Abstract
Introduction
Experiencing the death of a spouse during late life is associated with an increased risk of developing debilitating mental health problems. Healthy lifestyle practices, such as regular exercise, healthy eating, and good sleep hygiene are promising strategies to influence the mental health and associated physical symptoms of late-life spousal bereavement.
Objective
This paper describes the design and rationale of an intervention development study addressing selective and indicated prevention of depression, anxiety, and/or complicated grief disorder(s) among adults 60 years and older who are grieving the recent loss (within 8 months) of a spouse or partner.
Methods
In Phase I, now complete, we developed and standardized behavioral self-monitoring of daily lifestyle choices via an electronic diary (BSM) and the combined BSM + motivational interviewing-based lifestyle coaching (BSM + MI) to be administered to participants grieving the loss of loved one. In Phase II, we have been implementing the interventions in a randomized controlled trial and addressing challenges related to recruitment. Randomization is to one of three cells: BSM, BSM + MI, or an enhanced usual care condition.
Discussion
Several challenges in implementing our lifestyle interventions to older widow(er)s who are at risk for common mental disorders have been identified. Direct outreach to hospice organizations is an effective way to identify older adults in the early months following spousal death. Results from study may advance the field of grief support and promote a healthy adaptation to widowhood.
Keywords: Grief (normal, Complicated); Bereavement; Aging; Sleep; Exercise; Diet
In this report we describe the design, rationale, and initial feasibility of a mental illness prevention intervention pilot study focused on older adults who are grieving the loss of a loved one, entitled “Widowed Elders' Lifestyle after Loss” (WELL). We focus on bereaved adults because experiencing the death of a spouse during later life is associated with an intense period of suffering and an increased risk of developing debilitating mental health problems. Approximately 10–20% of older adults are diagnosed with major depression, anxiety, and/or prolonged grief disorder(s) [1], [2], [3], [4] in the context of losing their spouse. Preventing mental health problems is important because these conditions are highly prevalent and have lasting adverse consequences for the well-being of the bereaved survivor including physical disability, morbidity, and excess mortality [1], [5].
Apart from the emotional strain of losing a spouse, there are profound changes to bereaved survivors' lifestyle and daily routine (wake time, meal time, activity time, bed time, etc.) [6], [7], [8]. Losing a spouse leads to the absence of social cues that once kept daily routines properly entrained; bereaved elders suddenly feel no reason to wake up, eat meals, or go to bed at a particular time [6], [9]. These changes disrupt the stability of individuals' internal chronobiological rhythms, placing them at high risk for mood disorders like depression [8]. Observational research shows that among the bereaved, changes in daily routines associated with physical activity, food preparation/eating, and sleep/wake regularity are correlated with symptoms of depression and complicated grief [10], [11], [12], [13], [14], [15], [16], [17], [18].
For this pilot intervention study, we chose to develop and implement an intervention that we hypothesized might prevent depression, anxiety, and/or prolonged grief disorders in bereaved elders via re-entraining a regular daily routine: behavioral self-monitoring of daily lifestyle choices and motivational interviewing-based lifestyle coaching. Interventions such as behavioral self-monitoring (BSM) offer a promising, nonstigmatised strategy to equally target physical activity, healthy eating, and good sleep hygiene practices [19], [20], [21]. BSM teaches older adults to become mindful of their daily lifestyle practices and the conditions in which they occur. The steps of BSM include: (1) selecting a goal, (2) paying attention to some aspect of behavior, and (3) recording that behavior in a diary. We implemented a technology-based approach using personalized tablets to decrease participant burden and improve participant retention [22], [23]. Improving lifestyle practices can be challenging, requiring effort and motivation. Therefore, the effects of BSM to promote healthy lifestyle change may be enhanced by using motivational interviewing (MI) – a patient-centered approach to strengthen bereaved elders' motivation and commitment to change. MI facilitates healthy lifestyle change by helping patients resolve ambivalence about lifestyle change in an empathetic and encouraging climate [24], [25], [26].
We designed this pilot study to inform researchers about whether a healthy lifestyle intervention designed to target the daily routine of bereaved elders is feasible and acceptable when offered alone, and when offered in combination with MI. In this report we: 1) describe the development of a BSM + MI intervention using personalized tablet technology to increase engagement in and adherence to physical activity, healthy eating, and good sleep practices; 2) report on our experience with participant recruitment and retention; and 3) discuss intervention implementation challenges.
1. Methods
1.1. Overall study design
We conducted an intervention development study to examine the following:
-
•
The feasibility and acceptability of behavioral self-monitoring (BSM) of physical activity, diet, and sleep behaviors for 12 weeks in adults 60 + who are at risk for common mental disorders (depression, anxiety, and prolonged grief) following the recent death of a spouse or partner (within 8 months).
-
•
The feasibility and acceptability of motivational interviewing-based lifestyle coaching (MI) added to BSM for 12 weeks.
-
•
The pattern of interrelationships between healthy lifestyle practices and symptoms of depression, anxiety, and/or complicated grief in adults 60 years and older who are grieving the recent loss of a spouse or partner.
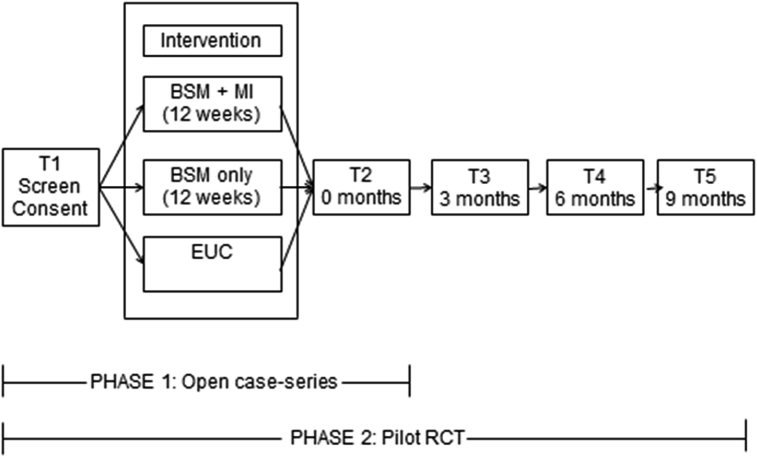
This project consists of two phases. Phase I, now complete, focused on developing and standardizing BSM and BSM + MI to be delivered to participants via an electronic diary (on a tablet device). In an open case-series (n = 10), we collected feasibility and acceptability data, monitored participant progress, and adjusted the delivery of BSM, alone and in combination with MI to meet the needs of older bereaved adults. Phase II focuses on implementing BSM and BSM + MI in a randomized clinical trial, relative to enhanced usual care (i.e., usual care enhanced by the provision of research assessment and monitoring).
The study design for Phase II is shown in Fig. 1. After the baseline assessment (T1), participants are randomized using a 2:2:1 allocation to behavioral self-monitoring (BSM), behavioral self-monitoring + motivational interviewing-based lifestyle coaching (BSM + MI), or enhanced usual care (EUC). We allocated fewer participants to enhanced usual care so that feasibility of intervention components could be evaluated. The interventions are described below. After the 12-week intervention period, participants complete a post-intervention interview (T2) and are assessed every 3 months (T3—T5) for up to 9 months (total study time = 12 months). The Institutional Review Board at the University of Pittsburgh and a data safety and monitoring board have reviewed all protocols and procedures. Recruitment began in June 2015, and follow-up will be completed in December 2018 (ClinicalTrials.gov; NCT02631291).
Fig. 1.
WELL study design.
1.2. Participants
Inclusion criteria required participants to be (1) aged 60 years and older; (2) having experienced the loss of a spouse or partner within the last 8 months; and (3) at-risk for mental illness, based on having at least one of the following high risk markers: high medical comorbidity (>=2 on at least two items of the CIRS-G), low social support (no family/friends to open up to or rely upon), functional disability (requiring help with at least 1 activity of daily living); or subthreshold symptoms of depression (HRSD of 9–14), anxiety (GAD-7 = 10), and/or complicated grief (ICG = 20), together with absence of current major depression, generalized anxiety, post-traumatic stress, or suicidality. These markers align with the Institute of Medicine's framework for selective and indicated prevention interventions (‘selective’ prevention focuses on persons at risk for developing the disorder, while ‘indicated’ prevention focuses on individuals who have subsyndromal symptoms, but are below the symptom threshold for clinical disease) [27], [28]. Exclusion criteria specify: (1) current DSM-5 criteria for syndromal mood, psychosis, anxiety, eating disorder, or substance abuse abuse/dependence; (2) cognitive impairment (3MS < 80); and (3) new psychotropic medication after spousal death (including antidepressants and benzodiazepines) or regular use of anxiolytics (low and/or stable dosage ok permitted, e.g., 1 mg Lorazepam prn ≤ 4 times/week).
1.3. Recruitment
Recruitment for both Phases I and II takes place in community senior centers, grief support centers, and primary care physician (PCP) offices. In addition, we collaborated with Pitt + Me, a research registry program of the University of Pittsburgh's Clinical and Translational Science Institute, who referred appropriate participants to our study.
1.4. Intervention
All participants receive written education about grief (NIA Age Page: Mourning the death of a Spouse) and healthy lifestyle choices for older adults (recommendations from the National Sleep Foundation, USDA's Choose My Plate, and the NIH's Getting Fit for Life) at baseline. All participants chose a personal health goal on which to focus over the course of the 12-week intervention. Personal health goals are related to physical activity, diet, and/or sleep hygiene; examples included: engage in 150 min of moderate-intensity activity/week; eat 2–3 servings of fruits and 3–4 servings of vegetables; and sleep 7–8 h per night, among others. After the 12-week intervention, participants receive a lifestyle ‘report’ that summarizes their typical activity and sleep patterns (using objective data collected from Actigraphy).
BSM. Participants randomized to BSM record their physical activity, diet, and sleep behaviors twice daily using a diary-like app on a tablet, for 12 weeks.
The diary includes a morning report, an evening report, and a section that provided the user feedback about their recorded behaviors. The diaries include questions to re-entrain a regular daily routine; including items from the Pittsburgh Sleep Diary [29]; the morning report asked about the previous night's sleep including 1) bedtime (hh:mm); 2) time to fall asleep (minutes); 3) number of nighttime awakenings; 4) mood at bedtime (alert, sleepy, worried, anxious; scale 1–10); 5) quality of sleep (scale 1–10); 6) time of morning awakening (hh:mm), 7) time out of bed to start daily activities (hh:mm); and 8) mood at morning awakening (scale 1–10; very good – very bad). The evening report asks about physical activity, diet, and sleep behaviors throughout the day including 1) time daily activities started (hh:mm); 2) time of first contact with another person via phone or in-person (hh:mm); 3) time of breakfast, lunch, and dinner (hh:mm); 4) number of servings of whole grains, fruits, vegetables, calcium-rich foods, proteins, and alcohol); 5) number of meals at a sit-down/take out restaurant; 6) number of naps; 7) time spent in light, moderate, and vigorous activity (minutes); time spent in sitting, walking, muscle strengthening, and volunteering activities (minutes); and 8) mood throughout the day (scale 1–10; very bad-very good).
Participants are trained on using the tablet and provided a manual prior to starting the intervention. We check-in with participants 1–2 days after training to ensure they are comfortable using the tablet. The morning report is available on the tablet from 4am until 11am; the evening report 6pm to 1am. Participants must complete reports within the specified time frame. Participants are instructed to complete the morning report when they get out of bed and the evening report right before they go to bed. Using data entered by the user, the feedback section includes a series of Figures illustrating the participants' sleep cycle (average bedtime and wake time over the last 7 days), daily plate (nutritional content of daily meals), and activity profile (average time spent in light, moderate, and vigorous activity over the last 7 days).
BSM + MI. Participants receive the same BSM intervention; in this condition, participants also talk to a ‘lifestyle coach’ about their health behaviors. A member of the research team call participants weekly and use motivational interviewing techniques to enhance older adults' confidence and intrinsic motivation to engage in healthy lifestyle practices. The MI techniques include reflective listening, rolling with resistance, agenda setting (deciding what behaviors to discuss), and eliciting self-motivational statements and change talk (cite). During these calls, participants often discuss their progress towards their personal health goals, self-care practices, and any grief symptoms affecting their ability to engage in a healthy lifestyle.
Enhanced Usual Care. Participants randomized to enhanced usual care do not receive an electronic diary to monitor their lifestyle choices, but are followed on the same assessment schedule. Participants who met criteria for depression, anxiety, and/or prolonged grief disorder(s) over follow-up (or reported new medical symptoms) are referred for medical care or grief counseling, as in the intervention arms.
1.5. Data safety and monitoring
Data on recruitment, retention, clinical progress, side effects and safety for each participant are reviewed on a weekly basis. The data safety and monitoring board consists of a geriatric psychiatrist, health psychologist, nurse practitioner, and mental health clinicians. Additional safety measures include access to a 24/7 answering service for clinical emergencies.
1.6. Schedule of assessments and planned outcomes
Participants in Phase I (the open case series of 10 subjects) were assessed twice: at baseline (T1) and postintervention (T2). Participants in Phase II are assessed at five time points: at baseline (T1), postintervention (T2), and then at 3, 6, and 9 months postintervention (T3–T5). Participants are assessed in-person at our research clinic or at their home. Table 1 shows our assessment battery.
Table 1.
Widowed elders' lifestyle after loss (WELL): Assessments.
| Domain | Measure |
|---|---|
| Depression | Hamilton Rating Scale for Depression, HRSD [30] |
| Patient Health Questionnaire-9, PHQ-9 [31] | |
| PRIME MD/MINI [34] | |
| Anxiety | Generalized Anxiety Disorder 7-item Scale, GAD-7 [32] |
| Complicated grief | Inventory of Complicated Grief, ICG [33] |
| Social isolation | Select items from the National Social Life, Health, and Aging Project [35] |
| Medical illness burden | Cumulative Illness Rating Scale – Geriatrics, CIRS-G [36] |
| Cognitive function | Modified Mini-Mental State Examination, 3MS [37] |
| Functional status, physical performance | Short Performance Physical Battery, SPPB [38] |
| Cardiometabolic health status | Heart rate, blood pressure, body mass index |
| Disability | Late-life Functional Disability Inventory, LLFDI [39] |
| Physical activity | CHAMPS Physical Activity Questionnaire [40] |
| Dietary behavior and intake | Rapid Eating Assessment for Patients, REAP [41] |
| Sleep | Pittsburgh Sleep Quality Index, PSQI [42] |
| Chronobiological function | Actigraphy and the Social Rhythm Metric, SRM [43] |
| Technology acceptance and usability | Technology Acceptance Measure [44] and Post-study Usability Questionnaire [45] |
| Biosignatures | Inflammatory cytokines (interleukin-6, tumor necrosis-α) |
Notes. Participants in Phase I and Phase II receive all of the above assessments.
Feasibility and Acceptability Endpoints: Our criteria for feasibility for Phase 2 stipulate ability to recruit the proposed sample, to retain 80% + of participants, and to collect 80% + of proposed measures. Our criteria for acceptability require attrition will be no higher than 20%; a brief questionnaire assesses participants' level of interest, enjoyment, and satisfaction with the intervention. We also assess the time to recruit and screen participants, which we define as the time between initial contact and enrollment; and retention at 6- and 12-weeks, which we define as the proportion of participants enrolled in the study that have not dropped out. We calculate compliance by examining the number of BSM sessions recorded in the tablet.
Mental Health Outcomes: We assessed symptom levels of depression (Hamilton Rating Scale for Depression, HRSD [30] and the Patient Health Questionnaire-9, PHQ-9 [31]), anxiety (7-item Generalized Anxiety Scale, GAD-7 [32]), and complicated grief (Inventory of Complicated Grief, ICG [33]); incidence of PRIME MD/MINI-defined major depression, generalized anxiety, and prolonged complex grief disorders over a 12-month period in the three arms of the study [34]. Symptom burden was measured at all assessments. Participants meeting diagnoses were referred for treatment but continued research follow-up.
2. Results
2.1. Phase I: developing and standardizing the study interventions
Approximately 12 months went into developing a diary-like app that was used for the recording of activity, diet, and sleep behaviors on a study tablet. The app was developed by programmers at the University of Pittsburgh's University Center for Social and Urban Research. We held meetings with programmers to design the style of the diary interface so that it was acceptable to older adults. We insured that large fonts and color contrasts were used to assist those with vision deficits. We added an instruction ‘button’ for each question to assist those needing assistance answering questions. Examples of instruction buttons included the definition of bedtime and wake time; the definition of moderate and vigorous activity; and the definitions of dietary serving sizes. Several participants had arthritis and/or dexterity problems and could not tap the tablet screen hard enough to record their activity. Therefore, we offered a paper version of the daily diary that consisted of two sheets per day for 12 weeks. Compliance was close to 100% for the paper version (vs. 66% in the tablet version). Participants wanted more information from the tablet in understanding their health behaviors, so we added a section that provided feedback about recorded behaviors. This feedback section was not a required part of the intervention protocol, but participants could view graphs and charts that depicted their daily activity, sleep, and diet behaviors.
Tablets were modified for maximal ease of use of the study app: quick links to other apps were deleted from the home screens and links to the study app were placed in easily accessible places. Participants trained in utilizing basic tablet functions such as returning to the home screen, swiping to log in, and accessing settings functions so that troubleshooting could be completed over the phone (if necessary). After training was complete, we used ‘screen pinning’ to lock the tablet display in a single view that allowed only the use of the diary app.
There were several unanticipated technology problems that impacted participants' satisfaction and data collection. When the tablets powered down, the time and/or the user profile would reset, inhibiting participants' ability to access the daily diaries. Random system updates and changes in daylight savings also inhibited access to the daily diaries. As a result, we were unable to collect compliance data on 3 of 10 participants in Phase 1. We now instruct participants to leave the tablet plugged in and at their bedside table. This method has proved successful and most participants contact us if they experience a technology issue, allowing us to troubleshoot over-the-phone. Finally, the algorithm used to calculate feedback needed to be reinstalled (due to a glitch in the system) and some participants were not able to view accurate feedback. Since implementing these strategies, we have collected complicate data on all participants.
We piloted the use of Actigraphy (ActiGraph GT9X) for the monitoring of physical activity and sleep patterns. Per manufacturer recommendations, we asked participants to wear the Actigraph sensor around their waist during the day and around their wrist while sleeping. Participants did not like wearing the sensor around their waist/belt, especially women who liked to wear dresses. Several participants misplaced the sensor when it was placed around their waist/belt. As a result, we instructed participants to wear the Actigraph sensor on their wrist at all times (except bathing and swimming) for the first and last 10 days of the intervention. The Actigraph watch has a large display (for time) and was not intrusive to participants' daily lifestyle.
We also piloted the MI intervention, in conjunction with BSM. We called participants weekly and focused on four components of MI: open-ended questions, affirmations, reflective listening, and summarizing statements. We anticipated needing to make changes to the timing and duration of calls, but all participants enjoyed weekly phone conversations. Participants' schedules varied week-to-week; therefore we let the participant decide the duration of calls (typically 15–30 min), as long as the four components of MI were met (via the Motivational Interviewing Treatment Integrity Coding Manual). Participants guided the content of conversations, but recurring themes included discussions about 1) barriers inhibiting participants from reaching health goals; 2) strategies to circumvent barriers; 3) grief symptoms; and 4) ideas for monitoring health behaviors beyond the intervention. After the first and second month of BSM we assessed mindfulness and talked about participants' awareness of (or changes to) their daily routine.
We enrolled 10 participants into Phase 1. Five participants were enrolled to standardize the BSM manual; 5 for the BSM + MI manual. There was 100% retention among those who started the intervention protocol; and we collected 100% of measures (see Table 1). Participants' median age was 78 years (range = 70–87 years); the median number of months bereaved at the time of enrollment was 3.5 (range = 1.0–6.5 months). Most were women (60%) and White (90%). Widow(er)s mental health symptomatology aligned with markers that indicated they were at high risk for depression (HRSD median score = 9, range = 4–14; PHQ-9 median score = 7, range = 3–18); and at lower risk for anxiety (GAD-7 median score = 2, range = 0–11), and complicated grief (ICG median score = 16, range = 3–39). After the 12-week intervention, PHQ-9 median scores decreased to 2.5 (range = 0–11), HRSD scores decreased to 5 (range = 4–6), GAD scores decreased to 2 (range = 0–4), and ICG scores remained relatively stable at 17 (range = 0–33). These change scores indicate that participants experienced fewer symptoms of depression (both self-report and clinician administered) while grief symptoms remained somewhat variable.
2.2. Phase II: implementing the study interventions
This prevention intervention project was conducted in preparation for a confirmatory trial; therefore, we expected to make many changes to the protocol during implementation. Table 2 summarizes the methodological changes made during the course of the study. The primary challenge in Phase II has been recruitment. A multifaceted approach is proving to be necessary. We initially contacted PCP offices, senior centers, grief centers, libraries, and other community centers to offer faculty presentations discussing grief and bereavement, followed by screening of individuals interested in the study participation. However, we found that older adults were not attending community presentations and/or senior centers in the early months following spousal death. Most people who attended our presentations were adults who had been widowed for several years. Other strategies included targeted mailings by nurses to family members of patients who died in the intensive care units of two different hospitals in Pittsburgh. Due to patient confidentiality, we did not know when older adults died in the ICU and whether letters were sent to their families. We contacted local funeral directors who agreed to have study fliers available in their office. We attempted to partner with local Cancer centers, but nurses and social workers often did not have contact with family members after cancer patients died. We also contacted local grief counselors, but they were hesitant to advertise our study in addition to the support/therapy they were providing to adults.
Table 2.
Methodological changes to the WELL study.
| Changes to inclusion/exclusion criteria | Rationale |
|
|
|
|
| Changes to intervention protocol | |
|
|
|
|
|
|
|
|
|
|
|
|
| Changes to assessment schedule | |
|
|
|
|
|
|
|
|
|
|
Four strategies proved successful. They include utilizing research registries, sending email blasts to employees at the University of Pittsburgh/UPMC, encouraging word of mouth by community champions and prior research participants, and partnering with local Hospice organizations. Our partnership with local Hospice organizations has been the most rewarding; we found several champion social workers who were passionate about WELL and recruited for us by 1) sending our study flyer in their support packet to spouses of older patients who died in the care of hospice, 2) describing WELL during follow-up phone calls with bereaved spouses, and 3) mentioning WELL during face-to-face interactions with bereaved (and soon-to-be bereaved) spouses.
Compliance, Uptake, and Fidelity. In general, participants were able to learn and use the tablet for the recording of physical activity, sleep, and dietary behaviors. Clinicians trained participants how to use the tablet during the baseline interview and provided a written manual that included instructions detailing how to complete the morning and evening reports. Compliance was measured by examining the total number of BSM sessions recorded in the tablet. For Phase I participants, an average of 104 BSM sessions (out of a possible 168 sessions [2 times per day for 84 days]) were recorded. This number is low (61% compliant) and likely reflects the technology problems we experienced when launching the electronic diary app to participants. Among those who have enrolled in and completed Phase II (n = 13 out of anticipated 50), compliance increased to 148 BSM sessions (88%). We tracked the uptake of BSM by asking participants whether after participating in WELL they became more aware of their daily routine and health behaviors on a scale of 1 (strongly disagree) to 5 (strongly agree). Participants mean score was 3.9 (SD = 1.2, range 1–5) indicating they believed they became more mindful of their daily routine, particularly their bedtime and wake time routine and daily activity. Fidelity to MI was measured by completing a checklist (to complete the 4 components of MI) after every phone session and recording the time spent discussing participants' weekly routine and/or health goals. Session notes (GRW) were discussed weekly with the PI (STS), who is certified in the use of MI for behavior change by the Motivational Interviewing Network of Trainers (MINT).
Participant Satisfaction. After 12 weeks of BSM or BSM + MI, participants were asked to complete a satisfaction survey. Items asked about participants' satisfaction regarding operational elements of WELL (flexibility and frequency of appointments, helpfulness of clinicians, frequency of phone calls) and the usefulness of WELL in managing grief and health behaviors. Reponses ranged from 1 (highly dissatisfied) to 4 (highly satisfied). Participants' median score was a 3.6 (range = 3.1–4.0) indicating high satisfaction with intervention components. We also administered a post-study usability of technology measure. Items asked about participants' satisfaction with using the daily diary to track health behaviors. Reponses ranged from 1 (strongly disagree) to 7 (strongly agree). Participants' median score was 6.5 (range = 3.3–7.0) indicating greater use and acceptability of the electronic diary and tablet. Participants' written feedback suggests that the BSM helped them “organize their life” and “promote social engagement.” One participant thought that the tablet allowed him to develop a renewed sense of self; stating that “it allowed me to focus on my own health.” One participant (who prior to the intervention avoided eating alone) appreciated that the tablet “reminded me to eat and prepare healthy meals.” Another participant noted that the “tablet became my boss, but in a good way.” Several participants told us they were glad to return the tablet after the 12 weeks because they felt like “they were answering the same thing every day.” This feedback likely reflects these participants' ability to return to a regular/routine schedule.
3. Discussion
We have presented the design, rationale, and issues related to implementation of a technology-based healthy lifestyle intervention for older adults at risk for common mental disorders following the death of their spouse/partner. The challenges we encountered were primarily due to technology for BSM and participant recruitment. Once we were able to problem solve technology issues and effectively reach and identify participants, widow(er)s were compliant with BSM and enjoyed monitoring their daily lifestyle choices via the electronic diary.
Two lessons learned in this intervention development study deserve highlighting. First, widow(er)s at risk for common mental disorders are interested in efforts to improve their health and wellbeing. This is important, because experiencing the death of a spouse is a major source of stress which can leave older adults vulnerable to future health problems. In the first few months post death, widow(er)s experience significant change to their daily routine and responsibilities (both personal and household) while simultaneously facing intense and unpredictable feelings of grief. Our goal was to deliver an intervention that was non-intrusive and could be easily integrated into participants' daily lives. Many participants were engaged in caregiving activities related to their physically-declining spouse prior to death. After spousal death, participants expressed a need for a purpose in life and/or a renewed sense of self. Engaging widow(er)s in a lifestyle intervention that may both promote their mental health but also has empowering qualities (via BSM and MI) appears to be an optimal approach to optimizing health and wellbeing.
The second lesson learned is where and how participants may be most effectively recruited. We initially relied heavily on community support centers and PCP offices to refer symptomatic participants. While these approaches were less successful in terms of identifying potential participants, our community presentations were well attended and we continue to give lectures on normal and pathological grief responses to better inform the community. Our most productive partnership was with local hospice organizations who integrated the WELL study into their bereavement support services for family members. This partnership has been mutually productive; we work closely with hospice social workers who consult with us about the mental health status of their patients and we connect hospice social workers to community mental health professionals who specialize in complicated grief and major depression in older adults. Given the challenges of recruitment for prevention studies (e.g., adults are not treatment seeking because they do not know they are at high-risk), effective recruitment strategies are critical for continuing along the intervention development pipeline (feasibility → efficacy → effectiveness, etc.).
WELL may lead to improvements in prevention strategies, thereby not only influencing mental health, but improving everyday physical health and physical functioning among aging widows and widowers. We are currently evaluating the feasibility and acceptability of WELL on the outcomes of interest, mental health. We plan to monitor participants for 1 year. These findings will be reported after study completion in December 2018.
Funding
This work was supported in part by Grants from NIH K01MH103467, P30MH90333, UL1 TR000005, and the UPMC Endowment in Geriatric Psychiatry (CFR).
Conflicts of interest
Dr. Reynolds reports being supported by the NIH, and the UPMC Endowment in Geriatric Psychiatry; having received medication supplies for investigator-initiated trials from Bristol Meyers Squibb, Forrest Labs, Lily, and Pfizer; and receives royalties for industry sponsored use of the Pittsburgh Sleep Quality Index (PSQI), to which he holds intellectual property rights. The other authors have nothing to disclose.
Acknowledgements
We thank Rebecca Tritchinger Ragni and Randy Hebert, MD for their help in the conduct of this study.
References
- 1.Stroebe M., Schut H., Stroebe W. Health outcomes of bereavement. Lancet. Dec 8 2007;370(9603):1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- 2.Utz R.L., Caserta M., Lund D. Grief, depressive symptoms, and physical health among recently bereaved spouses. Gerontologist. 2012;52(4):460–471. doi: 10.1093/geront/gnr110. 2012-09-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newson R.S., Boelen P.A., Hek K., Hofman A., Tiemeier H. The prevalence and characteristics of complicated grief in older adults. J. Affect. Disord. 2011;132(1–2):231–238. doi: 10.1016/j.jad.2011.02.021. 2012-09-10. [DOI] [PubMed] [Google Scholar]
- 4.Shear M.K., Simon N., Wall M. Complicated grief and related bereavement issues for DSM-5. Depress. Anxiety. 2011;28(2):103–117. doi: 10.1002/da.20780. 2012-09-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okereke O., Lyness J.M., Lotrich F.E., Reynolds C.F. Depression in late life: a focus on prevention. Lifelong Learn. Psychiatry. 2013;11:22–31. doi: 10.1176/appi.focus.11.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monk T.H., Germain A., Reynolds C.F. Sleep disturbance in bereavement. Psychiatr. Ann. 2008;38:671–675. doi: 10.3928/00485713-20081001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahl S.T., Schulz R. Changes in routine health behaviors following late-life bereavement: a systematic review. J. Behav. Med. 2014;37:736–755. doi: 10.1007/s10865-013-9524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl S.T., Schulz R. The effect of widowhood on husbands' and wives' physical activity: the Cardiovascular Health Study. J. Behav. Med. 2014;37:806–817. doi: 10.1007/s10865-013-9532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk T.H., Begley A.E., Billy B.D., Fletcher M.E., Germain A., Mazumdar S., Moul D.E., Shear M.K., Thompson W.K., Zarotney J.R. Sleep and circadian rhythms in spousally bereaved seniors. Chronobiol Int. 2008;25:83–98. doi: 10.1080/07420520801909320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers C.L., Frank E., Kupfer D.J. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch. Gen. Psychiatry. 1998;45(10):948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- 11.Janke M.C., Nimrod G., Kleiber D.A. Reduction in leisure activity and well-being during the transition to widowhood. J. Women Aging. 2008;20(1–2):83–98. doi: 10.1300/J074v20n01_07. 2012-09-10. [DOI] [PubMed] [Google Scholar]
- 12.Janke M.C., Nimrod G., Kleiber D.A. Leisure patterns and health among recently widowed adults. Activities Adapt. Aging. 2008;32(1):19–39. 2012-09-10. [Google Scholar]
- 13.Janke M.C., Nimrod G., Kleiber D.A. Leisure activity and depressive symptoms of widowed and married women in later life. J. Leis. Res. 2008;40(2):250–266. 2012-09-10. [Google Scholar]
- 14.Rosenbloom C.A., Whittington F.J. The effects of bereavement on eating behaviors and nutrient intakes in elderly widowed persons. J. Gerontol. 1993;48(4):S223–S229. doi: 10.1093/geronj/48.4.s223. 2013-05-29. [DOI] [PubMed] [Google Scholar]
- 15.Shahar D.R., Schultz R., Shahar A., Wing R.R. The effect of widowhood on weight change, dietary intake, and eating behavior in the elderly population. J. Aging Health. May 2001;13(2):189–199. doi: 10.1177/089826430101300202. [DOI] [PubMed] [Google Scholar]
- 16.Pasternak R.E., Reynolds C.F., 3rd, Hoch C.C. Sleep in spousally bereaved elders with subsyndromal depressive symptoms. Psychiatry Res. Jul 1992;43(1):43–53. doi: 10.1016/0165-1781(92)90140-x. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds C.F., 3rd, Hoch C.C., Buysse D.J. Electroencephalographic sleep in spousal bereavement and bereavement-related depression of late life. Biol. Psychiatry. Jan 1 1992;31(1):69–82. doi: 10.1016/0006-3223(92)90007-m. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds C.F., 3rd, Hoch C.C., Buysse D.J. Sleep after spousal bereavement: a study of recovery from stress. Biol. Psychiatry. Dec 1 1993;34(11):791–797. doi: 10.1016/0006-3223(93)90068-o. [DOI] [PubMed] [Google Scholar]
- 19.Akers J.D., Cornett R.A., Savla J.S., Davy K.P., Davy B.M. Daily self-monitoring of body weight, step count, fruit/vegetable intake, and water consumption: a feasible and effective long-term weight loss maintenance approach. J. Acad. Nutr. Diet. May 2012;112(5):685–692. doi: 10.1016/j.jand.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke L.E., Styn M.A., Glanz K. SMART trial: a randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemp. Clin. Trials. Nov 2009;30(6):540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke L.E., Wang J., Sevick M.A. Self-monitoring in weight loss: a systematic review of the literature. J. Am. Diet. Assoc. Jan 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke L.E., Styn M.A., Sereika S.M. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am. J. Prev. Med. Jul 2012;43(1):20–26. doi: 10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz R., Beach S.R., Matthews J.T., Courtney K.L., De Vito Dabbs A.J. Designing and evaluating quality of life technologies: an interdisciplinary approach. Proc. IEEE. 2012;100(8):2397–2409. [Google Scholar]
- 24.Britt E., Hudson S.M., Blampied N.M. Motivational interviewing in health settings: a review. Patient Educ. Couns. May 2004;53(2):147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 25.Martins R.K., McNeil D.W. Review of Motivational Interviewing in promoting health behaviors. Clin. Psychol. Rev. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Purath J., Keck A., Fitzgerald C.E. Motivational interviewing for older adults in primary care: a systematic review. Geriatr. Nurs. 2014;35:219–244. doi: 10.1016/j.gerinurse.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine . National Academy Press; Washington, DC: 1994. Committee on Prevention of Mental Disorders, Division of Biobehavorial Science and Mental Disorders. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. [Google Scholar]
- 28.Cuijpers P., Beekman A.T.F., Reynolds C.F., III Preventing depression. JAMA. 2012;307:1033–1034. doi: 10.1001/jama.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monk T.H.1, Reynolds C.F., 3rd, Kupfer D.J., Buysse D.J., Coble P.A., Hayes A.J., Machen M.A., Petrie S.R., Ritenour A.M. The Pittsburgh sleep diary. J. Sleep. Res. 1994;3:111–120. [PubMed] [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. Feb 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzer R.L., Kroenke K., Williams J.B., Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives Intern. Med. May 22 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 33.Prigerson H.G., Maciejewski P.K., Reynolds C.F., 3rd Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. Nov 29 1995;59(1–2):65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer R.L., Williams J.B.W., Kroenke K., Linzer M., deGruy F.V., 3rd, Hahn S.R., Brody D., Johnson J.G. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 35.Cornwell E.Y., Waite L.J. Measuring social isolation among older adults using multiple indicators from the NSHAP study. J. Gerontol. Ser. B. Nov 2009;64(Suppl 1):i38–46. doi: 10.1093/geronb/gbp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller M.D., Paradis C.F., Houck P.R., Mazumdar S., Stack J.A., Rifai A.H., Mulsant B., Reynolds C.F., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 37.Teng E.L., Chui H.C. The modified mini-mental state (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 38.Guralnik J.M., Simonsick E.M., Ferrucci L. A short physical performance battery ssessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 39.Jette A.M., Haley S.M., Coster W.J. Late life function and disability instrument: development and evaluation of the disability component. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 40.Stewart A.L., Mills K.M., King A.C., Haskell W.L., Gillis D., Ritter P.L. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. Jul 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Block G., Woods M., Potosky A., Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J. Clin. Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 42.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Monk T.H., Flaherty J.F., Frank E., Hoskinson K., Kupfer D.J., Davis F.D. The Social Rhythm Metric. An instrument to quantify the daily rhythms of life. J. Nerv. Ment. Dis. 1990 Feb;178(2):120–126. doi: 10.1097/00005053-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 1989;13:319–340. [Google Scholar]
- 45.Fruhling A., Lee S. Proceedings of the 9th Americas Conference on Information Systems. 2005. Assessing the reliability, validity and adaptability of PSSUQ. Omaha, Nebraska, USA, August 2005. [Google Scholar]