Abstract
Objectives
The clinical course and prognosis of follicular lymphoma (FL) are diverse and associated with the patient’s immune response. We investigated the lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) as prognostic factors in patients with FL, including those receiving radiotherapy.
Design
A retrospective cohort study.
Setting
Regional cancer centre in Hong Kong.
Participants
88 patients with histologically proven FL diagnosed between 2000 and 2014.
Materials and methods
The best LMR and NLR cut-off values were determined using cross-validated areas under the receiver operating characteristic curves. The extent to which progression-free survival (PFS) and overall survival differed by NLR and LMR cut-off values was assessed using Kaplan-Meier analysis and log-rank tests. A Cox proportional hazards model was fitted to adjust for confounders.
Results
The best cut-off values for LMR and NLR were 3.20 and 2.18, respectively. The 5-year PFS was 73.6%. After multivariate adjustment, high LMR (>3.20) at diagnosis was associated with superior PFS, with a HR of 0.31 (95% CI 0.13 to 0.71), whereas high NLR at relapse was associated with poorer postprogression survival (HR 1.24, 95% CI 1.04 to 1.49).
Conclusions
Baseline LMR and NLR at relapse were shown to be independent prognostic factors in FL. LMR and NLR are cheap and widely available biomarkers that could be used in combination with the Follicular Lymphoma International Prognostic Index by clinicians to better predict prognosis.
Keywords: neutrophils, monocytes, lymphocytes, lymphoma, survival, prognosis
Strengths and limitations of this study.
We obtained strong evidence in support of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio as prognostic factors that possess practical clinical utility and significance in follicular lymphoma.
Sensitivity analysis was performed to determine the robustness of the main findings in different scenarios.
Association between cell count ratios and systemic treatment choices, duration and number of cycles, and salvage treatment on progression were not analysed because of a limited sample size for subgroup analysis.
Introduction
Follicular lymphoma (FL) accounts for approximately 20% of all incident lymphoma cases, making it the most common indolent non-Hodgkin’s lymphoma (NHL). The clinical course and prognosis of FL are diverse.1–6 Clinical and laboratory parameters assist in predicting prognosis, allow for tailoring appropriate therapies and aid in selecting patients for appropriate clinical trials. The commonly used criteria include the Groupe d’Etude des Lymphomes Folliculaires criteria,7 Follicular Lymphoma International Prognostic Index (FLIPI)2 and Follicular Lymphoma International Prognostic Index 2 (FLIPI2).8 FLIPI is a clinical prognostic score and classifies patients into risk categories: low, intermediate and high risk. It does not include parameters associated with tumour microenvironment or host antitumour immune response.
About 20% of patients with FL do not respond to or experience progression within 2 years of treatment; early relapse manifests in a subgroup of patients who are at a substantially greater risk of death, and their median overall survival (OS) is only 5 years.9 These cases of high-risk FL may have a distinct biology, but it is not easily identified at diagnosis; even patients with high-risk disease defined by the commonly employed FLIPI2 could have prolonged survival with modern therapy. A biological rationale to account for this heterogeneity in patient outcomes would provide insights that may influence disease monitoring and treatment strategy.
Advances in gene expression profiling allow us to elucidate the role of stromal, non-malignant cells in the pathogenesis and progression of lymphoma. Immune response-1 and immune response-2 are two types of immune responses.10 Dave et al discovered that most of the component genes in prognostically unfavourable immune response-2 signatures are expressed more strongly in the non-malignant component of tumours.10 Many genes in the immune response-2 signature are highly expressed by peripheral blood monocytes. Furthermore, monocyte chemoattractant protein, a potent chemotactic factor for monocytes, and its receptor CC chemokine receptor 2 are shown to play roles in modulating inflammatory responses, tumour proliferation, angiogenesis and metastasis.11 12 Their levels of expression are correlated with cancer prognosis. In addition, myeloid-derived suppressor cells are reported to have immune-suppressive functions.13–15 Increasing numbers of monocytes, macrophages or their precursors have been detected in lymphomatous nodes.13 16 Recent studies have indicated that the peripheral blood lymphocyte-to-monocyte ratio (LMR) at diagnosis can predict long-term outcome in patients with diffuse large B-cell lymphoma,17 FL,18 19 and Hodgkin lymphoma (HL).20–22 This evidence indicates that monocytes are an important component of the tumour microenvironment.
On the other hand, absolute neutrophil count, a surrogate marker of inflammation produced by the tumour,23–26 is used in the form of peripheral blood neutrophil-to-lymphocyte ratio (NLR) at diagnosis to predict survival in diffuse large B-cell lymphoma17 27 and HL.28 The rationale behind using these cell count ratios is to consider the interaction among components of host immunity represented by lymphocytes, inflammation produced by the tumour and the tumour microenvironment. However, studies on FL mainly focus on patients who were treated with rituximab-containing chemotherapy, with little emphasis on those who underwent radiotherapy (RT) as a component of or as a primary treatment. Moreover, the prognostic role of NLR in FL in terms of survival outcomes has not been studied. Therefore, we aimed to investigate the extent to which NLR at diagnosis predicts survival outcomes in patients with FL, including those who were treated with RT. We also evaluated whether NLR can be used in combination with FLIPI to improve prognosis prediction.
Materials and methods
Study design, setting and participants
We performed a longitudinal study using retrospective information from electronic medical records of patients with incident FL treated in Tuen Mun Hospital, Hong Kong. All FL incident cases from 2000 to 2014 were identified (n=88). We restricted the analysis to patients with complete laboratory, pathology and radiological data in the medical records (online supplementary figure S1). The sociodemographic information of the excluded patients was not different from that of the included patients in the final sample. Patients were followed up for a median of 5.88 (range: 0.49–16.45) years. The peripheral blood count results were obtained from a standard automated complete blood count machine. This study was approved by the Clinical and Research Ethics Committee of the Tuen Mun Hospital, Tuen Mun, Hong Kong (NTWC/CREC/16107). The research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
bmjopen-2017-017904supp001.pdf (7.4KB, pdf)
Data and variables
OS and progression-free survival (PFS) were the main outcomes of the study. These outcomes were defined and measured as per criteria from the International Harmonization Project.29 OS was defined as the time from diagnosis until death as a result of any cause or the last follow-up visit for censored patients. PFS was defined as the time from diagnosis until lymphoma progression (first date of documentation of a new lesion or enlargement of a previous lesion) or death as a result of any cause or last follow-up visit for censored patients. Postprogression survival (PPS) was defined as the time from progression or relapse to the date of death as a result of any cause or last follow-up visit for censored patients. For the survival endpoints, patients were censored at their last follow-up visit. Patients’ demographics and disease characteristics were collected. The FLIPI score was then calculated using those factors (nodal sites, age, serum lactate dehydrogenase, stage and haemoglobin) (see online supplementary table S1).2 Chemotherapy involved cyclophosphamide, vincristine and prednisolone or cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) or CHOP-like regimens.
bmjopen-2017-017904supp002.pdf (241.2KB, pdf)
Statistical methods
We initially described the cohort of patients using ranges (minimum, maximum), means and SD for continuous variables and proportions for categorical variables. To evaluate LMR and NLR performance in predicting mortality, we fitted two logistic models with cancer-related death as the outcome and LMR and NLR as continuous independent predictors. Data-adaptive methods based on Bayesian and Akaike information criteria were used to determine the impact of any associated factors and to identify the best fitting model. Subsequently, we computed marginal probabilities for the outcome based on two different weighted logistic models, with time and NLR as independent predictors for the first model and time and LMR for the second model. Weights were accounted for the inverse probability of censoring.30 Then we derived cross-validated areas under the curve (AUC)31; afterwards we chose the best cut-off values based on the cross-validated sensitivity, specificity and the Youden’s indices (sensitivity+specificity−1). Respective LMR and NLR cut-off values were determined at a point with the maximum Youden’s index on the receiver operating characteristic (ROC) curve.32 33 To evaluate the extent to which OS and PFS differ by LMR and NLR cut-off values, we used incidence, rate ratios, Kaplan-Meier analysis and log-rank tests34 35 for statistical inference. We also used semiparametric Cox proportional hazards models to evaluate OS and PFS for the computed LMR and NLR cut-off values adjusted for FLIPI, use of rituximab and sex.36 37 Finally, we developed a sensitivity analysis to evaluate the robustness of our findings in the multivariate analysis including different model specifications to account for non-linearities and the interaction between rituximab and LMR/NLR levels. The proportional hazard assumption for multivariate-adjusted Cox models was also assessed based on the analysis of the Schoenfeld residuals. We used Stata V.14.2 (StataCorp) for the statistical analysis.
Results
Description of the cohort
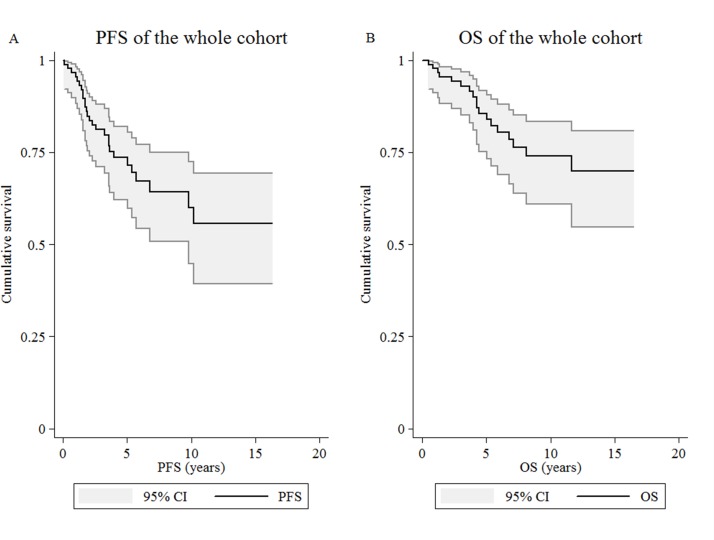
The median age at diagnosis of the patients included in the study was 54 years (range: 22–87 years). Among them, 18 died during the follow-up period. Thirteen patients died due to the lymphoma. Five deaths were non-lymphoma related: one patient developed prostate cancer and died of pneumonia, another three died of community-acquired pneumonia and one died of acute coronary syndrome and renal failure. The estimated 5-year PFS and OS were 73.6% and 85.6%, respectively (figure 1). At diagnosis, 18.2%, 21.6% and 60.2% were classified as being at low, intermediate and high risk according to the FLIPI score. Table 1 shows the descriptive summary statistics of patients included in the study.
Figure 1.
Kaplan-Meier curves. Kaplan-Meier estimate for (A) progression-free survival (PFS) and (B) overall survival (OS) of the whole study cohort (n=88).
Table 1.
Descriptive summary statistics for the best cut-offs of LMR and NLR according to patient clinical characteristics, n=88
| Characteristics | All patients (n=88) |
LMR >3.20 (n=49) |
LMR ≤3.20 (n=39) |
NLR >2.18 (n=57) |
NLR ≤2.18 (n=31) |
| Age, years | |||||
| Median (range) | 54 (22–87) | 53 (22–87) | 54 (31–78) | 55 (31–87) | 52 (22–77) |
| >60, n (%) | 29 (33.0) | 16 (32.7) | 13 (33.3) | 24 (42.1) | 5 (16.1) |
| ≤60, n (%) | 59 (67.0) | 33 (67.4) | 26 (66.7) | 33 (57.9) | 26 (83.9) |
| Sex, n (%) | |||||
| Male | 47 (53.4) | 29 (59.2) | 18 (46.2) | 27 (47.4) | 20 (64.5) |
| Female | 41 (46.6) | 20 (40.8) | 21 (53.9) | 30 (52.6) | 11 (35.5) |
| FLIPI, n (%) | |||||
| Low risk (scores 0–1) | 16 (18.2) | 9 (18.4) | 7 (18.0) | 6 (10.5) | 10 (32.3) |
| Intermediate risk (score 2) | 19 (21.6) | 13 (26.5) | 6 (15.4) | 10 (17.5) | 9 (29.0) |
| High risk (scores 3–5) | 53 (60.2) | 27 (55.1) | 26 (66.7) | 41 (71.9) | 12 (38.7) |
| ANC (109/L), median (range) | 4.2 (1.9–10.7) | 3.7 (1.9–7.9) | 4.6 (2.1–10.7) | 4.6 (2.1–10.7) | 3.5 (1.9–7.9) |
| ALC (109/L), median (range) | 1.6 (0.6–11.3) | 1.9 (0.7–11.3) | 1.1 (0.6–3.1) | 1.2 (0.6–3.1) | 2.1 (1.4–11.3) |
| AMC (109/L), median (range) | 0.4 (0.1–1.2) | 0.4 (0.1–0.9) | 0.5 (0.2–1.2) | 0.4 (0.1–1.2) | 0.4 (0.2–1.1) |
| NLR, median (range) | 2.76 (0.59–9.91) | 2.15 (0.59–8.50) | 3.83 (1.81–9.91) | 3.50 (2.20–9.91) | 1.73 (0.59–2.18) |
| LMR, median (range) | 3.80 (0.55–22.60) | 5.00 (3.43–22.60) | 2.33 (0.55–3.20) | 3.00 (0.55–8.00) | 5.33 (2.82–22.60) |
| LDH >220 IU/L, n (%) | 70 (80.5) | 39 (81.3) | 31 (79.5) | 47 (82.5) | 23 (76.7) |
| Stage, n (%) | |||||
| I/II | 24 (27.3) | 14 (28.6) | 10 (25.6) | 11 (19.3) | 13 (41.9) |
| III/IV | 64 (72.7) | 35 (71.4) | 29 (74.4) | 46 (80.7) | 18 (58.1) |
| Hb <12 g/dL, n (%) | 23 (26.1) | 14 (28.6) | 9 (23.1) | 15 (26.3) | 8 (25.8) |
| Number of nodal sites >4, n (%) | 50 (56.8) | 24 (49.0) | 26 (66.7) | 37 (64.9) | 13 (41.9) |
| Use of rituximab, n (%) | 38 (43.2) | 19 (38.8) | 19 (48.7) | 27 (47.4) | 11 (35.5) |
| Treatment, n (%) | |||||
| Chemotherapy plus RT | 14 (15.9) | 9 (10.2) | 5 (5.7) | 8 (9.1) | 6 (6.8) |
| Chemotherapy alone | 54 (61.4) | 26 (29.5) | 28 (31.8) | 40 (45.5) | 14 (15.9) |
| RT alone | 14 (15.9) | 9 (10.2) | 5 (5.7) | 6 (6.8) | 8 (9.1) |
ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; FLIPI, Follicular Lymphoma International Prognostic Index; Hb, haemoglobin; LDH, lactate dehydrogenase; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; RT, radiotherapy.
Only 18 patients underwent RT as a definitive treatment for limited stages I and II. Involved-field irradiation, in which the RT fields were limited to the involved nodal region, was mostly administered with two parallel opposed fields; the median radiation dose was 40 Gy (range: 30–54 Gy). Ten other patients with stage III or IV disease received RT during their disease course, as part of palliation or as consolidation therapy to sites demonstrating an inadequate response to systemic treatment. High-grade transformation occurred in 6 out of 27 patients with relapse. Peripheral blood counts were available at the time of the relapse.
Progression-free survival
The AUCs of LMR and NLR were 0.90 (95% CI 0.84 to 0.97) and 0.87 (95% CI 0.77 to 0.96), respectively, and they did not differ in terms of predictive performance for PFS (test equality of ROC areas, p value 0.470). An LMR cut-off value of 3.20 (positive predictive value 22.4% and negative predictive value 82.1%; sensitivity 61.1% and specificity 45.7%) and NLR cut-off value of 2.18 (positive predictive value 21.1% and negative predictive value 80.6%; sensitivity 66.7% and specificity 35.7%) showed the greatest Youden’s index, corresponding to maximum joint sensitivity and specificity on the ROC curve (online supplementary table S2).
bmjopen-2017-017904supp003.pdf (70.1KB, pdf)
NLR and LMR mortality predictive performance
The median NLR and LMR at diagnosis were 2.77 (range: 0.59–9.91) and 3.80 (range: 0.55–22.60), respectively. The median NLR and LMR at relapse were 2.67 (range: 0.95–17.25) and 3.33 (range: 0.48–8.5), respectively.
NLR at relapse was associated with PPS as a continuous variable (HR 1.24, 95% CI 1.04 to 1.49). In the univariate analysis presented in table 2, high LMR (>3.20) had a superior PFS with a HR of 0.34 (95% CI 0.16 to 0.74). Patients with a high FLIPI score had a 2.5 times greater risk of death or relapse than patients with a lower score (HR 2.52, 95% CI 1.10 to 5.75). However, patients treated with rituximab had a 72% lower risk of death or relapse (HR 0.28, 95% CI 0.10 to 0.81). We found evidence of a linear association between PFS and the calendar period (p value of trend=0.003). Compared with the period 2000–2005, those patients diagnosed during 2010–2014 had a 90% lower risk of death or relapse (HR 0.10, 95% CI 0.01 to 0.75). Furthermore, there was no evidence of differences in PFS by sex (male vs female, HR 1.09, 95% CI 0.51 to 2.32). LMR at relapse showed a weak association with PPS (HR 1.06, 95% CI 0.77 to 1.45).
Table 2.
HRs of PFS events, n=88
| Cases/1000 person-years | Rate (per 1000 person-years) | HR | 95% CI | p Value | |
| LMR | |||||
| >3.20 | 11 | 34.66 | 0.34 | 0.16 to 0.74 | 0.004 |
| ≤3.20 | 16 | 101.28 | 1 | ||
| NLR | |||||
| >2.18 | 18 | 67.37 | 1.56 | 0.70 to 3.47 | 0.273 |
| ≤2.18 | 9 | 43.23 | 1 | ||
| FLIPI | |||||
| High risk | 19 | 82.30 | 2.52 | 1.10 to 5.75 | 0.023 |
| Low/intermediate risk | 8 | 32.72 | 1 | ||
| Sex | |||||
| Male | 15 | 58.96 | 1.09 | 0.51 to 2.32 | 0.831 |
| Female | 12 | 54.29 | 1 | ||
| Rituximab | |||||
| Yes | 4 | 22.02 | 0.28 | 0.10 to 0.81 | 0.012 |
| No | 23 | 78.31 | 1 | ||
| Year of diagnosis | |||||
| 2010–2014 | 1 | 8.71 | 0.10 | 0.01 to 0.75 | 0.006 |
| 2006–2010 | 5 | 43.05 | 0.50 | 0.19 to 1.33 | 0.157 |
| 2000–2005 | 21 | 85.91 | 1 | ||
FLIPI, Follicular Lymphoma International Prognostic Index; LMR, lymphocyte-to-monocyte ratio; NLR neutrophil-to-lymphocyte ratio; PFS, progression-free survival.
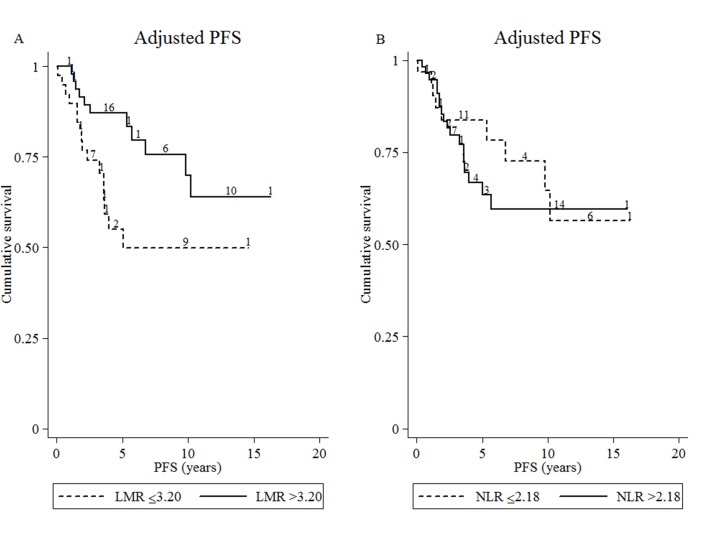
In the multivariate analysis, patients with a high LMR (>3.20) at diagnosis had a longer PFS, with an adjusted HR of 0.31 (95% CI 0.13 to 0.71) (figure 2). However, NLR cut-off levels did not show strong evidence of an association with PFS (adjusted HR 1.33, 95% CI 0.57 to 3.10) (table 3).
Figure 2.
Adjusted progression-free survival (PFS). Estimate of (A) high and low lymphocyte-to-monocyte ratio (LMR) at diagnosis and (B) high and low neutrophil-to-lymphocyte ratio (NLR) at diagnosis (n=88).
Table 3.
Multivariate analyses of PFS with LMR and NLR at diagnosis, n=88
| Adjusted HR* | 95% CI | p Value | |
| LMR: >3.20 vs ≤3.20 (reference) | 0.31 | 0.13 to 0.71 | 0.006 |
| FLIPI: high risk vs low/intermediate risk (reference) | 2.17 | 0.92 to 5.10 | 0.075 |
| Sex: male vs female (reference) | 1.50 | 0.67 to 3.34 | 0.321 |
| Rituximab use: yes vs no (reference) | 0.16 | 0.05 to 0.48 | 0.001 |
| Adjusted HR* | 95% CI | p Value | |
| NLR: >2.18 vs ≤2.18 (reference) | 1.33 | 0.57 to 3.10 | 0.511 |
| FLIPI: high risk vs low/intermediate risk (reference) | 2.47 | 1.03 to 5.89 | 0.042 |
| Sex: male vs female (reference) | 1.15 | 0.53 to 2.48 | 0.721 |
| Rituximab use: yes vs no (reference) | 0.19 | 0.07 to 0.57 | 0.003 |
*Adjusted for all other covariates in the table.
FLIPI, Follicular Lymphoma International Prognostic Index; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival.
Sensitivity analysis
Sensitivity analysis showed that LMR was consistently associated with PFS under different model specifications and multivariate adjustments. We evaluated the multivariate analyses, and found that the assumption for the proportional hazard was met. However, the strength of the evidence for differences in OS by LMR and NLR levels was weak.
PFS tended to increase with LMRs above the cut-off and low FLIPI scores (HR LMR >3.2 and low FLIPI 0.17, 95% CI 0.04 to 0.70 vs HR LMR >3.2 and high FLIPI 0.60, 95% CI 0.24 to 1.50), but evidence of a statistical interaction was weak (interaction p value 0.171). There was some evidence of a statistical interaction between LMR and rituximab (interaction p value 0.024, HR of the interaction term 17.1, 95% CI 1.46 to 199.36).
Discussion
To our knowledge, this study is the first to report the clinical and prognostic implications of pretreatment NLR in patients with FL. Our findings demonstrated notable differences in clinical behaviour and outcome between the low and high LMR groups at diagnosis and NLR groups at the time of relapse. Previous studies reported that NLR is a predictor of mortality in several cancer types, including gastric38 39 and colorectal cancer.40 One possible underlying mechanism is the inflammatory reaction, which has been reported to be involved in tumour growth, invasion, metastasis and resistance to treatment.23–26
The factors included in FLIPI2 are primarily related to tumour burden (stage, serum lactate dehydrogenase, and number of nodal site involvement) and patient characteristics (age and haemoglobin). Cell count ratio at diagnosis is a simple tool that assesses the host’s immune homoeostasis, inflammatory state23 24 and the tumour microenvironment.14 15 We obtained strong evidence in support of these prognostic factors possessing practical clinical utility and significance. In the present study, LMR played a significant role in predicting the PFS and NLR in PPS; however, the strength of the evidence for OS was weak. This weak evidence may be attributed to the inadequate sample size and the few deaths observed, along with the interaction with other parameters or unknown confounding. Moreover, the availability of salvage treatments on progression makes the difference in OS difficult to demonstrate.
Cell count or its ratio at diagnosis may be used to decide which treatment strategy is most appropriate, including watchful waiting, RT or systemic treatment. Previous studies showed that lymphocytes have an important role in mediating the antitumour effect of rituximab.41–43 For those with low LMR, the disease may progress earlier, and closer follow-up may be indicated. We separated patients into FLIPI-based low/intermediate and high-risk groups and then incorporated a biological factor (LMR) into a known clinical prognostic factor (FLIPI). Based on the findings of our study, future studies should aim to understand the utility of cell count ratios with other established prognostic factors in an independent validation cohort and to explore the therapeutic strategies based on cell count ratio (ie, observation alone vs early initiation of treatment), ideally in a prospective manner.
Most studies are subject to a certain degree of misclassification related to measurement error.44 However, our study population followed standardised investigation procedures; any misclassification is likely to be non-differential. The incidence and spectrum of NHL cases differ between the Chinese and Western populations, and the risk of FL is lower in the former group.45–47 However, our sample size is comparable to those in other retrospective FL studies in Asia, ranging between 40 and 50 patients.45 48 Both genetic and environmental factors play a part in governing the overall incidence, as shown by migration studies.46 Our lymphoma treatment regimens were not completely uniform in this analysis and involved a modest sample size, which may have introduced selection bias. We analysed a group of patients receiving definitive RT as treatment, with a sample size of only 18. The results did not reach statistical significance; a bigger cohort or even a dedicated prospective study would be interesting. In our data, the complete blood count did not differentiate the subtypes of B and T lymphocytes and monocytes. Therefore, information regarding patient outcomes with a combination of different subtypes of immune cells was not explored in this study. Furthermore, in our study, the distribution of blood cells may be different when leucocytosis or leucopenia is present. Moreover, evidence of a correlation between age and circulating white cell counts has been reported, and a decrease in total lymphocyte counts is observed more frequently in the elderly than in younger adults.49 Also, the treatments may interact with other factors, such as age and performance status. There appears to be an interaction between rituximab and LMR. However, the 95% CI of the interaction term was wide; this reflected the small numbers and data sparsity for secondary analysis, and therefore, no conclusive evidence can be extrapolated. We did not analyse the association between cell count ratios and systemic treatment choices, duration and number of cycles and salvage treatment on progression because of the limited sample size for the subgroup analysis. Given the unavailability of beta-2 microglobulin in most patients, we did not analyse FLIPI2.
One merit of our study is the performance of a sensitivity analysis to determine the robustness of the main findings in different scenarios. A sensitivity analysis was not conducted in numerous studies assessing the relationship between cell count ratio and survival. Furthermore, we explored the effect of calendar year of diagnosis to account for potential improvement in life expectancy over the study period due to changes in the environment or technological advancement in general medical care. Also, we accounted for the impact of the inclusion of rituximab as a therapeutic option since early 2005, which is strongly correlated with an improved OS and PFS.
The external validity of this study is limited to a single institution. Thus, further evidence for validation of our results and multi-institutional studies with larger sample sizes are warranted. However, the strength of the evidence of our findings is still important given the clinical relevance of LMR and NLR capability to predict prognosis.
Conclusion
In this study, we demonstrated that LMR and NLR may provide independent and additional prognostic information for risk classification when used along with FLIPI in FL. These can be determined using widely available complete blood count tests, which can be used as non-invasive and cost-effective alternatives to complement prognosis data for FL. Future prospective studies are necessary to validate the results of our study and evaluate the exact clinical significance.
Supplementary Material
Footnotes
Contributors: SFL developed the concept and design of the study. SFL analysed the data with MAL-F guidance. SFL wrote the manuscript. Both authors interpreted the data, drafted and revised the manuscript critically, and approved the final version of the manuscript. SFL is the guarantor of the paper.
Competing interests: None declared.
Ethics approval: New Territories West Cluster Clinical & Research Ethics Committee of Tuen Mun Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Raw data can be obtained by contacting the authors at the corresponding address.
References
- 1. Anon. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909–18. [PubMed] [Google Scholar]
- 2. Solal-Céligny P, Roy P, Colombat P, et al. . Follicular lymphoma international prognostic index. Blood 2004;104:1258–65. 10.1182/blood-2003-12-4434 [DOI] [PubMed] [Google Scholar]
- 3. Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin’s lymphomas. N Engl J Med 1984;311:1471–5. 10.1056/NEJM198412063112303 [DOI] [PubMed] [Google Scholar]
- 4. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1998;16:2780–95. 10.1200/JCO.1998.16.8.2780 [DOI] [PubMed] [Google Scholar]
- 5. Glas AM, Knoops L, Delahaye L, et al. . Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol 2007;25:390–8. 10.1200/JCO.2006.06.1648 [DOI] [PubMed] [Google Scholar]
- 6. Solal-Céligny P, Cahu X, Cartron G. Follicular lymphoma prognostic factors in the modern era: what is clinically meaningful? Int J Hematol 2010;92:246–54. 10.1007/s12185-010-0674-x [DOI] [PubMed] [Google Scholar]
- 7. Brice P, Bastion Y, Lepage E, et al. . Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 1997;15:1110–7. 10.1200/JCO.1997.15.3.1110 [DOI] [PubMed] [Google Scholar]
- 8. Federico M, Bellei M, Marcheselli L, et al. . Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009;27:4555–62. 10.1200/JCO.2008.21.3991 [DOI] [PubMed] [Google Scholar]
- 9. Casulo C, Byrtek M, Dawson KL, et al. . Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol 2015;33:2516–22. 10.1200/JCO.2014.59.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dave SS, Wright G, Tan B, et al. . Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 2004;351:2159–69. 10.1056/NEJMoa041869 [DOI] [PubMed] [Google Scholar]
- 11. Liu GX, Zhang X, Li S, et al. . Monocyte chemotactic protein-1 and CC chemokine receptor 2 polymorphisms and prognosis of renal cell carcinoma. Tumour Biol 2013;34:2741–6. 10.1007/s13277-013-0827-7 [DOI] [PubMed] [Google Scholar]
- 12. Deshmane SL, Kremlev S, Amini S, et al. . Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009;29:313–26. 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tadmor T, Fell R, Polliack A, et al. . Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma-possible link with monocytic myeloid-derived suppressor cells. Hematol Oncol 2013;31:65–71. 10.1002/hon.2019 [DOI] [PubMed] [Google Scholar]
- 14. Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clin Proc 2010;85:656–63. 10.4065/mcp.2010.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagaraj S, Collazo M, Corzo CA, et al. . Regulatory myeloid suppressor cells in health and disease. Cancer Res 2009;69:7503–6. 10.1158/0008-5472.CAN-09-2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farinha P, Masoudi H, Skinnider BF, et al. . Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 2005;106:2169–74. 10.1182/blood-2005-04-1565 [DOI] [PubMed] [Google Scholar]
- 17. Ho CL, Lu CS, Chen JH, et al. . Neutrophil/Lymphocyte Ratio, Lymphocyte/Monocyte Ratio, and Absolute Lymphocyte Count/Absolute Monocyte Count Prognostic Score in Diffuse Large B-Cell Lymphoma: Useful Prognostic Tools in the Rituximab Era. Medicine 2015;94:e993 10.1097/MD.0000000000000993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belotti A, Doni E, Bolis S, et al. . Peripheral blood lymphocyte/monocyte ratio predicts outcome in follicular lymphoma and in diffuse large B-cell lymphoma patients in the rituximab era. Clin Lymphoma Myeloma Leuk 2015;15:208–13. 10.1016/j.clml.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 19. Kumagai S, Tashima M, Fujikawa J, et al. . Ratio of peripheral blood absolute lymphocyte count to absolute monocyte count at diagnosis is associated with progression-free survival in follicular lymphoma. Int J Hematol 2014;99:737–42. 10.1007/s12185-014-1576-0 [DOI] [PubMed] [Google Scholar]
- 20. Porrata LF, Ristow K, Colgan JP, et al. . Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica 2012;97:262–9. 10.3324/haematol.2011.050138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koh YW, Kang HJ, Park C, et al. . The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin’s lymphoma: correlation with tumor-associated macrophages. Oncologist 2012;17:871–80. 10.1634/theoncologist.2012-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porrata LF, Ristow K, Habermann TM, et al. . Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in nodular lymphocyte-predominant Hodgkin lymphoma. Br J Haematol 2012;157:321–30. 10.1111/j.1365-2141.2012.09067.x [DOI] [PubMed] [Google Scholar]
- 23. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 24. Moore MM, Chua W, Charles KA, et al. . Inflammation and cancer: causes and consequences. Clin Pharmacol Ther 2010;87:504–8. 10.1038/clpt.2009.254 [DOI] [PubMed] [Google Scholar]
- 25. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colotta F, Allavena P, Sica A, et al. . Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–81. 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 27. Porrata LF, Ristow K, Habermann T, et al. . Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol 2010;85:896–9. 10.1002/ajh.21849 [DOI] [PubMed] [Google Scholar]
- 28. Marcheselli R, Bari A, Tadmor T, et al. . Neutrophil-lymphocyte ratio at diagnosis is an independent prognostic factor in patients with nodular sclerosis Hodgkin lymphoma: results of a large multicenter study involving 990 patients. Hematol Oncol 2016. 10.1002/hon.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheson BD, Pfistner B, Juweid ME, et al. . Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 30. Shen W, Ning J, Yuan Y. A direct method to evaluate the time-dependent predictive accuracy for biomarkers. Biometrics 2015;71:439–49. 10.1111/biom.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luque-Fernandez MA, Maringe C, Nelson P. CVAUROC: Stata module to compute Cross-validated area under the curve for ROC analysis after predictive modelling for binary outcomes. 2017.
- 32. Skendzel LP, Youden WJ. A graphic display of interlaboratory test results. Am J Clin Pathol 1969;51:161–5. 10.1093/ajcp/51.2.161 [DOI] [PubMed] [Google Scholar]
- 33. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 34. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 35. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163–70. [PubMed] [Google Scholar]
- 36. Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. 4th ed Oxford: Blackwell Science, 2002. [Google Scholar]
- 37. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972;34:187–220. [Google Scholar]
- 38. Mohri Y, Tanaka K, Ohi M, et al. . Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg 2010;34:285–90. 10.1007/s00268-009-0302-1 [DOI] [PubMed] [Google Scholar]
- 39. Yamanaka T, Matsumoto S, Teramukai S, et al. . The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007;73:215–20. 10.1159/000127412 [DOI] [PubMed] [Google Scholar]
- 40. Walsh SR, Cook EJ, Goulder F, et al. . Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–4. 10.1002/jso.20329 [DOI] [PubMed] [Google Scholar]
- 41. Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010;47:115–23. 10.1053/j.seminhematol.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Decaudin D, Des Guetz G, Mathiot C, et al. . Absolute lymphocyte count as a predictive factor for response to monoclonal anti-CD20 antibody therapy. Ann Oncol 2003;14:171–2. 10.1093/annonc/mdg015 [DOI] [PubMed] [Google Scholar]
- 43. Behl D, Ristow K, Markovic SN, et al. . Absolute lymphocyte count predicts therapeutic efficacy of rituximab therapy in follicular lymphomas. Br J Haematol 2007;137:409–15. 10.1111/j.1365-2141.2007.06596.x [DOI] [PubMed] [Google Scholar]
- 44. Lambert J. Statistics in brief: how to assess bias in clinical studies? Clin Orthop Relat Res 2011;469:1794–6. 10.1007/s11999-010-1538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang R, Todd D, Chan TK, et al. . Follicular non-Hodgkin’s lymphoma in Hong Kong Chinese: a retrospective analysis. Hematol Oncol 1988;6:29–37. 10.1002/hon.2900060106 [DOI] [PubMed] [Google Scholar]
- 46. Au WY, Gascoyne RD, Klasa RD, et al. . Incidence and spectrum of non-Hodgkin lymphoma in Chinese migrants to British Columbia. Br J Haematol 2005;128:792–6. 10.1111/j.1365-2141.2005.05387.x [DOI] [PubMed] [Google Scholar]
- 47. Herrinton LJ, Goldoft M, Schwartz SM, et al. . The incidence of non-Hodgkin’s lymphoma and its histologic subtypes in Asian migrants to the United States and their descendants. Cancer Causes Control 1996;7:224–30. 10.1007/BF00051298 [DOI] [PubMed] [Google Scholar]
- 48. Yamashita H, Izutsu K, Nakamura N, et al. . Treatment results of chemoradiation therapy for localized aggressive lymphomas: a retrospective 20-year study. Ann Hematol 2006;85:523–9. 10.1007/s00277-006-0114-4 [DOI] [PubMed] [Google Scholar]
- 49. Diaz-Jauanen E, Strickland RG, Williams RC. Studies of human lymphocytes in the newborn and the aged. Am J Med 1975;58:620–8. 10.1016/0002-9343(75)90497-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-017904supp001.pdf (7.4KB, pdf)
bmjopen-2017-017904supp002.pdf (241.2KB, pdf)
bmjopen-2017-017904supp003.pdf (70.1KB, pdf)