Abstract
Background
Disrupted rest-activity circadian rhythm (RAR) patterns have been associated with poor health outcomes (i.e. diminished cognitive function, increased risk of dementia and falls). Circadian time cues in bone influence the differentiation of osteoblasts and osteoclasts, and bone turnover markers exhibit circadian variation; relationships between bone outcomes and RAR are emerging areas of research. We evaluated associations between RAR and areal bone mineral density (aBMD) at the total hip and femoral neck in older men from the Osteoporotic Fractures in Men (MrOS) cohort. We hypothesized that weaker RAR patterns would be associated with lower aBMD.
Methods
MrOS is an ongoing prospective cohort study following ambulatory men ≥ 65 years (n = 5994) at 6 U.S. clinics (baseline enrollment 3/2000–4/2002); participants for this analysis are from an ancillary study, Outcomes of Sleep Disorders in Older Men (MrOS Sleep). We included data from men who had technically adequate measures of RAR and aBMD at Sleep Visit 1 (12/2003–3/2005), with repeat aBMD at core Visit 3 (3/2007–3/2009) (n = 2412; mean age at Sleep Visit 1: 75.7 ± 5.2 years). aBMD was measured by dual energy x-ray absorptiometry (DXA). Actigraphs worn on the non-dominant wrist were used to collect circadian activity data over 4.8 ± 0.8 consecutive 24-hour periods. An extension of the traditional cosine curve was used to fit RAR to the activity data [Ancoli-Israel et al., 2003; Marler et al., 2006]. Six RAR parameters were evaluated: acrophase (time of peak activity), amplitude (rhythm strength), mesor (mean of activity fitted curve), pseudo F-statistic (overall circadian rhythmicity of rest and activity), alpha statistic (daytime to nighttime activity ratio), and beta statistic (daytime activity). Associations between RAR and aBMD (Sleep Visit 1), and RAR and ΔaBMD (Sleep Visit 1-Visit 3) were assessed with generalized linear models. Covariates included age, clinic site, physical activity, race, comorbidity, body mass index (BMI), smoking, alcohol, caffeine, beta blocker use, serum 25(OH) vitamin D and urinary melatonin and calcium.
Results
Pseudo F-statistic was significantly associated with total hip aBMD (p-trend = 0.009), femoral neck aBMD (p-trend = 0.007) and total hip ΔaBMD (p-trend = 0.017) in minimally adjusted models but not after multivariate (MV) adjustment. Alpha statistic was significantly associated with femoral neck aBMD (p-trend = 0.029) and femoral neck ΔaBMD (p-trend = 0.019) in minimally adjusted models; significance was retained in the femoral neck ΔaBMD model (p-trend = 0.034) after MV adjustment. There were no consistent, significant associations between the other RAR variables and aBMD or ΔaBMD.
Conclusions
The data demonstrate modest associations between overall circadian rhythmicity of rest and activity (measured by pseudo F-statistic), as well as daytime to nighttime activity ratio (measured by alpha statistic), aBMD and ΔaBMD, but adjustment for covariates related to lifestyle, BMI and comorbidities attenuated most of these associations. These results suggest that RAR patterns are not independently associated with aBMD or four-year ΔaBMD at the total hip or femoral neck in older men, but additional research is needed.
Abbreviations: aMT6s, 6-sulfatoxymelatonin; BMD, bone mineral density; MrOS, Osteoporotic Fractures in Men prospective cohort study; MrOS Sleep, Outcomes of Sleep Disorders in Men, a MrOS ancillary study; PASE, Physical Activity Scale for the Elderly; RAR, rest-activity circadian rhythm; TST, total sleep time
Keywords: Circadian, Rest-activity rhythm, Actigraphy, Pseudo F-statistic, Alpha statistic, Areal BMD
Highlights
-
•
Disrupted rest-activity circadian rhythm (RAR) undermines health outcomes.
-
•
We modeled RAR patterns and areal bone mineral density (aBMD) in older men.
-
•
Most associations lost significance after multivariate adjustment.
-
•
RAR patterns are not independently associated with aBMD or ΔaBMD in older men.
1. Introduction
Increasing evidence suggests possible relationships between sleep, circadian rhythms and bone health in older men and women, related in part to the presence of circadian clock genes in bone cells, as well as melatonin (Albayrak et al., 2016, Cunningham and Di Pace, 2015, Stone et al., 2006, Stone et al., 2014, Swanson et al., 2015, Dudek and Meng, 2014, Amstrup et al., 2013). Circadian rhythm influences sleep and wakefulness (Saper et al., 2005, Dijk and Lockley, 2002), and rest-activity circadian rhythm (RAR) patterns can be measured with wrist-worn actigraphs; data can be fitted on a cosine curve for analysis (Martin and Hakim, 2011, Ancoli-Israel et al., 2003). We recently reported that later acrophase was associated with a modestly greater risk of falls in a cohort of elderly men enrolled in the Osteoporotic Fractures in Men (MrOS) prospective cohort study (Rogers et al., 2017). Common RAR measures include acrophase (time of peak activity level), amplitude (strength of rhythm), mesor (mean of activity fitted curve), pseudo F-statistic (a measure of overall circadian rhythmicity), alpha statistic (a ratio of daytime to nighttime activity), and beta statistic (a measure of daytime activity) (Paudel et al., 2010).
To further elucidate potential relationships between circadian rhythm and bone, we examined associations between RAR patterns and areal bone mineral density (aBMD) at the total hip and femoral neck. By utilizing data from MrOS and the MrOS ancillary study, Outcomes of Sleep Disorders in Men (MrOS Sleep), we had the opportunity to examine aBMD and RAR patterns cross-sectionally and longitudinally in a large cohort of community-dwelling older men. Participants were well-characterized for the RAR and aBMD variables, as well as confounding factors such as lifestyle habits, body composition and comorbidities.
Based on the previous associations between disrupted RAR patterns and deleterious health outcomes in the elderly (Paudel et al., 2010, Tranah et al., 2010, Tranah et al., 2011, Paudel et al., 2011, Rogers et al., 2017), we hypothesized that dysregulated RAR patterns would be associated with lower aBMD at MrOS Sleep Visit 1 and with greater aBMD loss over four years (MrOS Sleep Visit 1- MrOS Visit 3) in older men in the MrOs cohort.
2. Materials and methods
2.1. Study participants
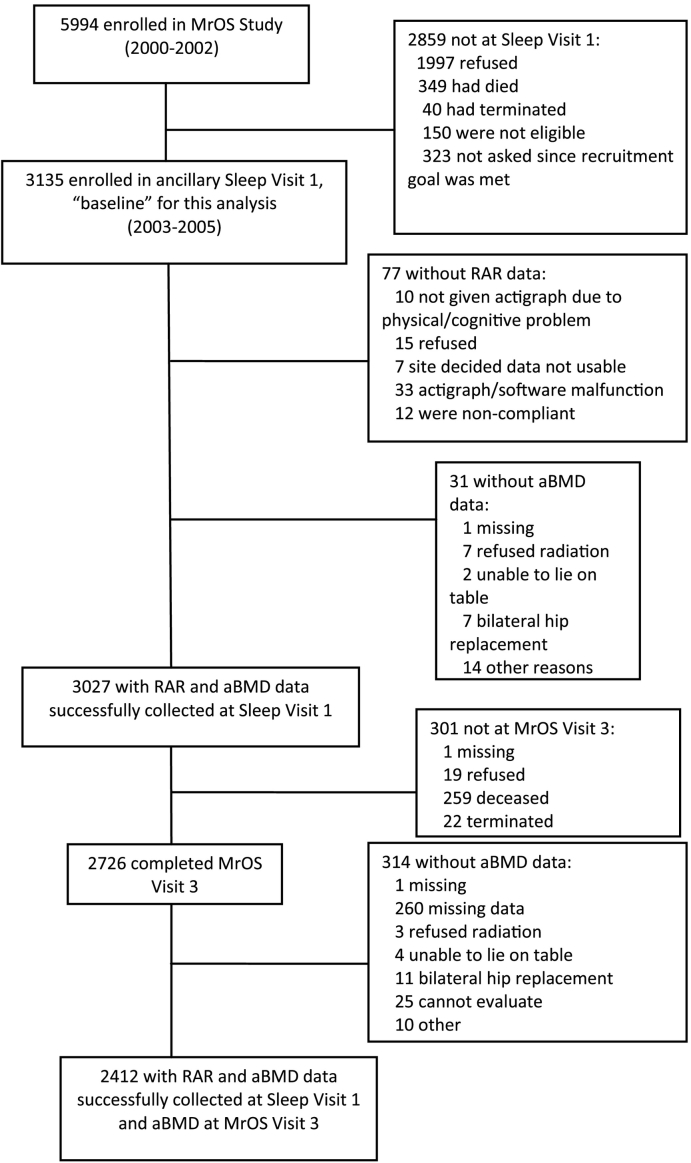
The Osteoporotic Fractures in Men (MrOS) prospective cohort study follows ambulatory men ≥ 65 years of age (n = 5994) at six U.S. clinics (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California). Enrollment in MrOS took place from March 2000 through April 2002, at which time men had to be 65 years of age or older. Exclusion criteria included the inability to walk without assistance and history of bilateral hip replacement (Orwoll et al., 2005, Blank et al., 2005). The MrOS Sleep ancillary study focuses on sleep disorders in older men; 3135 men (of the 5994 MrOS participants) were enrolled (105% of recruitment goal), for MrOS Sleep Visit 1 from December 2003 through March 2005. For the present study, we included data from men with technically adequate measures of RAR patterns and aBMD measurements at MrOS Sleep Visit 1 and aBMD at MrOS Visit 3 (March 2007–March 2009); the final sample size was 2412 (Fig. 1).
Fig. 1.
Flow diagram of participant recruitment and inclusion. aBMD, areal bone mineral density. RAR, rest-activity circadian rhythm. MrOS, Osteoporotic Fractures in Men prospective cohort study.
The study was approved by the Institutional Review Board at each clinic site, and all participants provided written informed consent.
2.2. Assessment of aBMD
Areal bone mineral density (aBMD, g/cm2) of the total hip and femoral neck was measured by dual energy x-ray absorptiometry (DXA) (QDR 4500 W; Hologic Inc., Bedford, MA) at both visits.
2.3. Assessment of rest-activity patterns
Activity count data were collected from participants over 4.8 ± 0.8 consecutive 24-hour periods using wrist-worn actigraphy (SleepWatch-O; Ambulatory Monitoring, Inc., Ardsley, New York, USA). This time period met the minimum three day requirement for actigraphy monitoring according to the Centers for Medicare Services (CMS) (American Academy of Sleep Medicine, 2014). Participants wore the actigraph on the non-dominant wrist, and they were instructed to wear the actigraph at all times unless performing activities that would submerge it in water. Participants also used a sleep diary to record bedtime, wake time and any times the device was removed (Blackwell et al., 2017). The actigraph detected participants' movements via a piezoelectric bimorph-ceramic cantilever beam which generated a voltage every time the actigraph was moved. Voltages were aggregated continuously and then summarized over intervals of 1 min; movement was measured in counts per minute (Ambulatory Monitoring, Inc., n.d.). Actigraphy data are reported here using proportional integration mode (PIM), the preferred mode of measuring activity for this device (Blackwell et al., 2011). Because activity data often assume a shape more similar to a squared wave than a cosine curve, a five parameter extension of the traditional cosine curve was used to fit RAR to the activity data (Ancoli-Israel et al., 2003, Marler et al., 2006).
Six RAR parameters were evaluated in this analysis. Amplitude, which is measured in activity counts per minute, is defined as the peak to nadir difference in the fitted curve and illustrates the strength of the circadian rhythm. Mesor, which is also measured in activity counts per minute, indicates the mean level of the fitted curve. The pseudo F-statistic, which has no unit, illustrates how well the activity fits the cosine model; higher pseudo F-statistic denotes a better fit and increased overall circadian rhythmicity of rest and activity. Acrophase reflects the time of day of peak activity, and earlier or later acrophase suggests a tendency toward advanced or delayed activity rhythm. The alpha statistic indicates the daytime to nighttime activity ratio and is a measure of peak-to-trough width. The beta statistic measures curve steepness and reflects stability of daytime activity levels. Like the pseudo F-statistic, the alpha and beta statistics have no units (Paudel et al., 2010, Paudel et al., 2011).
This analysis also included total sleep time (TST), which is a sleep-wake activity variable and is defined as the hours spent after “lights off” (Stone et al., 2014).
2.4. Other measures
Additional measures assessed at MrOS Sleep Visit 1 included self-reported diagnoses of co-morbid conditions, depressive symptoms (Geriatric Depression Scale, GDS), current prescription and over the counter medications, self-reported alcohol and caffeine intake, self-reported smoking, physical activity (Physical Activity Scale for the Elderly, PASE), and height and body weight, which were used to calculate body mass index (BMI; kg/m2) (Yesavage and Sheikh, 1986, Almeida and Almeida, 1999, Pahor et al., 1994, Barone and Roberts, 1996, Washburn et al., 1993, Saksvik-Lehouillier et al., 2015, Rogers et al., 2017). The major urinary metabolite of melatonin, 6-sulfatoxymelatonin (aMT6s), was measured from first morning void urine specimens. Participants received instruction and supplies during clinic visits and were told to keep the specimen refrigerated. Assays to measure aMT6s were performed in duplicate at the Oregon Health and Science University (OHSU) Oregon Clinical and Translational Research Institute (OCTRI) Core Laboratory with the Buhlman 6-sulphatoxymelatonin ELISA (ALPCO Diagnostics, Windham, NH, USA). The inter-assay coefficient of variation was 12.5%, and the intra-assay coefficient of variation was 5.0%. All aMT6s levels were standardized by creatinine, which was measured in the same urine samples (Roche COBAS Integra 6000 automated analyzer; Roche Diagnostics Corp., Indianapolis, IN, USA). Details of the aMT6s measurements in MrOS have been described previously (Saksvik-Lehouillier et al., 2015). Urinary calcium was also measured from the urine samples (Roche COBAS Integra 6000 automated analyzer; Roche Diagnostics Corp., Indianapolis, IN, USA). The inter-assay coefficient of variation for urinary calcium was 1.57%, and the intra-assay coefficient of variation was 1.22%.
Nocturia was defined as self-reported difficulty sleeping due to nocturnal bathroom use at least three times per week (Rogers et al., 2017). Serum 25(OH) vitamin D was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (ThermoFisher Scientific, Franklin, Massachusetts 02038 and Applied Biosystems-MDS Sciex, Foster City, CA 94404).
2.5. Statistical analyses
We compared characteristics of the participants across quartiles of pseudo F-statistic using the Kruskal-Wallis tests for skewed continuous variables, analysis of variance (ANOVA) for continuous normally distributed variables, and chi-square tests for categorical variables. Additionally, we examined Spearman correlations between the RAR variables and aMT6s, as well as between RAR variables and TST.
We examined associations between RAR parameters and aBMD at Sleep Visit 1, as well as associations between RAR and ΔaBMD (Sleep Visit 1 to MrOS Visit 3) using generalized linear models, reporting adjusted means with p-values for trends. Currently RAR variables do not have standard clinical cut-points (Rogers et al., 2017), and we assessed amplitude, mesor, pseudo F-statistic, alpha and beta in quartiles, with the lowest quartile of each RAR variable serving as the referent. Acrophase was analyzed in terms of deviation from the population mean (± 1.5 SD). The mean acrophase group served as the referent since earlier or later acrophase indicates a shifted circadian rhythm (Walsh et al., 2014, Paudel et al., 2010, Paudel et al., 2011). Data for the beta variable were log transformed.
Models were initially adjusted for age and clinic site. Multivariate models were adjusted for race (white vs. non-white), BMI, medical conditions (hypertension, congestive heart failure), depression (GDS ≥ 6), physical activity (PASE score), caffeine intake, alcohol intake (< 1 drink/week, 1–13 drinks/week, ≥ 14 drinks/week), smoking (none, past, current), beta blocker use, serum 25 (OH) vitamin D, urinary melatonin (aMT6s), and urinary calcium. Covariates for multivariate models were chosen by selecting those that were related to the RAR variable at p < 0.10 and then put into a backwards selection model at p < 0.05.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC), and two-sided significance levels were set at p < 0.05.
3. Results
In this analysis, we included data from men who had technically adequate measures of RAR and aBMD at Sleep Visit 1 and aBMD at Visit 3. The analytic cohort included 2412 primarily white (91.3%) men with an average age of 75.7 ± 5.2 years at the RAR assessment (Sleep Visit 1) and an average BMI of 27.2 ± 3.7 kg/m2.
Participant characteristics stratified by quartile of pseudo F-statistic are presented in Table 1. Men in higher quartiles of pseudo F-statistic (i.e. men with greater circadian rhythmicity) were more likely to be younger, have a lower BMI and higher levels of serum 25 (OH) vitamin D, as well as higher urinary aMT6s and calcium, compared to men in lower quartiles of pseudo F-statistic. Additionally, men with greater pseudo F-statistic were also less likely to report comorbidities, beta blocker use or nocturia, but were more likely to have higher alcohol and caffeine intake and higher physical activity (higher PASE score). There were no significant differences across quartiles of pseudo F-statistic in race, smoking or use of anti-depressants, benzodiazepines, bisphosphonates or corticosteroids (Table 1).
Table 1.
Characteristics of older men in the MrOS cohort by quartiles of pseudo F-statistic (rest-activity rhythm robustness).
| Characteristic | Quartile 1 (8.3 to < 689.6, n = 541) | Quartile 2 (689.6 to < 959.6, n = 587) | Quartile 3 (959.6 to < 1311.9, n = 628) | Quartile 4 (1311.9 to 4501.4, n = 651) | p-Value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 76.8 ± 5.51 | 75.97 ± 5.34 | 75.53 ± 5.02 | 74.76 ± 4.68 | < 0.001 |
| White race, n (%) | 487 (90.0) | 535 (91.1) | 575 (91.6) | 605 (92.9) | 0.342 |
| Body mass index (kg/m2), mean ± SD | 28.1 ± 4.1 | 27.4 ± 3.7 | 30.0 ± 3.5 | 26.4 ± 3.3 | < 0.001 |
| Urinary melatonina (ng/mg), mean ± SD | 9.7 ± 8.8 | 10.6 ± 8.8 | 11.7 ± 8.8 | 11.4 ± 9.4 | < 0.001 |
| Diabetes, n (%) | 87 (16.1) | 77 (13.1) | 67 (10.7) | 70 (10.8) | 0.016 |
| Heart attack, n (%) | 114 (21.1) | 87 (14.8) | 84 (13.4) | 95 (14.6) | 0.002 |
| Congestive heart failure, n (%) | 51 (9.4) | 25 (4.3) | 30 (4.8) | 22 (3.9) | < 0.001 |
| Hypertension, n (%) | 302 (55.8) | 291 (49.6) | 321 (51.1) | 260 (39.9) | < 0.001 |
| Smoking status, n (%) | 0.192 | ||||
| Never | 212 (39.2) | 248 (42.3) | 255 (40.6) | 254 (39.0) | |
| Past | 310 (57.3) | 330 (56.2) | 364 (58.0) | 383 (58.8) | |
| Current | 19 (3.5) | 9 (1.5) | 9 (1.4) | 14 (2.2) | |
| Drinks per week, n (%) | 0.000 | ||||
| None | 209 (38.9) | 202 (34.7) | 196 (31.3) | 179 (27.6) | |
| 1–13 | 307 (57.1) | 351 (60.2) | 395 (63.1) | 416 (64.2) | |
| 14 + | 22 (4.1) | 30 (5.2) | 35 (5.6) | 53 (8.2) | |
| Caffeine consumption (mg/day), mean ± SD | 215.0 ± 258.4 | 238.0 ± 248.9 | 228.6 ± 235.0 | 261.4 ± 252.1 | 0.001 |
| PASEb score, mean ± SD | 130.2 ± 68.2 | 143.7 ± 68.1 | 156.6 ± 68.8 | 169.6 ± 70.0 | < 0.001 |
| Depression (GDSc > 6), n (%) | 53 (9.8) | 35 (6.0) | 18 (2.9) | 28 (4.3) | < 0.001 |
| Anti-depressant use, n (%) | 41 (7.6) | 47 (8.0) | 39 (6.2) | 40 (6.1) | 0.467 |
| Benzodiazepine use, n (%) | 30 (5.6) | 25 (4.3) | 20 (3.2) | 26 (4.0) | 0.246 |
| Beta blocker use, n (%) | 204 (37.8) | 168 (28.6) | 151 (24.0) | 136 (20.9) | < 0.001 |
| Bisphosphonate use, n (%) | 29 (5.4) | 19 (3.2) | 29 (4.6) | 31 (4.8) | 0.353 |
| Corticosteroid use, n (%) | 54 (10.0) | 51 (8.7) | 61 (9.8) | 55 (8.5) | 0.749 |
| Nocturia, n (%) | 190 (35.12) | 194 (33.05) | 185 (29.46) | 182 (27.96) | 0.031 |
| Serum 25(OH) vitamin D (ng/ml) | 27.98 ± 9.05 | 28.91 ± 8.9 | 29.2 ± 8.86 | 29.97 ± 8.75 | 0.002 |
| Urinary calcium (mg/dl) | 9.08 ± 7.14 | 9.65 ± 6.84 | 9.78 ± 6.94 | 11.12 ± 8.2 | < 0.001 |
p-Values for continuous variables are from ANOVA for normally distributed data; the Kruskal Wallis test was used for skewed data. p-Values for categorical data from a chi-square test.
6-Sulfatoxymelatonin (aMT6s), the major urinary metabolite of melatonin.
Physical Activity Scale for the Elderly.
Geriatric Depression Scale.
Amplitude and pseudo F-statistic correlated with aMT6s, although correlation coefficients were quite small. Pseudo F-statistic and alpha were positively correlated with TST, and mesor was negatively correlated with TST (Table 2).
Table 2.
Spearman correlations between 6-sulfatoxymelatonin (aMT6s), total sleep time (TST) and rest-activity circadian rhythm variables in older men. Correlation coefficient (p-value).
| 6-Sulfatoxymelatonin (aMT6s) | Total sleep time (TST) | |
|---|---|---|
| aMT6s | – | 0.044 (0.037) |
| TST | 0.044 (0.037) | – |
| Acrophase | 0.031 (0.148) | 0.005 (0.780) |
| Amplitude | 0.086 (< 0.0001) | 0.004 (0.855) |
| Pseudo F-statistic | 0.092 (< 0.0001) | 0.220 (< 0.0001) |
| Mesor | 0.035 (0.104) | − 0.228 (< 0.0001) |
| Alpha | − 0.041 (0.053) | 0.284 (< 0.0001) |
| Beta | − 0.028 (0.184) | − 0.024 (0.240) |
Cross-sectional associations at Sleep Visit 1 between RAR parameters and total hip aBMD are presented in Table 3. Pseudo F-statistic and aBMD were associated in models adjusted for age and clinical site, but this association lost significance after further adjustment for BMI, heart attack, diabetes mellitus, alcohol, caffeine, aMT6s, PASE score, depression, beta blocker use, 25 (OH) vitamin D and urinary calcium. No other significant associations were observed between RAR parameters and total hip aBMD at Sleep Visit 1.
Table 3.
Associations of rest-activity circadian rhythm parameters and total hip areal bone mineral density (aBMD) in older men at MrOS Sleep Visit 1.
| Predictor | aBMD at Sleep Visit 1 (age and site adjusted) |
aBMD at Sleep Visit 1 (multivariate adjusted) |
||
|---|---|---|---|---|
| LS means (95% CI) | p-Trend | LS means (95% CI) | p-Trend | |
| Amplitude (counts/min) | ||||
| Q1 (ref): 278.83 to < 2893.84 | 0.96 (0.95, 0.97) | 0.457 | 0.95 (0.94, 0.96) | 0.116 |
| Q2: 2893.84 to < 3536.64 | 0.96 (0.95, 0.97) | 0.96 (0.95, 0.97) | ||
| Q3: 3536.64 to < 4190.04 | 0.96 (0.94, 0.97) | 0.96 (0.95, 0.97) | ||
| Q4: 4190.04 to 12,357.70 | 0.95 (0.94, 0.97) | 0.96 (0.95, 0.97) | ||
| Mesor (counts/min) | ||||
| Q1 (ref): 567.86 to < 1847.12 | 0.96 (0.95, 0.97) | 0.620 | 0.95 (0.94, 0.97) | 0.486 |
| Q2: 1847.12 to < 2134.04 | 0.96 (0.95, 0.97) | 0.96 (0.95, 0.97) | ||
| Q3: 2134.04 to < 2424.78 | 0.95 (0.94, 0.96) | 0.95 (0.94, 0.96) | ||
| Q4: 424.78 to 7592.08 | 0.96 (0.95, 0.97) | 0.96 (0.95, 0.97) | ||
| Pseudo F-statistic (no unit) | ||||
| Q1 (ref): 8.27 to < 689.559 | 0.96 (0.95, 0.98) | 0.009 | 0.95 (0.94, 0.97) | 0.784 |
| Q2: 689.56 to < 959.56 | 0.96 (0.95, 0.98) | 0.96 (0.95, 0.97) | ||
| Q3: 959.56 to < 1311.94 | 0.95 (0.94, 0.97) | 0.96 (0.95, 0.97) | ||
| Q4: 1311.94 to 4501.43 | 0.94 (0.93, 0.96) | 0.95 (0.94, 0.96) | ||
| Alpha statistic (no unit) | ||||
| Q1 (ref): − 0.82 to <− 0.47 | 0.96 (0.95, 0.97) | 0.223 | 0.96 (0.95, 0.97) | 0.610 |
| Q2: − 0.47 to <− 0.35 | 0.95 (0.94, 0.96) | 0.95 (0.94, 0.96) | ||
| Q3: − 0.35 to <− 0.18 | 0.95 (0.94, 0.96) | 0.95 (0.94, 0.96) | ||
| Q4: − 0.18 to 1.00 | 0.97 (0.96, 0.98) | 0.96 (0.95, 0.97) | ||
| Beta statistic (no unit) | ||||
| Q1 (ref): 0.71 to < 4.94 | 0.96 (0.95, 0.97) | 0.229 | 0.95 (0.94, 0.96) | 0.463 |
| Q2: 4.94 to < 8.82 | 0.96 (0.95, 0.97) | 0.97 (0.96, 0.98) | ||
| Q3: 8.82 to < 20.85 | 0.95 (0.94, 0.96) | 0.95 (0.94, 0.97) | ||
| Q4: 20.85 to 746.20 | 0.95 (0.94, 0.96) | 0.95 (0.94, 0.96) | ||
| Acrophase (hours, min) | ||||
| − 1.5 SD | 0.94 (0.92, 0.97) | 0.618 | 0.94 (0.92, 0.97) | 0.898 |
| Mean (ref) | 0.96 (0.95, 0.96) | 0.96 (0.95, 0.96) | ||
| + 1.5 SD | 0.93 (0.91, 0.96) | 0.94 (0.92, 0.97) | ||
Bold values indicate significance at p < 0.05.
Multivariate model adjustments: amplitude, pseudo F-statistic, mesor multivariate models adjusted for: BMI, heart attack, diabetes, alcohol, caffeine, PASE, depression, melatonin, beta blocker use, 25(OH) vitamin D, urinary calcium. Alpha and beta multivariate models adjusted for: BMI, smoking, alcohol, depression. Acrophase multivariate model adjusted for: race, BMI, melatonin, alcohol, smoking, caffeine, PASE, depression.
Associations between RAR parameters and four-year change in total hip aBMD (ΔaBMD) are presented in Table 4. Similarly to the cross-sectional models, pseudo F-statistic was associated with ΔaBMD after adjustment for age and clinical site, but not after further adjustments for covariates (depression, hypertension, congestive heart failure and 25 (OH) vitamin D). None of the other RAR parameters were associated with ΔaBMD in either of the models.
Table 4.
Associations of rest-activity circadian rhythm parameters and four year change in total hip areal bone mineral density (ΔaBMD) in older men (MrOS Sleep Visit 1 to MrOS Visit 3).
| Predictor | ΔaBMD at Sleep Visit 1 (age and site adjusted) |
ΔaBMD at Sleep Visit 1 (multivariate adjusted) |
||
|---|---|---|---|---|
| LS means (95% CI) | p-Trend | LS means (95% CI) | p-Trend | |
| Amplitude (counts/min) | ||||
| Q1 (ref): 278.83 to < 2893.84 | − 1.60 (− 1.93, − 1.28) | 0.360 | − 1.48 (− 1.81, − 1.15) | 0.772 |
| Q2: 2893.84 to < 3536.64 | − 1.37 (− 1.67, − 1.07) | − 1.43 (− 1.73, − 1.13) | ||
| Q3: 3536.64 to < 4190.04 | − 1.31 (− 1.61, − 1.02) | − 1.31 (− 1.60, − 1.02) | ||
| Q4: 4190.04 to 12,357.70 | − 1.39 (− 1.68, 1.10) | 1.45 (− 1.74, − 1.15) | ||
| Mesor (counts/min) | ||||
| Q1 (ref): 567.86 to < 1847.12 | − 1.66 (− 1.98, − 1.34) | 0.612 | − 1.62 (− 1.94, − 1.30) | 0.834 |
| Q2: 1847.12 to < 2134.04 | − 1.25 (− 1.55, − 0.95) | − 1.24 (− 1.55, − 0.94) | ||
| Q3: 2134.04 to < 2424.78 | − 1.23 (− 1.52, − 0.93) | − 1.26 (− 1.55, − 0.97) | ||
| Q4: 424.78 to 7592.08 | − 1.53 (− 1.82, − 1.23) | − 1.54 (− 1.84, − 1.25) | ||
| Pseudo F-statistic (no unit) | ||||
| Q1 (ref): 8.27 to < 689.56 | − 1.68 (− 1.99, − 1.36) | 0.017 | − 1.56 (− 1.88, − 1.24) | 0.163 |
| Q2: 689.56 to < 959.56 | − 1.56 (− 1.86, − 1.26) | − 1.54 (− 1.84, − 1.23) | ||
| Q3: 959.56 to < 1311.94 | − 1.24 (− 1.53, − 0.94) | − 1.26 (− 1.55, − 0.97) | ||
| Q4: 1311.94 to 4501.43 | − 1.22 (− 1.52, − 0.93) | − 1.33 (− 1.62, − 1.03) | ||
| Alpha statistic (no unit) | ||||
| Q1 (ref): − 0.82 to <− 0.47 | − 1.23 (− 1.53, − 0.94) | 0.297 | − 1.23 (− 1.53, − 0.93) | 0.453 |
| Q2: − 0.47 to <− 0.35 | − 1.54 (− 1.84, − 1.25) | − 1.60 (− 1.90, − 1.30) | ||
| Q3: − 0.35 to <− 0.18 | − 1.32 (− 1.62, − 1.02) | − 1.33 (− 1.63, − 1.03) | ||
| Q4: − 0.18 to 1.00 | − 1.55 (− 1.85, − 1.24) | − 1.49 (− 1.80, − 1.19) | ||
| Beta statistic (no unit) | ||||
| Q1 (ref): 0.71 to < 4.94 | − 1.70 (− 1.99, − 1.40) | 0.306 | − 1.70 (− 1.99, − 1.40) | 0.407 |
| Q2: 4.94 to < 8.82 | − 1.19 (− 1.48, − 0.89) | − 1.13 (− 1.43, 0.84)* | ||
| Q3: 8.82 to < 20.85 | − 1.34 (− 1.64, − 1.04) | − 1.40 (− 1.70, − 1.01) | ||
| Q4: 20.85 to 746.20 | − 1.42 (− 1.73, − 1.11) | − 1.43 (− 1.74, − 1.11) | ||
| Acrophase (hours, min) | ||||
| − 1.5 SD | − 1.80 (− 2.45, − 1.16) | 0.783 | − 1.78 (− 2.43, − 1.13) | 0.555 |
| Mean (ref) | − 1.37 (− 1.53, − 1.21) | − 1.39 (− 1.54, 1.23) | ||
| + 1.5 SD | − 1.67 (− 2.32, − 1.03) | − 1.51 (− 2.16, − 0.86) | ||
Bold values indicate significance at p < 0.05.
Multivariate model adjustments: amplitude and pseudo F-statistic multivariate models adjusted for depression, hypertension, congestive heart failure, 25(OH) vitamin D. Mesor multivariate model adjusted for depression, hypertension, 25(OH) vitamin D. Alpha statistic multivariate model adjusted for hypertension, smoking, depression, 25(OH) vitamin D. Beta statistic multivariate model adjusted for smoking, depression, 25(OH) vitamin D. Acrophase multivariate model adjusted for depression, 25(OH) vitamin D.
Cross-sectional associations at Sleep Visit 1 between RAR parameters and femoral neck aBMD are presented in Table 5. Pseudo F-statistic and alpha statistic were associated with femoral neck aBMD after adjusting for age and clinic site, but significance was not retained after multivariate adjustment.
Table 5.
Associations of rest-activity circadian rhythm parameters and femoral neck areal bone mineral density (aBMD) in older men at MrOS Sleep Visit 1.
| Predictor | aBMD at Sleep Visit 1 (age and site adjusted) |
aBMD at Sleep Visit 1 (multivariate adjusted) |
||
|---|---|---|---|---|
| LS means (95% CI) | p-Trend | LS means (95% CI) | p-Trend | |
| Amplitude (counts/min) | 0.366 | 0.280 | ||
| Q1 (ref): 278.83 to < 2893.84 | 0.79 (0.78, 0.80) | 0.78 (0.77, 0.79) | ||
| Q2: 2893.84 to < 3536.64 | 0.79 (0.78, 0.80) | 0.78 (0.77, 0.79) | ||
| Q3: 3536.64 to < 4190.04 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Q4: 4190.04 to 12,357.70 | 0.78 (0.77, 0.79) | 0.79 (0.78, 0.80) | ||
| Mesor (counts/min) | 0.913 | 0.232 | ||
| Q1 (ref): 567.86 to < 1847.12 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Q2: 1847.12 to < 2134.04 | 0.79 (0.78, 0.80) | 0.79 (0.77, 0.80) | ||
| Q3: 2134.04 to < 2424.78 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Q4: 424.78 to 7592.08 | 0.79 (0.78, 0.80) | 0.79 (0.78, 0.80) | ||
| Pseudo F-statistic (no unit) | 0.007 | 0.407 | ||
| Q1 (ref): 8.27 to < 689.559 | 0.79 (0.78, 0.80) | 0.78 (0.77, 0.79) | ||
| Q2: 689.56 to < 959.56 | 0.78 (0.78, 0.80) | 0.79 (0.78, 0.80) | ||
| Q3: 959.56 to < 1311.94 | 0.78 (0.77, 0.79) | 0.78 (0.78, 0.79) | ||
| Q4: 1311.94 to 4501.43 | 0.77 (0.76, 0.78) | 0.78 (0.77, 0.79) | ||
| Alpha statistic (no unit) | 0.029 | 0.657 | ||
| Q1 (ref): − 0.82 to <− 0.47 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.80) | ||
| Q2: − 0.47 to <− 0.35 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Q3: − 0.35 to <− 0.18 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Q4: − 0.18 to 1.00 | 0.80 (0.79, 0.81) | 0.79 (0.78, 0.80) | ||
| Beta statistic (no unit) | 0.324 | 0.443 | ||
| Q1 (ref): 0.71 to < 4.94 | 0.79 (0.78, 0.80) | 0.78 (0.77, 0.79) | ||
| Q2: 4.94 to < 8.82 | 0.79 (0.78, 0.80) | 0.79 (0.78, 0.80) | ||
| Q3: 8.82 to < 20.85 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Q4: 20.85 to 746.20 | 0.78 (0.77, 0.79) | 0.78 (0.77, 0.79) | ||
| Acrophase (hours, min) | 0.444 | 0.887 | ||
| − 1.5 SD | 0.77 (0.75, 0.79) | 0.77 (0.75, 0.79) | ||
| Mean (ref) | 0.79 (0.78, 0.79) | 0.78 (0.78, 0.79) | ||
| + 1.5 SD | 0.76 (0.74, 0.78) | 0.77, 0.75, 0.79) | ||
Bold values indicate significance at p < 0.05.
Multivariate model adjustments: amplitude, pseudo F-statistic, mesor multivariate models adjusted for: BMI, heart attack, diabetes, alcohol, caffeine, PASE, depression, melatonin, beta blocker use, 25(OH) vitamin D, urinary calcium. Alpha and beta multivariate models adjusted for: BMI, smoking, alcohol, depression. Acrophase multivariate model adjusted for: race, BMI, melatonin, alcohol, smoking, caffeine, PASE, depression.
Associations between RAR parameters and femoral neck ΔaBMD are presented in Table 6. Alpha statistic was significantly associated with femoral neck ΔaBMD in the minimally adjusted model. After further adjustment for covariates (hypertension, smoking, depression and 25(OH) vitamin D), the association between alpha statistic and femoral neck ΔaBMD was still significant. None of the other associations between RAR parameters and femoral neck ΔaBMD were significant in the multivariate models.
Table 6.
Associations of rest-activity circadian rhythm parameters and four year change in femoral neck areal bone mineral density (ΔaBMD) in older men (MrOS Sleep Visit 1 to MrOS Visit 3).
| Predictor | ΔaBMD at Sleep Visit 1 (age and site adjusted) |
ΔaBMD at Sleep Visit 1 (multivariate adjusted) |
||
|---|---|---|---|---|
| LS means (95% CI) | p-Trend | LS means (95% CI) | p-Trend | |
| Amplitude (counts/min) | 0.572 | 0.923 | ||
| Q1 (ref): 278.83 to < 2893.84 | − 1.83 (− 2.25, − 1.40) | − 1.68 (− 2.11, − 1.25) | ||
| Q2: 2893.84 to < 3536.64 | − 1.47 (− 1.85, − 1.08) | − 1.49 (− 1.89, − 1.10) | ||
| Q3: 3536.64 to < 4190.04 | − 1.25 (− 1.63, − 0.87) | − 1.24 (− 1.62, − 0.86) | ||
| Q4: 4190.04 to 12,357.70 | − 1.69 (− 2.07, − 1.31) | − 1.70 (− 2.09, − 1.32) | ||
| Mesor (counts/min) | 0.400 | 0.318 | ||
| Q1 (ref): 567.86 to < 1847.12 | − 1.55 (− 1.96, − 1.15) | − 1.51 (− 1.92, − 1.09) | ||
| Q2: 1847.12 to < 2134.04 | − 1.47 (− 1.86, − 1.08) | − 1.43 (− 1.82, − 1.03) | ||
| Q3: 2134.04 to < 2424.78 | − 1.30 (− 1.68, − 0.91) | − 1.31 (− 1.69, − 0.93) | ||
| Q4: 424.78 to 7592.08 | − 1.86 (− 2.24, − 1.47) | − 1.84 (− 2.23, − 1.45) | ||
| Pseudo F-statistic (no unit) | 0.092 | 0.439 | ||
| Q1 (ref): 8.27 to < 689.56 | − 1.81 (− 2.22, − 1.39) | − 1.64 (− 2.06, − 1.22) | ||
| Q2: 689.56 to < 959.56 | − 1.68 (− 2.08, − 1.29) | − 1.63 (− 2.03, − 1.23) | ||
| Q3: 959.56 to < 1311.94 | − 1.33 (− 1.72, − 0.95) | − 1.35 (− 1.73, − 0.96) | ||
| Q4: 1311.94 to 4501.43 | − 1.40 (− 1.78, − 1.03) | − 1.49 (− 1.88, − 1.11) | ||
| Alpha statistic (no unit) | 0.019 | 0.034 | ||
| Q1 (ref): − 0.82 to <− 0.47 | − 1.32 (− 1.71, − 0.94) | − 1.28 (− 1.67, − 0.89) | ||
| Q2: − 0.47 to <− 0.35 | − 1.34 (− 1.73, − 0.95) | − 1.36 (− 1.75, − 0.97) | ||
| Q3: − 0.35 to <− 0.18 | − 1.60 (− 1.99, − 1.21) | − 1.63 (− 2.01, − 1.24) | ||
| Q4: − 0.18 to 1.00 | − 1.94 (− 2.33, − 1.54) | − 1.83 (− 2.24, − 1.43) | ||
| Beta statistic (no unit) | 0.336 | 0.389 | ||
| Q1 (ref): 0.71 to < 4.94 | − 1.90 (− 2.28, − 1.51) | − 1.87 (− 2.26–1.48) | ||
| Q2: 4.94 to < 8.82 | − 1.28 (− 1.66, − 0.89) | − 1.27 (− 1.60, − 0.82) | ||
| Q3: 8.82 to < 20.85 | − 1.43 (− 1.82, − 1.04) | − 1.46 (− 1.87, − 1.09) | ||
| Q4: 20.85 to 746.20 | − 1.57 (− 1.98, − 1.17) | − 1.55 (− 1.94, − 1.12) | ||
| Acrophase (hours, min) | 0.436 | 0.275 | ||
| − 1.5 SD | − 2.42 (− 3.26, − 1.58) | − 2.35 (− 3.20, − 1.51) | ||
| Mean (ref) | − 1.47 (− 1.67, − 1.26) | − 1.46 (− 1.67, − 1.25) | ||
| + 1.5 SD | − 1.95 (− 2.78, − 1.11) | − 1.69 (− 2.54, − 0.84) | ||
Bold values indicate significance at p < 0.05.
Multivariate model adjustments: amplitude and pseudo F-statistic multivariate models adjusted for depression, hypertension, congestive heart failure, 25(OH) vitamin D. Mesor multivariate model adjusted for depression, hypertension, 25(OH) vitamin D. Alpha statistic multivariate model adjusted for hypertension, smoking, depression, 25(OH) vitamin D. Beta statistic multivariate model adjusted for smoking, depression, 25(OH) vitamin D. Acrophase multivariate model adjusted for depression, 25(OH) vitamin D.
4. Discussion
The main findings of the present analysis involved pseudo F-statistic, the RAR variable suggestive of overall circadian rhythmicity of rest and activity. Pseudo F-statistic, which was correlated with urinary melatonin levels (aMT6s) and total sleep time, was modestly associated with total hip aBMD and femoral neck aBMD in this cohort of relatively healthy older American men. In addition, a more robust circadian rhythm (greater pseudo F-statistic) was associated with attenuated aBMD decline. However, adjustment for BMI and covariates related to comorbidities and lifestyle eliminated the significance of these associations. The alpha statistic, which indicates the ratio of daytime to nighttime activity, was associated with femoral neck aBMD and ΔaBMD, and significance was retained in the fully adjusted femoral neck ΔaBMD model. We found no other significant associations between RAR parameters and aBMD or ΔaBMD at the total hip or femoral neck. Contrary to our hypothesis, in older men in the MrOS cohort, dysregulated RAR patterns were not strongly and independently associated with reduced total hip aBMD or femoral neck aBMD at MrOS Sleep Visit 1 or with greater aBMD loss over four years.
Understanding of the relationships between circadian rhythm and bone health outcomes is currently nascent. Some previous studies have focused on melatonin, a hormone that is produced by the pineal gland and regulates circadian rhythm. The present study found modest correlations between RAR variables (amplitude and pseudo F-statistic) and urinary melatonin (aMT6s) in older men, and aMT6s was a covariate in multivariate models of associations between RAR and aBMD. These findings corroborate previous work suggesting a key role for melatonin as a mediator in the relationship between circadian rhythm and bone (Amstrup et al., 2013). Melatonin influences bone by several mechanisms, including promotion of osteoblastogenesis, suppression of osteoclastogenesis, free-radical savaging and antioxidant activity (Maria and Witt-Enderby, 2014). Two small clinical trials of women have measured the effects of 3 mg/day of melatonin supplementation on bone in humans. Exogenous melatonin has resulted in reduction of the ratio of resorption marker N-telopeptide of type 1 collagen (NTX) to formation marker osteocalcin (Kotlarczyk et al., 2012), dose-dependent increases in femoral neck aBMD compared to placebo, and increased volumetric aBMD of the spine (Amstrup et al., 2015).
To our knowledge, this is the first study to examine RAR parameters and aBMD in any population. Low pseudo F-statistic has been associated with negative health outcomes such as increased atherosclerotic and all-cause mortality (Tranah et al., 2010) and likelihood of mild cognitive impairment and dementia (Tranah et al., 2011) in older women, as well as greater cardiovascular disease (CVD) mortality (Paudel et al., 2010) and increased risk of peripheral vascular disease (Paudel et al., 2011) in older men. In our previous analysis, later acrophase, reduced amplitude and lower pseudo F-statistic were associated with increased risk of falls but not fractures in minimally adjusted models for age and clinic site, though after multivariate adjustment, significance was retained only for associations between later acrophase and falls (Rogers et al., 2017). Few other studies have examined associations with the alpha statistic. In the MrOS cohort, higher alpha statistic was modestly associated with increased CVD-mortality (Paudel et al., 2010) and in models adjusted for age, clinic site and race, greater alpha statistic was associated with an increased risk of incident CVD events (Paudel et al., 2011). Our data suggest a greater decline in femoral neck aBMD with higher alpha statistic. However, it is possible that the significant association between alpha statistic and femoral neck ΔaBMD may be a spurious finding. Additional research is needed to confirm this result and to better understand the clinical significance of increased alpha statistic.
Evidence of relationships between circadian rhythm and bone is more robust at the cellular level. Calcium, parathyroid hormone (PTH), calcitonin, C-telopeptide of type 1 collage (CTX) and bone specific alkaline phosphatase (BSAP) are known to exhibit circadian variation (Dudek and Meng, 2014). Key genetic mediators include the transcriptional factors brain and muscle Arnt-like protein 1 (Bmal1) and circadian locomotor output cycles kaput (CLOCK), which activate clock control genes period 1 and 2 (Per1-Per2) and cryptochrome 1 and 2 (Cry1-Cry2). Compared to wild type mice, Per and Cry knockout (KO) mice, as well as Bmal1 KO mice, have a higher bone mass phenotype and greater bone formation, suggesting that the circadian clock inhibits osteoblast proliferation and negatively regulates bone formation (Dudek and Meng, 2014). Recently it has also been shown that Bmal1 regulates bone resorption via transcription of nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 (Nfatc1) (Xu et al., 2016), as well as via the RANKL-OPG pathway (Takarada et al., 2017).
Because clinical research of circadian rhythm and bone is currently limited, at this time we can only speculate possible reasons why these circadian-bone connections were less apparent at the human level in the present analysis. First, dysregulated RAR patterns may directly affect neuromuscular function more than bone, as evidenced by the association with falls but not fractures which we reported previously (Rogers et al., 2017) and the generally non-significant associations between RAR and ΔaBMD in the present analysis. Secondly, since adjustment for BMI and covariates related to lifestyle and comorbidities attenuated associations between pseudo F-statistic and total hip aBMD, it is possible that the relationship between RAR and aBMD is mediated by co-morbidities that limit one's ability to be active and maintain aBMD. Lastly, rather than actually reflecting reduced circadian rhythmicity, low pseudo F-statistic results may instead suggest that the RAR patterns did not fit the model well in this particular statistical approach (Paudel et al., 2011).
This study has a number of strengths. As noted previously (Rogers et al., 2017), the MrOS cohort has a large sample size of community-dwelling men with nearly complete follow up of surviving cohort participants, and outcome measures were well-validated. The actigraphy measures of circadian rhythm were obtained on a large number of subjects, making this dataset exceptionally unique and important for evaluating risk factors for aging. However, we also acknowledge several limitations. First, there was a fairly low prevalence of severely disrupted circadian rhythms in this cohort, making it more difficult to observe the full spectrum of associations between RAR parameters and aBMD (Paudel et al., 2010). Secondly, this analysis was restricted to participants for whom there were at least three days of continuous actigrpahy measurements since three days is the minimum current requirement for actigraphy monitoring for the Centers for Medicare Services (CMS) (American Academy of Sleep Medicine, 2014). It is possible that RAR assessed over several days and may not be reflective of RARs patterns over the four year period that aBMD change was assessed. Finally, the use of wrist actigraphy is one approach to measure RAR and may be more influenced by behavior and therefore may be less reliable than 24 hour melatonin or temperature rhythms (Ancoli-Israel et al., 2003).
Because these and previous data (Rogers et al., 2017) do not suggest strong, independent associations between RAR parameters and aBMD or fracture in healthy older men, future studies could examine these variables in other populations such as shift workers, post-menopausal women and persons with chronic conditions and more severe functional limitations, who are at greater risk for falls (Nevitt et al., 1989). Future studies could also examine RAR patterns and bone turnover markers (since both bone formation and resorption display circadian rhythmicity (Dudek and Meng, 2014, Xu et al., 2016, Takarada et al., 2017)), as well as RAR patterns and ΔaBMD at other sites such as the lumbar spine. Additionally, future studies could examine associations between aBMD and sleep quality variables (e.g. sleep duration, sleep efficiency). The correlation results of the present analysis, as well as work by Saksvik-Lehouillier (Saksvik-Lehouillier et al., 2015), could provide a basis for future analyses of urinary melatonin, sleep and circadian variables in the MrOS cohort. Lastly, future studies could utilize statistical techniques such as functional principal components analysis (fPCA). In contrast to 5-parameter cosine extension, which assumes a specific shape of activity (i.e. cosine curve), fPCA is shape-naïve and may be more appropriate in populations with physical or psychological limitations such as the elderly (Zeitzer et al., 2017).
5. Conclusion
Overall circadian rhythmicity of rest and activity (measured by pseudo F-statistic) and daytime-to- nighttime activity ratio (measured by alpha statistic) were modestly associated with aBMD and four-year ΔaBMD in minimally adjusted models. Adjustment for BMI and covariates related to lifestyle and comorbidities attenuated most of these associations. These data suggest that RAR is not independently associated with aBMD or ΔaBMD at the total hip and femoral neck in older men.
Transparency document
Transparency document.
Acknowledgments
Acknowledgements
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
CMS is supported by K23 AR070275.
Additional funding was provided by NIH/NIAMS grant P50 AR063043.
Footnotes
The Transparency document associated with this article can be found, in online version.
Contributor Information
Tara S. Rogers, Email: tsrogers@ucdavis.edu.
Stephanie Harrison, Email: SLitwack@psg.ucsf.edu.
Christine Swanson, Email: CHRISTINE.SWANSON@ucdenver.edu.
Jane A. Cauley, Email: jcauley@edc.pitt.edu.
Elizabeth Barrett-Connor, Email: ebarrettconnor@ucsd.edu.
Eric Orwoll, Email: orwoll@ohsu.edu.
Katie L. Stone, Email: KStone@psg.ucsf.edu.
Nancy E. Lane, Email: nelane@ucdavis.edu.
References
- Albayrak I., Aydogmus M., Ozerbil O.M., Levendoglu F. The association between bone mineral density, quality of life, quality of sleep and fatigue. Acta Clin. Belg. 2016:1–7. doi: 10.1179/2295333715Y.0000000061. [DOI] [PubMed] [Google Scholar]
- Almeida O.P., Almeida S.A. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int. J. Geriatr. Psychiatry. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ambulatory Monitoring, Inc. Motionlogger User's Guide: Act Millenium, (Ardsley, NY).
- American Academy of Sleep Medicine Coding FAQ. 2014. http://www.aasmnet.org/codingfaq.Aspx
- Amstrup A.K., Sikjaer T., Mosekilde L., Rejnmark L. Melatonin and the skeleton. Osteoporos. Int. 2013;24(12):2919–2927. doi: 10.1007/s00198-013-2404-8. [DOI] [PubMed] [Google Scholar]
- Amstrup A.K., Sikjaer T., Heickendorff L., Mosekilde L., Rejnmark L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: a randomized controlled trial. J. Pineal Res. 2015;59(2):221–229. doi: 10.1111/jpi.12252. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S., Cole R., Alessi C., Chambers M., Moorcroft W., Pollak C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Barone J.J., Roberts H.R. Caffeine consumption. Food Chem. Toxicol. 1996;34(1):119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Blackwell T., Ancoli-Israel S., Redline S., Stone K.L. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J. Clin. Sleep Med. 2011;7(4):357–367. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T., Paudel M., Redline S., Ancoli-Israel S., Stone K.L. A novel approach using actigraphy to quantify the level of disruption of sleep by in-home polysomnography: the MrOS Sleep Study: sleep disruption by polysomnography. Sleep Med. 2017;32:97–104. doi: 10.1016/j.sleep.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank J.B., Cawthon P.M., Carrion-Petersen M.L., Harper L., Johnson J.P., Mitson E., Delay R.R. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp. Clin. Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Cunningham T.D., Di Pace B.S. Is self-reported sleep duration associated with osteoporosis? Data from a 4-year aggregated analysis from the National Health and Nutrition Examination Survey. J. Am. Geriatr. Soc. 2015;63(7):1401–1406. doi: 10.1111/jgs.13477. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Lockley S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. (Bethesda, Md: 1985) 2002;92(2):852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- Dudek M., Meng Q.J. Running on time: the role of circadian clocks in the musculoskeletal system. Biochem. J. 2014;463(1):1–8. doi: 10.1042/BJ20140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarczyk M.P., Lassila H.C., O'Neil C.K., D'Amico F., Enderby L.T., Witt-Enderby P.A., Balk J.L. Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J. Pineal Res. 2012;52(4):414–426. doi: 10.1111/j.1600-079X.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- Maria S., Witt-Enderby P.A. Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014;56(2):115–125. doi: 10.1111/jpi.12116. [DOI] [PubMed] [Google Scholar]
- Marler M.R., Gehrman P., Martin J.L., Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat. Med. 2006;25(22):3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- Martin J.L., Hakim A.D. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevitt M.C., Cummings S.R., Kidd S., Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663–2668. [PubMed] [Google Scholar]
- Orwoll E., Blank J.B., Barrett-Connor E., Cauley J., Cummings S., Ensrud K., Lewis C., Cawthon P.M., Marcus R., Marshall L.M., McGowan J., Phipps K., Sherman S., Stefanick M.L., Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp. Clin. Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Pahor M., Chrischilles E.A., Guralnik J.M., Brown S.L., Wallace R.B., Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur. J. Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- Paudel M.L., Taylor B.C., Ancoli-Israel S., Blackwell T., Stone K.L., Tranah G., Redline S., Cummings S.R., Ensrud K.E. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol. Int. 2010;27(2):363–377. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel M.L., Taylor B.C., Ancoli-Israel S., Stone K.L., Tranah G., Redline S., Barrett-Connor E., Stefanick M.L., Ensrud K.E. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol. Int. 2011;28(3):258–266. doi: 10.3109/07420528.2011.553016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.S., Blackwell T.L., Lane N.E., Tranah G., Orwoll E.S., Cauley J.A., Ancoli-Israel S., Stone K.L., Cummings S.R., Cawthon P.M. Rest-activity patterns and falls and fractures in older men. Osteoporos. Int. 2017;28(4):1313–1322. doi: 10.1007/s00198-016-3874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksvik-Lehouillier I., Harrison S.L., Marshall L.M., Tranah G.J., Ensrud K., Ancoli-Israel S., Clemons A., Redline S., Stone K.L., Schernhammer E.S. Association of urinary 6-sulfatoxymelatonin (aMT6s) levels and objective and subjective sleep measures in older men: the MrOS Sleep Study. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70(12):1569–1577. doi: 10.1093/gerona/glv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Stone K.L., Ewing S.K., Lui L.Y., Ensrud K.E., Ancoli-Israel S., Bauer D.C., Cauley J.A., Hillier T.A., Cummings S.R. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J. Am. Geriatr. Soc. 2006;54(8):1177–1183. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- Stone K.L., Blackwell T.L., Ancoli-Israel S., Cauley J.A., Redline S., Marshall L.M., Ensrud K.E. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J. Am. Geriatr. Soc. 2014;62(2):299–305. doi: 10.1111/jgs.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C.M., Shea S.A., Stone K.L., Cauley J.A., Rosen C.J., Redline S., Karsenty G., Orwoll E.S. Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J. Bone Miner. Res. 2015;30(2):199–211. doi: 10.1002/jbmr.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T., Xu C., Ochi H., Nakazato R., Yamada D., Nakamura S., Kodama A., Shimba S., Mieda M., Fukasawa K., Ozaki K., Iezaki T., Fujikawa K., Yoneda Y., Numano R., Hida A., Tei H., Takeda S., Hinoi E. Bone resorption is regulated by circadian clock in osteoblasts. J. Bone Miner. Res. 2017;32(4):872–881. doi: 10.1002/jbmr.3053. [DOI] [PubMed] [Google Scholar]
- Tranah G.J., Blackwell T., Ancoli-Israel S., Paudel M.L., Ensrud K.E., Cauley J.A., Redline S., Hillier T.A., Cummings S.R., Stone K.L. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J. Am. Geriatr. Soc. 2010;58(2):282–291. doi: 10.1111/j.1532-5415.2009.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranah G.J., Blackwell T., Stone K.L., Ancoli-Israel S., Paudel M.L., Ensrud K.E., Cauley J.A., Redline S., Hillier T.A., Cummings S.R., Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.M., Blackwell T., Tranah G.J., Stone K.L., Ancoli-Israel S., Redline S., Paudel M., Kramer J.H., Yaffe K. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37(12):2009–2016. doi: 10.5665/sleep.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R.A., Smith K.W., Jette A.M., Janney C.A. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J. Clin. Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Xu C., Ochi H., Fukuda T., Sato S., Sunamura S., Takarada T., Hinoi E., Okawa A., Takeda S. Circadian clock regulates bone resorption in mice. J. Bone Miner. Res. 2016;31(7):1344–1355. doi: 10.1002/jbmr.2803. [DOI] [PubMed] [Google Scholar]
- Yesavage J.A., Sheikh J.I. 9/Geriatric Depression Scale (GDS) Clin. Gerontol. 1986;5(1–2):165–173. [Google Scholar]
- Zeitzer J.M., Blackwell T., Hoffman A.R., Cummings S., Ancoli-Israel S., Stone K. Daily patterns of accelerometer activity predict changes in sleep, cognition, and mortality in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2017 doi: 10.1093/gerona/glw250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.