Abstract
We treated a case of acute kidney injury and nephrotic syndrome after malathion inhalation. A 69-year-old Japanese man presented with oedema 15 days after inhalation of malathion, a widely used pesticide. Serum albumin was 2.4 g/dL, urinary protein 8.6 g/gCr and serum creatinine 2.5 mg/dL. Kidney biopsy revealed tubular cell damage, epithelial cell damage in glomeruli and diffuse foot process effacement in electron microscopy. Acute kidney injury progressed to treatment with dialysis. Renal function recovered after corticosteroid administration from the 43rd day after admission. Malathion inhalation should be ruled out as a differential diagnosis in individuals who develop acute kidney injury and nephrotic syndrome, especially in rural-dwelling patients.
Keywords: toxicology, renal medicine, acute renal failure, nephrotic syndrome, proteinurea
Background
Malathion is an organophosphorus pesticide, used widely all over the world.1 Organophosphate intoxication is characterised by cholinergic effects,2 and has also been reported to cause motor paralysis,2 haemorrhagic panesophagitis,3 necrotising pancreatitis4 and genetic toxicity.5 Although several case reports have described acute kidney injury in poisoning by organophosphates other than malathion (chlorpyrifos, methamidophos),6 7 there have been only two case reports regarding nephrotoxicity of malathion in humans.8 9 Here, we report a third case of acute kidney injury induced by malathion.
Case presentation
A 69-year-old Japanese man with no remarkable medical history presented with oedema at our hospital. He had annual health check-ups of urine dipstick up to 3 years before the first visit, without any history of proteinuria or haematuria. Fifteen days before the first visit, he had deeply inhaled malathion, a widely used pesticide, when spraying it. He sprayed 20 mL of 50% malathion liquid mistakenly dissolved into 2 L of water, which was a 10-fold higher concentration than the manufacturer’s recommendation. He sprayed it on his outdoor vegetable garden for 10 min. The patient did not have gross haematuria and did not express any other symptom of organophosphate toxicity (bradycardia, miosis, lacrimation, salivation, bronchorrhoea, bronchospasm, urination, emesis, diarrhoea or neurological disorder) at the time of exposure. He developed oedema the day after the inhalation. Twelve days after inhaling malathion, he had visited another clinic, and was found to have an elevated serum creatinine concentration of 1.48 mg/dL, hypoalbuminaemia of 2.4 g/dL, proteinuria and haematuria. Thus, he was referred to our hospital.
Investigations
At his first visit, the patient’s height was 166 cm and weight was 54.6 kg; he had gained 6.6 kg in 2 weeks. His blood pressure was 147/92 mm Hg, and his body temperature was 36.7°C. His extremities and face were oedematous. The serum albumin was 2.4 g/dL, urinary protein was 8.55 g/gCr and total cholesterol was 308 mg/dL, findings consistent with nephrotic syndrome. Other laboratory results were haemoglobin concentration 15.0 g/dL, white blood cell count 5840, eosinophil 6.8% (normal range in our laboratory, 0.0%–7.0%), platelet count 382 000 per mm3, serum total protein 5.3 g/dL, blood urea nitrogen 95.3 mg/dL, serum creatinine 2.53 mg/dL, uric acid 8.7 mg/dL, serum sodium 136 mmol/L, serum potassium 5.4 mmol/L, serum chloride 106 mmol/L, serum calcium 7.9 mg/dL, serum phosphate 5.4 mg/dL, C-reactive protein 0.07 mg/dL, urinary beta-2 macroglobulin 92 mg/dL, haemoglobin A1c 5.9% and erythrocyte sedimentation rate 61 mm. The urine sediment showed a red blood cell count of 30–50 per high-power field and white cell count of 5–9 per high-power field, without eosinophiluria or white cell casts. Liver function tests were normal. The titre of antinuclear antibodies was 1:40. Anti-double-stranded DNA antibodies were negative. Serum IgA level was 458 mg/dL (reference range: 90–400 mg/dL), C3 level 102 mg/dL (reference range: 80–140 mg/dL) and C4 level 20.6 mg/dL (reference range: 11.0–34.0 mg/dL). myeloperoxidase (MPO)-antinuclear cytoplasmic antibody (ANCA) and proteinase 3 (PR3)-ANCA were negative. CT revealed pleural effusion and ascites. The kidneys were normal in size (right, 11.3×5.2 cm; left, 11.3×5.9 cm) on CT.
The patient was admitted the next day, and a kidney biopsy was performed.
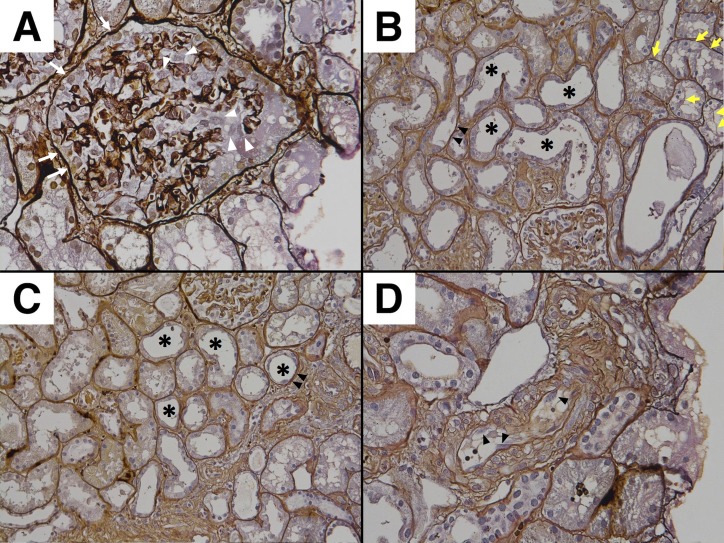
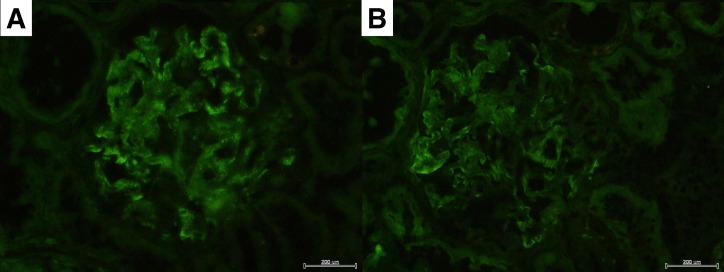
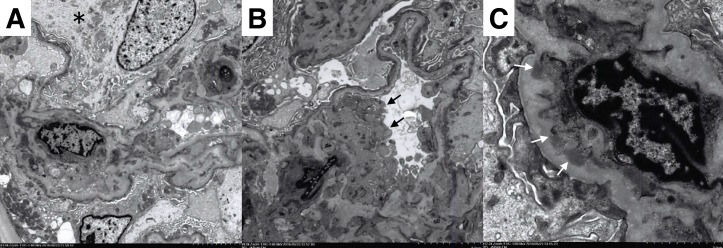
Twenty-nine glomeruli were obtained. Two glomeruli were obliterated by global sclerosis, and one glomerulus was globally collapsed. The remaining glomeruli showed neither mesangial hypercellularity nor basement membrane thickening. Seven glomeruli showed prominent swelling of podocytes and Bowman’s capsule epithelial cells (figure 1A). There was mild cell infiltration in the interstitium. Focal infiltration was observed in five parts in the specimen. The infiltrated cells were mostly lymphocytes, and there were very few eosinophils. The proximal convoluted tubules were irregularly dilated, and focally showed epithelial flattening, vacuolar degeneration and decrease in brush border. The epithelial cells also showed focal detachment from the basement membrane (figure 1B). The distal convoluted tubules were also irregularly dilated and showed epithelial detachment (figure 1C). Interlobular arteries showed marked fibrous intimal thickening. Focal endothelial swelling was also observed (figure 1D). These findings were consistent with the diagnosis of acute tubular necrosis. Immunofluorescent staining showed IgM and IgA depositions in the mesangium and capillary wall (figure 2A,B). Immunofluorescence for IgG, complement, fibrinogen were negative. The findings of electron microscopy revealed diffuse foot process effacement (figure 3A,B), epithelial swelling (figure 3A) and endothelial swelling (figure 3B). Mesangial electron-dense deposits were also observed (figure 3C).
Figure 1.
(A) Glomerulus with swelling of podocytes (white arrow head) and Bowman’s capsule epithelial cells (white arrow) (×400). (B) Irregularly dilated proximal convoluted tubules with decreased brush border (*), epithelial swelling with vacuolar degeneration (yellow arrow) and detachment from the basement membrane (black arrow head) (×200). (C) Irregularly dilated distal convoluted tubules (*) with epithelial detachment (black arrow head) (×200). (D) Interlobular arteries with focal endothelial swelling (black arrow head) (×400).
Figure 2.
(A) Immunofluorescent staining for IgM, showing deposition in the mesangium and capillary wall.(B) Immunofluorescent staining for IgA, showing deposition in the mesangium and capillary wall.
Figure 3.
(A) Diffuse foot process effacement and epithelial swelling (*). (B) Diffuse foot process effacement and endothelial swelling (black arrow). (C) Mesangial electron-dense deposits (white arrow).
Treatment
The patient’s clinical course is shown in figure 4. On the day of admission, the urine volume was 120 mL/day. The next day, the urine volume decreased to <50 mL/day. We, therefore, started haemodialysis. As the anuria persisted, an arteriovenous fistula was created on the 22nd day. On the 43rd day, we initiated prednisolone (40 mg/day) expecting an effect on the nephrotic syndrome.
Figure 4.
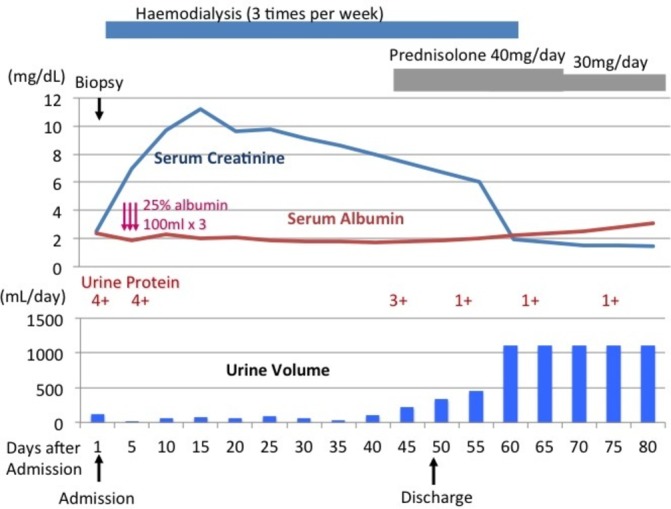
The clinical course of the patient, a 69-year-old man.
Outcome and follow-up
The urine volume gradually increased. Haemodialysis was discontinued on the 61 st day, and the prednisolone was gradually tapered. The patient’s renal function was stabilised, and his creatinine level was 1.35 mg/dL on the 122nd day.
Discussion
We treated a patient presenting with acute kidney injury with proteinuria after malathion inhalation. To the best of our knowledge, this is the third case report of such a case.
We did not initially prescribe corticosteroids because the first case report of acute kidney failure induced by malathion showed a spontaneous recovery.8 In that first case report by Albright and colleagues,8 a 65-year-old man developed weight gain and oedema 1 week after inhaling malathion. Five weeks later, his renal function recovered, and the proteinuria disappeared spontaneously with only conservative treatment. The patient’s kidney biopsy revealed segmental membranous nephropathy, with sparse deposits of IgG in glomeruli shown by immunofluorescence staining, and segmental epithelial electron-dense deposits and diffuse effacement of the foot processes shown by electron microscopy. Albright and colleagues8 speculated that malathion caused immune complex formation, resulting in the acute kidney injury and massive proteinuria, although this should be interpreted cautiously because it was based on 1983 technology.
The second reported article on malathion renal toxicity in humans involved a 65-year-old woman who had ingested 100 mL of 15% malathion, experiencing transient renal dysfunction (creatinine clearance 36 mL/min) and proteinuria (maximal value 2.1 g/day on day 10). The renal dysfunction and proteinuria recovered spontaneously without any specific treatment. Kidney biopsy was not undertaken.9
A postmortem study of nine human acute organophosphate poisonings showed that organophosphates were deposited in the kidney in relatively large amounts compared with other organs, clearly indicating the tendency of malathion to accumulate in the kidney.10
Our patient’s case exhibited pathological findings of diffuse epithelial cell damage in glomeruli with swelling of podocytes and Bowman’s capsule epithelial cells, in addition to tubular cell damage. These findings could explain both the nephrotic syndrome and the acute kidney injury. Endothelial cells were also damaged since some glomeruli and interlobular arteries showed swelling of endothelial cells. Malathion seems to be cytotoxic.
In an experimental study with rats treated with malathion, similar pathological findings as in our case were reported: vacuolar degeneration and diminished brush border of proximal convoluted tubules, fusion of foot processes and mesangial electron-dense deposits.11 There are many animal studies showing the toxic effect of malathion on the kidney due to oxidative stress and negative alterations on antioxidant enzyme activities,12–20 leading to reduced total kidney cell numbers,21 histological and functional impairment of renal tubules,22–25 glomerular inflammation26 and proteinuria.25 26
In our patient’s case, IgA and IgM were positive in immunofluorescence staining, suggesting the presence of immune complex. Although the precise mechanism of nephrotic syndrome is not clear in the present case, it is not likely that immune complex caused nephrotic syndrome, because: (1) unlike the first case report, oedema developed rapidly after malathion inhalation, and (2) membranous nephropathy was ruled out by the absence of subepithelial deposits. We thus suspect that podocyte injury by the malathion caused nephrotic syndrome in this case. The presence of collapsed glomeruli also raises the possibility of collapsing focal segmental glomerulosclerosis (FSGS) as a cause of nephrotic syndrome.
The agreement between IgA deposits in immunofluorescence and electron microscopy could suggest that this case was a worsening of existing IgA nephropathy with exposure to environmental agents (malathion). However, this is unlikely, as there was extensive tubular injury, extensive foot process fusion and no crescents in 29 glomeruli. It seems more likely that the IgA deposition was incidental.
Acute tubulointerstitial nephritis is an important differential diagnosis. However, lack of marked interstitial infiltrates and the presence of heavy proteinuria made acute tubulointerstitial nephritis less likely.
Since the first reported case of malathion-induced acute kidney injury and nephrotic syndrome improved spontaneously, the cells damaged by the malathion may have recovered. Possible causes of acute kidney injury in the present case include the direct toxic effect of malathion on tubular epithelial cells, decreased intravascular volume due to nephrotic syndrome, or both. Our patient’s recovery may have been a natural course as in the first reported case, or the glucocorticoid may have had a beneficial effect on podocytes.27
In summary, we reported the third case of proteinuria and acute kidney injury after malathion exposure. Although it is not possible to exclude other cause of the nephrotic syndrome, malathion toxicity should be considered in patients with proteinuria and acute kidney injury after pesticide exposure.
Learning points.
Malathion inhalation may cause acute kidney injury and nephrotic syndrome.
Malathion may be a nephrotoxic chemical and should be used only at the recommended concentrations.
Malathion inhalation should be ruled out as a cause of acute kidney injury and nephrotic syndrome, especially in rural-dwelling patients.
Corticosteroids may be beneficial for patients with nephrotic syndrome and acute kidney injury associated with malathion exposure. Dialysis may be needed.
Footnotes
Contributors: All authors listed have contributed sufficiently to the report to be included as authors, and all those who are qualified to be authors are listed in the author byline. KY made a substantial contribution to the conception of the work and the writing of the manuscript. MF treated the patient and made a contribution to the discussion. RK made a contribution to the interpretation of pathology findings. TO made a contribution to the discussion.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Singh B, Kaur J, Singh K. Microbial degradation of an organophosphate pesticide, malathion. Crit Rev Microbiol 2014;40:146–54. 10.3109/1040841X.2013.763222 [DOI] [PubMed] [Google Scholar]
- 2.Sudakin DL, Mullins ME, Horowitz BZ, et al. Intermediate syndrome after malathion ingestion despite continuous infusion of pralidoxime. J Toxicol Clin Toxicol 2000;38:47–50. 10.1081/CLT-100100915 [DOI] [PubMed] [Google Scholar]
- 3.Koga H, Yoshinaga M, Aoyagi K, et al. Hemorrhagic panesophagitis after acute organophosphorus poisoning. Gastrointest Endosc 1999;49:642–3. 10.1016/S0016-5107(99)70397-4 [DOI] [PubMed] [Google Scholar]
- 4.Zamir DL, Novis BN. Organophosphate poisoning and necrotizing pancreatitis. Isr J Med Sci 1994;30:855–6. [PubMed] [Google Scholar]
- 5.Flessel P, Quintana PJ, Hooper K. Genetic toxicity of malathion: a review. Environ Mol Mutagen 1993;22:7–17. 10.1002/em.2850220104 [DOI] [PubMed] [Google Scholar]
- 6.Cavari Y, Landau D, Sofer S, et al. Organophosphate poisoning-induced acute renal failure. Pediatr Emerg Care 2013;29:646–7. 10.1097/PEC.0b013e31828e9e45 [DOI] [PubMed] [Google Scholar]
- 7.Agostini M, Bianchin A. Acute renal failure from organophospate poisoning: a case of success with haemofiltration. Hum Exp Toxicol 2003;22:165–7. 10.1191/0960327103ht343cr [DOI] [PubMed] [Google Scholar]
- 8.Albright RK, Kram BW, White RP. Malathion exposure associated with acute renal failure. JAMA 1983;250:2469 10.1001/jama.1983.03340180031010 [DOI] [PubMed] [Google Scholar]
- 9.Dive A, Mahieu P, Van Binst R, et al. Unusual manifestations after malathion poisoning. Hum Exp Toxicol 1994;13:271–4. 10.1177/096032719401300409 [DOI] [PubMed] [Google Scholar]
- 10.Tsatsakis AM, Aguridakis P, Michalodimitrakis MN, et al. Experiences with acute organophosphate poisonings in Crete. Vet Hum Toxicol 1996;38:101–7. [PubMed] [Google Scholar]
- 11.Mossalam HH, Abd-El Aty OA, Morgan EN, et al. Biochemical and ultra structure studies of the antioxidant effect of aqueous extract of Hibiscus sabdariffa on the nephrotoxicity induced by organophosphorous pesticide (malathion) on the adult albino rats. Life Sci J 2011;8:561–74. [Google Scholar]
- 12.Karmakar S, Patra K, Jana S, et al. Exposure to environmentally relevant concentrations of malathion induces significant cellular, biochemical and histological alterations in Labeo rohita. Pestic Biochem Physiol 2016;126:49–57. 10.1016/j.pestbp.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Ural MŞ, Yonar ME, Mişe Yonar S. Protective effect of ellagic acid on oxidative stress and antioxidant status in Cyprinus carpio during malathion exposure. Cell Mol Biol 2015;61:58–63. [PubMed] [Google Scholar]
- 14.Yonar SM, Ural MŞ, Silici S, et al. Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio carpio: protective role of propolis. Ecotoxicol Environ Saf 2014;102:202–9. 10.1016/j.ecoenv.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 15.Yonar SM. Toxic effects of malathion in carp, Cyprinus carpio carpio: protective role of lycopene. Ecotoxicol Environ Saf 2013;97:223–9. 10.1016/j.ecoenv.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Huculeci R, Dinu D, Staicu AC, et al. Malathion-induced alteration of the antioxidant defence system in kidney, gill, and intestine of Carassius auratus gibelio. Environ Toxicol 2009;24:523–30. 10.1002/tox.20454 [DOI] [PubMed] [Google Scholar]
- 17.Selmi S, Jallouli M, Gharbi N, et al. Hepatoprotective and Renoprotective Effects of Lavender (Lavandula stoechas L.) Essential Oils Against Malathion-Induced Oxidative Stress in Young Male Mice. J Med Food 2015;18:1103–11. 10.1089/jmf.2014.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coban FK, Ince S, Kucukkurt I, et al. Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem Toxicol 2015;38:391–9. 10.3109/01480545.2014.974109 [DOI] [PubMed] [Google Scholar]
- 19.Selmi S, El-Fazaa S, Gharbi N. Oxidative stress and alteration of biochemical markers in liver and kidney by malathion in rat pups. Toxicol Ind Health 2015;31:783–8. 10.1177/0748233713475507 [DOI] [PubMed] [Google Scholar]
- 20.Alp H, Aytekin I, Hatipoglu NK, et al. Effects of sulforophane and curcumin on oxidative stress created by acute malathion toxicity in rats. Eur Rev Med Pharmacol Sci 2012;16(Suppl 3):144–8. [PubMed] [Google Scholar]
- 21.Beaman JR, Finch R, Gardner H, et al. Mammalian immunoassays for predicting the toxicity of malathion in a laboratory fish model. J Toxicol Environ Health A 1999;56:523–42. 10.1080/00984109909350175 [DOI] [PubMed] [Google Scholar]
- 22.Bosco C, Rodrigo R, Diaz S, et al. Renal effects of chronic exposure to malathion in Octodon degus. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1997;118:247–53. 10.1016/S0742-8413(97)00140-0 [DOI] [PubMed] [Google Scholar]
- 23.Baiomy AA, Attia HF, Soliman MM, et al. Protective effect of ginger and zinc chloride mixture on the liver and kidney alterations induced by malathion toxicity. Int J Immunopathol Pharmacol 2015;28:122–8. 10.1177/0394632015572083 [DOI] [PubMed] [Google Scholar]
- 24.Keadtisuke S, Dheranetra W, Fukuto TR. Detection of kidney damage by malathion impurities using a microdissection technique. Toxicol Lett 1989;47:53–9. 10.1016/0378-4274(89)90085-4 [DOI] [PubMed] [Google Scholar]
- 25.Keadtisuke S, Fukuto TR. Dysproteinuria induced in rats by O,O,S-trimethyl phosphorothioate. Toxicol Lett 1987;37:33–9. 10.1016/0378-4274(87)90164-0 [DOI] [PubMed] [Google Scholar]
- 26.Rodgers KE. Effects of oral administration of malathion on the course of disease in MRL-lpr mice. J Autoimmun 1997;10:367–73. 10.1006/jaut.1997.0145 [DOI] [PubMed] [Google Scholar]
- 27.Schönenberger E, Ehrich JH, Haller H, et al. The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant 2011;26:18–24. 10.1093/ndt/gfq617 [DOI] [PubMed] [Google Scholar]