Abstract
Penile squamous cell carcinoma (pSCC) is a relatively rare disease in Western world but is a significant health problem in developing countries like India. We report here a case of successful multimodality management of recurrent pSCC with pelvic lymphadenopathy in a 56-year-old male patient with poorly controlled diabetes. The patient presented with ulceroproliferative growth over the residual penile stump clinically involving root of penis and with right pelvic lymphadenopathy. The patient had a history of partial penectomy done elsewhere 20 months ago. In view of the comorbidities, locally recurrent disease and presence of right Iliac lymphadenopathy, the patient was treated with nanosomal docetaxel lipid suspension (NDLS), cisplatin and 5-fluorouracil (TPF regimen) in the neoadjuvant setting followed by staged surgical resection. This is the first case report showing successful treatment of recurrent pSCC with NDLS-based TPF regimen in the neoadjuvant setting followed by staged surgery in a patient with poorly controlled diabetes.
Keywords: cancer intervention, urological cancer, surgical oncology
Background
Penile squamous cell carcinoma (pSCC) is the most common histological subtype (>95%) of penile malignancy.1 Though pSCC is relatively rare in the Western world, it is a significant health problem in developing countries like India.
The definitive treatment for early penile cancer is wide surgical excision or penectomy (partial/total), usually accompanied with bilateral inguinal lymph node dissection with/without pelvic lymph node dissection. In patients with advanced/recurrent pSCC or with bulky (>4 cm) inguinal lymph nodes, multimodality treatment, comprising surgery, chemotherapy and/or radiation therapy should be considered. Cisplatin-containing chemotherapy with/without taxanes remains the mainstay of combination chemotherapy with significant clinical benefit on technically unresectable and recurrent penile cancer.2 We report here a case of a locally recurrent pSCC with pelvic lymphadenopathy, in a patient with poorly controlled diabetes, treated with nanosomal docetaxel lipid suspension (NDLS) based cisplatin and 5-fluorouracil (TPF) regimen in the neoadjuvant setting. The patient subsequently achieved surgical operability and underwent total radical penectomy with bilateral ilioinguinal lymphadenectomy, in two stages. This is the first case report showing efficacy and safety of NDLS-based TPF regimen in a patient with recurrent penile cancer.
Case presentation
A 56-year-old man with hypertension and poorly controlled type II diabetes mellitus presented to our tertiary care hospital with complaints of ulceroproliferative growth over penile stump with bleeding, purulent discharge, pain and progressive difficulty in micturition for the last 3 months. The patient had a history of partial penectomy 20 months back for primary pSCC, reported as stage II (pT2Nx). He refused to undergo inguinal lymphadenectomy at the time of primary surgery.
Physical examination revealed an ulceroproliferative growth measuring 4×4 cm over distal part of residual penile stump and base of penis with induration reaching up to bulbar urethra. There were clinically palpable mobile right side inguinal lymph nodes, with no palpable opposite inguinal or iliac lymphadenopathy; scrotum and testes were normal. A MRI scan of the pelvis revealed a 9.5×6×4.5 cm lesion over penile stump, involving remaining part of corpora cavernosa, extending up to bulbar urethra and root of penis with distorted soft tissue contour at the site of amputation and enlarged ilioinguinal lymph nodes on the right side, the largest iliac lymph node measuring 2.5×1.5 cm (figure 1). Punch biopsy revealed a moderately differentiated squamous cell carcinoma. The patient was clinically staged as IIIA (T2N1 M0).
Figure 1.
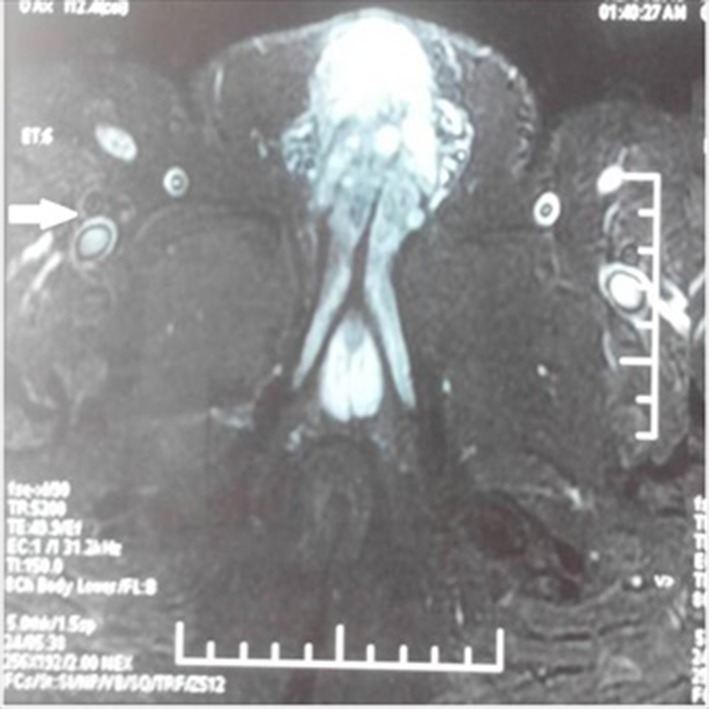
Prechemotherapy MRI showing penile lesion along with enlarged iliac lymph nodes (bold arrow) abutting iliac vessels.
This patient had poorly controlled diabetes on regular insulin. In view of comorbidities, extensive locally recurrent disease and presence of iliac lymphadenopathy, neoadjuvant chemotherapy (NACT) was planned with docetaxel+displatin+5 fluorouracil (5-FU) (TPF regimen) every 3 weeks followed by surgery in two stages. Since it is known that premedication with steroids worsens hyperglycaemia in diabetes and increase the risk of infections postsurgery,3 it was planned to administer NDLS to him, thereby obviating the need for steroid premedication. The chemotherapy regimen consisted of NDLS 75 mg/m² administered over 1 hour on day 1; 5-FU 750 mg/m² on days 1–2 and cisplatin 75 mg/m² in divided doses on day 1–2. Following first cycle of NACT, there was good clinical response in primary tumour in terms of decreased pain, bleeding and the exudate in the first 20 days itself. The patient received 4 cycles of TPF regimen with progressive decrease in size of penile ulcer and symptomatic pain relief.
A contrast-enhanced CT done post-NACT showed a 6.5×4.2×2.1 cm ill-defined necrotic lesion involving the remaining corpus cavernosa and extending up to root of penis (bulbar urethra). Few lymph nodes maximum size up to 1.2 cm were present in right inguinal and iliac region. The tumour showed decrease by ~30% as per response evaluation criteria in solid tumours (RECIST V.1.1) following four cycles of NACT (figure 2). In view of the patient’s reluctance to undergo bilateral ilioinguinal lymphadenectomy in the same setting as total penectomy, he was planned for staged surgery. He underwent total radical penectomy with permanent perineal urethrostomy (figure 3A). Histopathological examination revealed squamous cell carcinoma (7.5×4 cm circumferential lesion, with invasion into corpora cavernosa and spongiosum) and resection margins free from tumour at root of penis (figure 3B).
Figure 2.
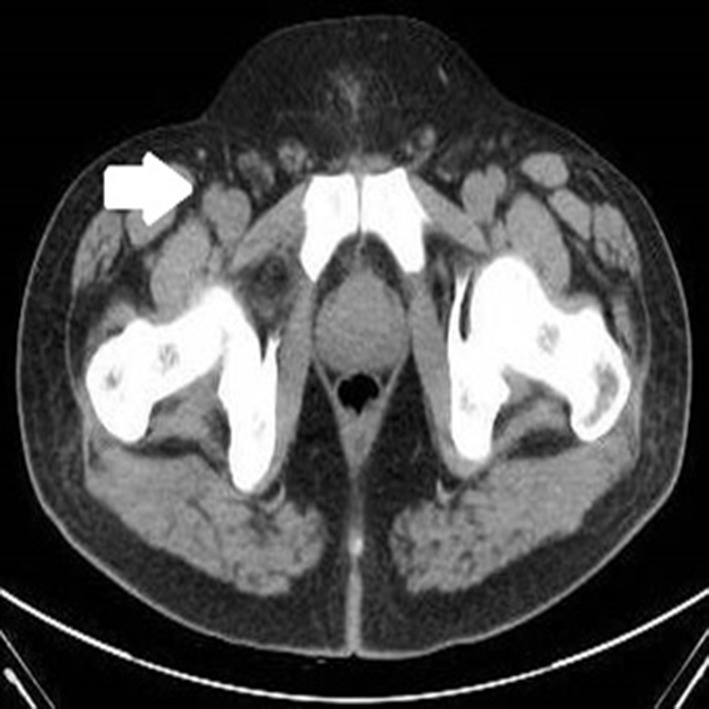
Postchemo contrast-enhanced CT showing residual disease with marked response (bold arrow) in iliac lymph nodes.
Figure 3.
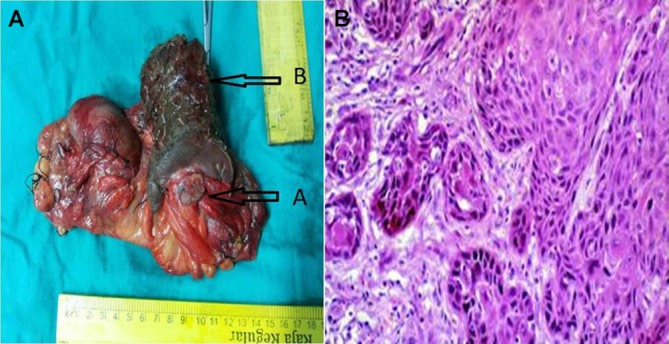
Intraoperative image showing (A) rest of the penis, (B) residual disease in partial penectomy specimen. (B) Squamous cell carcinoma, penis, histology, low power.
The patient received two more cycles of NDLS containing TPF regimen postsurgery followed by bilateral ilioinguinal block dissection. He tolerated the chemotherapy very well without any serious adverse effects. The postoperative course was uneventful except for minor right inguinal wound infection, which was managed with topical antibiotics and dressing. All lymph nodes were free from tumour invasion (right ilioinguinal—0/24 and left ilioinguinal—0/14).
Outcome and follow-up
The patient is in regular follow-up and is doing well nearly 26 months after completion of treatment (figure 4 and B).
Figure 4.
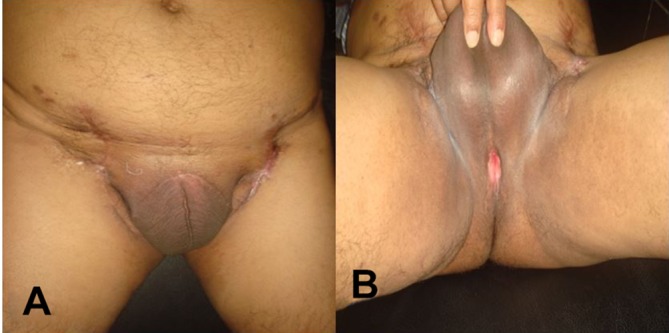
(A) Postoperative picture showing the healed bilateral inguinal block dissection scar. (B) Postoperative picture showing functional perineal urethrostomy.
Discussion
Squamous cell carcinoma of the penis is more prevalent in parts of Africa, South America and Asia, making it an imperative global health problem.1 Selected patients with regional lymph node metastases can be rendered free of disease with inguinal and pelvic lymphadenectomy and can enjoy long-term survival.
Surgery alone is curative with low recurrence rates reported (10%–20%) in patients with no or minimal nodal disease (<3 unilateral inguinal lymph node metastases, without extranodal extension or any pelvic lymph node involvement).4 However, high recurrence rate reported (as high as 80%–90%) in patients with extensive nodal involvement (bilateral lymph node metastases or extranodal extension and involved pelvic lymph nodes).5 Multimodality treatment incorporating neoadjuvant and/or adjuvant chemotherapy before/after lymphadenectomy is desirable in patients with advanced inguinal and pelvic lymph node metastases.
A response rate (RR) of 23% to cisplatin monotherapy has been reported,6–8 which is comparable to the overall response rate (ORR) of 30.8% reported for irinotecan and cisplatin in the single-arm phase II EORTC trial.9 Three single-institution case series have reported a pooled RR of 63% with cisplatin and 5-flurouracil (PF) combination in 19 patients (three complete remissions and nine partial remissions).10–12 Di Lorenzo et al conducted a retrospective analysis and found a RR of 32% for this regimen.13
The TIP regimen (cisplatin, paclitaxel and ifosfamide) has also been reported to downstage locally advanced disease in the neoadjuvant setting.14 15 The docetaxel, cisplatin and 5-FU (TPF) combination has produced high RRs and have improved survival when compared with PF in squamous carcinomas of the head and neck (SCCHN);.16 17 Though recent studies have shown that addition of induction chemotherapy may be an option in a select subset of patients with advanced disease with high risk for local or distant failure in SCCHN, this approach is still not the standard of care.18 SCCHN displays histological and clinical similarities to penile cancer. In a single-centre study, patients were treated with either paclitaxel or docetaxel-based TPF regimen in the adjuvant, neoadjuvant and palliative settings; one out of six patients with metastatic disease and seven out of 12 patients in neoadjuvant setting reported an objective response rate (ORR) of 44%.19 In a phase II study, 10 out of total 29 patients with locally advanced or metastatic pSCC receiving the TPF regimen, 38.5% achieved an objective response and two patients with locally advanced disease achieved radiological complete response.20
Conventional docetaxel is formulated in polysorbate 80 (and ethanol), which is known to cause infusion-related toxicities and hypersensitivity reactions. NDLS is a novel formulation of docetaxel, which is lipid-based drug delivery system that eliminates need of polysorbate 80 and ethanol. NDLS has been studied in patients with locally advanced and metastatic breast cancer, hormone refractory prostate cancer and locally advanced head and neck squamous cell cancer, where it has demonstrated a better efficacy and safety versus conventional docetaxel.21
Any major study with head to head comparison would be difficult because of limited disease prevalence, especially in Western countries. In study by Pizzocaro et al and Leijte et al, number of patients in trials for NACT in penile cancer were 6 and 20, respectively.3 22 In review by Hakenberg et al, author concluded that taxane-based NACT for N3 ilioinguinal disease is recommended and can result in long term survival but main problem still remains the lack of data.23
In our patient with local recurrence of pSCC, combination chemotherapy with NDLS-containing TPF regimen was responsible for the tumour shrinkage and the successful control of the local recurrence, which subsequently rendered the patient operable for staged surgical resection. Treatment of patients with locally advanced/recurrent penile cancer with NDLS-based combination chemotherapy may be considered as one of the options in locally advanced disease or patients with comorbid conditions precluding safe single stage surgery. Though this strategy needs to be validated in larger patient cohort, this is the first case report showing successful treatment of recurrent pSCC with NDLS-based TPF regimen in the neoadjuvant setting.
Learning points.
Penile squamous cell carcinoma (pSCC) is a relatively rare disease in Western world but is a significant health problem in countries like India.
We report here a case of successful multimodality management of recurrent pSCC in a patient with poorly controlled diabetes.
In view of the comorbidities, locally recurrent disease and presence of right Iliac lymphadenopathy, the patient was treated with nanosomal docetaxel lipid suspension (NDLS), cisplatin and 5-fluorouracil (TPF regimen) in the neoadjuvant setting followed by staged surgical resection.
This is the first case report showing successful treatment of recurrent pSCC with NDLS-based TPF regimen in the neoadjuvant setting.
Footnotes
Contributors: Planning SG, SSP; Conduct SG, SSP; Reporting SG, SSP; Design & Conception SG, SSP; Data acquisition SG, SSP; Manuscript preparation SG, SSP, DB; Manuscript editing SG, SSP, DB; Manuscript review SG, SSP, DB; Guarantor SG;
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Crook J, Mazeron J, cancer P : Gunderson L, Tepper J, Clinical radiation oncology. Philadelphia: Elsevier Saunders, 2012:1167. [Google Scholar]
- 2.Pizzocaro G, Nicolai N, Milani A. Taxanes in combination with cisplatin and fluorouracil for advanced penile cancer: preliminary results. Eur Urol 2009;55:546–51. 10.1016/j.eururo.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Yoo KE, Kang RY, Lee JY, et al. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer 2015;23:1969–77. 10.1007/s00520-014-2547-y [DOI] [PubMed] [Google Scholar]
- 4.Novara G, Galfano A, De Marco V, et al. Prognostic factors in squamous cell carcinoma of the penis. Nat Clin Pract Urol 2007;4:140–6. 10.1038/ncpuro0751 [DOI] [PubMed] [Google Scholar]
- 5.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol 2006;93:133–8. 10.1002/jso.20414 [DOI] [PubMed] [Google Scholar]
- 6.Sklaroff RB, Yagoda A. Cis-diamminedichloride platinum II (DDP) in the treatment of penile carcinoma. Cancer 1979;44:1563–5. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed T, Sklaroff R, Yagoda A. Sequential trials of methotrexate, cisplatin and bleomycin for penile cancer. J Urol 1984;132:465–8. 10.1016/S0022-5347(17)49693-5 [DOI] [PubMed] [Google Scholar]
- 8.Gagliano RG, Blumenstein BA, Crawford ED, et al. cis-Diamminedichloroplatinum in the treatment of advanced epidermoid carcinoma of the penis: a Southwest Oncology Group Study. J Urol 1989;141:66–7. 10.1016/S0022-5347(17)40590-8 [DOI] [PubMed] [Google Scholar]
- 9.Theodore C, Skoneczna I, Bodrogi I, et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992). Ann Oncol 2008;19:1304–7. 10.1093/annonc/mdn149 [DOI] [PubMed] [Google Scholar]
- 10.Fisher H, Barada J, Horton J. Neoadjuvant therapy with cisplatin and 5-fluorouracil for stage III squamous cell carcinoma of the penis. Acta Oncol 1990;27:A652. [Google Scholar]
- 11.Hussein AM, Benedetto P, Sridhar KS. Chemotherapy with cisplatin and 5-fluorouracil for penile and urethral squamous cell carcinomas. Cancer 1990;65:433–8. [DOI] [PubMed] [Google Scholar]
- 12.Shammas FV, Ous S, Fossa SD. Cisplatin and 5-fluorouracil in advanced cancer of the penis. J Urol 1992;147:630–2. 10.1016/S0022-5347(17)37327-5 [DOI] [PubMed] [Google Scholar]
- 13.Di Lorenzo G, Buonerba C, Federico P, et al. Cisplatin and 5-fluorouracil in inoperable, stage IV squamous cell carcinoma of the penis. BJU Int 2012;110(11 Pt B):E661–E666. 10.1111/j.1464-410X.2012.11453.x [DOI] [PubMed] [Google Scholar]
- 14.Bermejo C, Busby JE, Spiess PE, et al. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol 2007;177:1335–8. 10.1016/j.juro.2006.11.038 [DOI] [PubMed] [Google Scholar]
- 15.Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol 2010;28:3851–7. 10.1200/JCO.2010.29.5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705–15. 10.1056/NEJMoa070956 [DOI] [PubMed] [Google Scholar]
- 17.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007;357:1695–704. 10.1056/NEJMoa071028 [DOI] [PubMed] [Google Scholar]
- 18.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 2013;14:257–64. 10.1016/S1470-2045(13)70011-1 [DOI] [PubMed] [Google Scholar]
- 19.Salvioni R, Nicolai N, Piva L, et al. Pilot study of cisplatin, 5-fluorouracil, and a taxane (TPF) for advanced squamous cell carcinoma (SCC) of the penis. Journal of Clinical Oncology 2011;29:4639 10.1200/jco.2011.29.15_suppl.4639 [DOI] [Google Scholar]
- 20.Nicholson S, Hall E, Harland SJ, et al. Phase II trial of docetaxel, cisplatin and 5FU chemotherapy in locally advanced and metastatic penis cancer (CRUK/09/001). Br J Cancer 2013;109:2554–9. 10.1038/bjc.2013.620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad A, Sheikh S, Taran R, et al. Therapeutic efficacy of a novel nanosomal docetaxel lipid suspension compared with taxotere in locally advanced or metastatic breast cancer patients. Clin Breast Cancer 2014;14:177–81. 10.1016/j.clbc.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 22.Leijte JA, Kerst JM, Bais E, et al. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur Urol 2007;52:488–94. 10.1016/j.eururo.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 23.Hakenberg OW, Protzel C. Chemotherapy in penile cancer. Ther Adv Urol 2012;4:133–8. 10.1177/1756287212441235 [DOI] [PMC free article] [PubMed] [Google Scholar]


