
- 5.O’Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist 2015;8:217–30. 10.2147/IDR.S54125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9. 10.1086/321815 [DOI] [PubMed] [Google Scholar]
- 7.Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990;3:46–65. 10.1128/CMR.3.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid A, Hamilton SR. Necrosis of the gastrointestinal tract in uremic patients as a result of sodium polystyrene sulfonate (Kayexalate) in sorbitol: an underrecognized condition. Am J Surg Pathol 1997;21:60–9. 10.1097/00000478-199701000-00007 [DOI] [PubMed] [Google Scholar]
- 9.Bomback AS, Woosley JT, Kshirsagar AV. Colonic necrosis due to sodium polystyrene sulfate (Kayexalate). Am J Emerg Med 2009;27:e1–753. 10.1016/j.ajem.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naranjo CA, Busto U, Sellers EM, et al. . A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 11.Weiner LM, Webb AK, Limbago B, et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016;37:1288–301. 10.1017/ice.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]