Abstract
Micronutrients or non-energetic nutrients (NEN) are needed in reduced amounts, but are essential for many mosquito physiological processes that influence biological traits from vector competence to reproductive capacity. The NEN include amino acids (AA), vitamins, salts, metals and sterols. Free AA play critical roles controlling most physiological processes, from digestion to reproduction. Particularly proline connects metabolic pathways in energy production, flight physiology and ammonia detoxification. Metal, in particular iron and calcium, salts, sterol and vitamin homeostasis are critical for cell signaling, respiration, metabolism and reproduction. Micronutrient homeostasis influence the symbiotic relationships with microorganisms, having important implications in mosquitoes’ nutrition, physiology and behavior, as well as in mosquito immunity and vector competence.
Graphical abstract
Introduction
Adult female mosquitoes of several species are important vectors of human diseases. Due to a primarily hematophagous feeding strategy and the ability to produce offspring, most studies in mosquitoes have naturally focused on the adult female. Aedes aegypti, in particular, have received the majority of attention because of its ease of rearing, desiccation resistant eggs, cosmopolitan distribution and vector importance. Those factors have all converged to make Ae. aegypti a model organism. Nutrition strongly influences the physiology and behavior of mosquitoes. A sugar meal can sustain the energetic requirements of a female Ae. aegypti, and blood meals are required to produce eggs. However, much less is known about particular nutrients required during larval stages or about the importance of specific components of a sugar or blood meal in completing oogenesis.
For the purposes of this review, the nutritional requirements of mosquitoes can be divided into two main classes: energetic nutrients (so-called macronutrients) such as carbohydrates, fatty acids and some amino acids [1,2,3] and micronutrients or non-energetic nutrients (NEN). NEN are needed in reduced amounts but are essential for proper cellular and organismal function [4]. The NEN’s include vitamins, salts, metals as well as sterols. Many amino acids (AA) can also be considered micronutrients because they are not used as a main source of energy, yet they are indispensable [4,5]. While energy-providing nutrients have received full attention regarding their impact on the physiology, behavior and ecology of insects, NEN have been largely overlooked. This review summarizes the current knowledge on the influence of NEN homeostasis on physiology of mosquitoes, and stresses some major gaps in our understanding of these processes.
Non-energetic nutrient homeostasis
The physiology of adult female mosquitoes is markedly linked to the gonotrophic cycle. Ovarian development in Ae. aegypti can be divided into three distinct phases: previtellogenesis (PVG), oocyte state-of-arrest (ORS) and vitellogenesis (VG) [6,7]. To provide the energy, molecular building blocks and NEN for ovarian development, the female mosquito draws nutrition from three main feeding modalities: larval feeding, adult sugar feeding, and adult blood feeding. Each of these processes provides a unique repertoire of NENs. Mosquito larvae mostly filter feed particulate matter such as phytoplankton, microorganisms, and detritus [8]. The nutrition obtained during larval feeding is considered preimaginal or teneral reserves and is mainly utilized during metamorphosis and PVG. The most commonly recorded sugar sources for mosquitoes are floral nectaries and honeydew [4]. Floral nectar is a complex mixture of chemicals. Sucrose is the major carbohydrate constituent but nectar also includes AA, proteins, lipids, antioxidants, alkaloids, vitamins, organic acids, allantoin and allantoic acid, dextrins, and minerals [9]. Nutrients obtained during nectar feeding contributes critical reserves during the ORS. Finally, a blood meal provides amino acids, cholesterol, lipids and metals. In Ae. aegypti, a blood meal triggers VG, and produces up to 120 eggs in a single gonotrophic cycle; therefore, a tightly regulated control of nutrient allocations to the ovaries is critical [10,11].
Amino acids
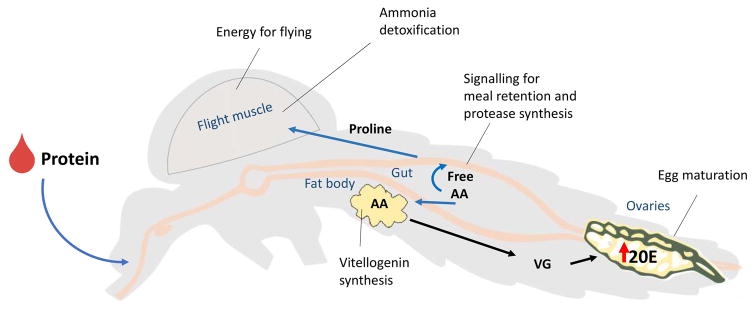
Vertebrate blood is rich in proteins that are digested into their constituent AA, with hemoglobin making up more than 90% of the dry mass of vertebrate blood [12]. Blood feeding on different host species affects mosquito female egg production [12]. The level of free isoleucine in blood has been shown to account for some of these differences, highlighting the importance of this essential amino acid in female mosquito oogenesis [13]; human blood is severely deficient in isoleucine; thus, female mosquitoes have to make full use of the human plasma-free Ile for their reproduction [14]. A large percentage of the blood-meal derived AA is metabolized and used for energy production, while the rest is used for the synthesis of yolk proteins. Free AA play critical roles controlling key physiological processes (Fig. 1). The presence of free AA in the midgut lumen is an important signal used by the mosquito to regulate the retention of the meal in the midgut [15], as well as for the induction of digestive proteases after a blood meal [16,17,18]. An increase in extracellular AA levels after a blood meal, is critical for 20-OH ecdysone stimulation of vitellogenin synthesis in the fat body [19,20].
Fig. 1. Amino acid effects on mosquito physiology.
Digestion of hemoglobin releases free amino acids in the midgut lumen that act as signaling molecules for meal retention and protease synthesis. Increases in AA titers in hemolymph, together with an increase in 20E synthesis in ovaries, stimulate vitellogenin synthesis by the fat body. An increase in proline connects metabolic pathways in energy production, flight physiology and ammonia detoxification. AA: amino acids; 20E: 20-hydroxyecdysone; VG: vitellogenin.
The amino acid proline plays a unique role in supporting both the flight and post-blood meal physiology of Ae. aegypti [21]. Proline participates in a proline-alanine shuttle system that transports acetyl units, in the form of proline, from the fat body and to the flight muscles [22]. In this way, proline serves as an energetic substrate for flight metabolism. The enzyme alanine aminotransferase (ALAT) is a key component of this shuttle system, which enables transferring of proline and alanine as well as the detoxification of ammonia [22].
One of the major by-products of blood digestion is ammonia. Ae. aegypti females have evolved strategies to efficiently detoxify ammonia via multiple metabolic pathways [23,24]. Proline again occupies a central role in nitrogen metabolism and ammonia detoxification by serving as a nitrogen sink during the rapid deamination of proteins and production of ammonia that occurs following a blood meal [25]. Preventing ammonia detoxification by RNAi knockdown of ALAT, causes a massive increase of uric acid in the midgut and a delay in digestion, excretion, and oviposition with a significant reduction in egg production [26]. Treatment with the ALAT inhibitor L-cycloserine resulted in significant increases in mortality and disruptions to motor activity in a dose-dependent manner [27]. Key enzymes components of this “shuttling/detoxification” system are up regulated in mosquitoes reared under nutrient-limited conditions [28]. Interestingly, proline has also been recognized as an osmolyte in osmoconforming mosquito larvae [29]. Could a haemolymph proline store serve yet another purpose in Ae. aegypti by helping to buffer osmotic changes after a blood meal? Together these results illustrate a central role for proline connecting metabolic pathways in energy production, flight physiology and ammonia detoxification.
Metals
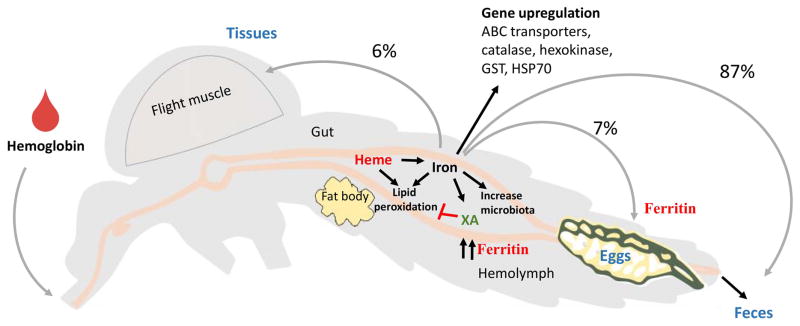
Iron is an important micronutrient for mosquitoes. A blood meal is the main source of iron for female mosquitoes, with the majority of iron bound to hemoglobin inside erythrocytes (98%), as well as transported by transferrin (2%) [30]. A blood meal provides a surplus of iron, and at the end of the first gonotrophic cycle in Ae. aegypti, 87% of the ingested heme iron is excreted, while 7% is incorporated into the eggs and 6% is stored in different tissues [30] (Fig. 2). Iron limited meals reduced egg number and progeny survivorship in Aedes albopictus [31].
Fig. 2. Iron effects on mosquito physiology.
Digestion of hemoglobin releases heme and iron that promote lipid peroxidation and increases in free radical formation. Heme promotes the generation of xanthurenic acid that binds heme and iron. Iron increases upregulate the expression of antioxidant proteins, which block lipid peroxidation. Iron stimulates the proliferation of gut microbiota and increases ferritin titers in hemolymph. Most iron is eliminated in the feces; smaller amounts are incorporated into the eggs or stored in tissues. XA: xanthurenic acid.
Hemoglobin digestion also releases large quantities of heme, which has potential pro-oxidant and cytotoxic effects when not bound to proteins [32]. Heme promotes the formation of free radicals; therefore, mosquitoes have evolved different mechanisms to avoid heme toxicity [33]; including the shutdown of the production of reactive oxygen species (ROS) in the gut [32] (Fig. 2). Heme also promotes the generation of xanthurenic acid (XA), a product of the oxidative metabolism of tryptophan, which acts as an antioxidant by binding both heme and iron [34]. Blood meal related increases of heme in Ae. aegypti triggers a wide range of pleiotropic changes in the expression of genes associated to antioxidant activities, energy metabolism, cell cycle, cellular signaling, immunity and blood meal digestion [35]. Ferritin is an iron-storage protein found free in hemolymph, as well as intracellularly in most tissues [36,37]. It is transcriptionally induced after a blood meal, and contributes to sequester iron released from heme digestion, preventing oxidative damage [38,39]. Ferritin accumulates into the eggs, providing a source of iron that functions as an antioxidant during the development of embryos [36,40] (Fig. 2).
Copper (Cu2+) is an essential component of key enzymes involved in respiration, oxidative stress protection and pigmentation; it is also a potentially toxic trace element in mosquitoes. Eukaryotic organisms use elaborated systems to regulate copper homeostasis, with the involvement of copper importers, copper chaperones, transcription factors, small metal binding proteins called metallothioneins and copper exporters [41]. Cu2+ tolerance physiology also serves as a model for mercury, silver, zinc and cadmium metabolism, because each of these share similar detoxification pathways via metallothionein binding. In Ae. aegypti, copper stress exerts multiple effects on larvae and adult physiologies [42,43,44]. Metal stressed larvae emerge with decreased lipid reserves, lowered adult body mass, increased starvation tolerance, reduced fecundity, and show an increase in starvation tolerance of offspring compared with non-metal-stressed individuals [44]. How these physiological changes due to copper exposure may affect transmission dynamics of diseases vectored by Ae. aegypti is currently unknown. Metal homeostasis is critical to many additional physiological and behavioral processes. Calcium signaling and regulation in the Malpighian tubules play essential roles in osmoregulation and ion homeostasis, detoxification and immunity [45,46]. Excess of calcium in the diet or defects in calcium homeostasis results in the formation of calcium oxalate crystals in the Malpighian tubules of flies; establishing insects as good models for studying human kidney diseases [47]. The homeostatic regulation of intraneuronal levels of divalent cations plays an important role in the development and functions of the dopaminergic system and associated behaviors [48]. Manganese homeostasis is essential for mitochondrial respiration and cellular metabolism [49].
Salts, vitamins and sterols
Salts are common constituents of larval diet or imbibed by adult mosquitoes as either primary or secondary metabolites from floral nectar and honeydew [1]. In the absence of inorganic salts in the diet, only 30% of Ae. aegypti larvae completed development; however, the addition of eight inorganic elements (Ca, Cl, Fe, K, Mg, Na, S, P) in the diet was sufficient for normal growth [4,50, 51]. Studies on Ae. aegypti mosquitoes reported better acceptance of sucrose diet containing salt concentrations isotonic to hemolymph [52,53]. Sodium is an essential ion in extracellular fluids that must be kept in a narrow range to maintain osmoregulatory functions in animal cells. Hypoosmotic and isosmotic conditions have shown to affect the expression of aquaporins in the anal papillae of larvae of Ae. aegypti, which are important osmo and ion regulatory organs [54].
Vitamins such as thiamine, riboflavin, nicotinic acid, pyridoxine, pantothenic acid and biotin are essential for full growth of mosquito larvae and most metabolic adult functions, while folic acid is required for pupation [4,50,51]. Vitamin A (retinol) or its precursor, β-carotene, are essential for the formation of mosquito visual pigments; deficiency in vitamin A results in abnormal development of photoreceptor cells, altering the compound eye response to light [55]. The B vitamins are chemically heterogeneous, but all function as essential coenzymes that are vital to insects [4]. Studies on Culex quinquefasciatus have shown that sucrose diets fortified with vitamin B stimulate ovarian development, and increase viability and longevity [56].
Insects lack the ability to synthesize sterols de novo; therefore, they acquire this essential nutrient from their food. Mosquitos use cholesterol, 7-dehydrocholesterol, sitosterol and stigmasterol to satisfy their metabolic demands [8, 57]. Cholesterol in insects is vital to membrane stability and cellular signaling and serves as the precursor to ecdysteroid hormones [58]. Dietary cholesterol in mosquitoes has a positive effect on egg development; small female mosquitoes with low cholesterol reserves show an increased number of eggs after feeding with a blood meal supplemented with cholesterol [59].
Insects that have restricted diets often develop intimate symbioses with bacteria, and it has long been hypothesized that a basis for the symbioses is nutritional [60]. The symbiotic relationships between mosquitoes and several microorganisms have important implications in mosquitoes’ nutrition, physiology and behavior [61], as well as in mosquito immunity and vector competence [62,63]. In mosquitoes, antibiotic treatment affects reproductive output, indirectly implying an important role of microbiota in reproductive fitness [64,65]. The endosymbiotic bacteria of the genus Wolbachia are one of the most successful symbiont bacteria in the terrestrial ecosystem. Wolbachia infects several mosquitoes, including Aedes and Culex species [65,66]. There is evidence of competition between Wolbachia and a mosquito host over AA and dietary cholesterol [67,68]. Ae. aegypti infected with Wolbachia have reduced cholesterol levels [68], as well as reduced Zika virus transmission [69], suggesting yet another source of competition between mosquito and Wolbachia to the detriment of viral propagation.
Conclusions
Larval and adult sugar and blood feedings provide distinct groups of energetic and non-energetic nutrients. Only now are we beginning to learn how non-energetic nutrients contribute to the overall physiology of mosquitoes. Evidence is beginning to accumulate that NEN influence everything from vector competence to the reproductive capacity of female mosquitoes. However, important questions remain in many areas. For example, little is known about the influence of NEN’s in the interplay between virus and mosquito host. Another area worthy of study includes the relationship between the mosquito microbiome and NEN’s. Finally, the role of most metals, vitamins and sterols is only superficially understood but potentially important. Early work in each of these areas is beginning to illustrate that there is much more to mosquito nutrition than only energetic molecules such as lipids and carbohydrates.
Highlights.
Non-energetic nutrients or micronutrients are essential for mosquito physiology.
Free amino acids play important roles in digestion, flight physiology and ammonia detoxification.
Metal, sterol and vitamin homeostasis are critical for cell signaling, respiration, metabolism and reproduction.
Micronutrient homeostasis influence the symbiotic relationships with microorganisms.
Acknowledgments
We would like to thank Marten Edwards, Patricia Pietrantonio, Andrea Batisti, Sassan Asgari and Marcela Nouzova for critical reading and feedback on the manuscript. This work was supported by the National Institute of Health [grant number 2R01AI045545].
Footnotes
The authors have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- 2.Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutrit. 2011;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Friend WG, Dadd RH. Insect Nutrition: A comparative perspective. Adv Nutr Res. 1982;4:205–247. doi: 10.1007/978-1-4613-9934-6_8. This is a good basic review on micronutrients and macronutrients requirements in insects. [DOI] [PubMed] [Google Scholar]
- 5.Dadd RH. Insect nutrition: current developments and metabolic implications. Annu Rev Entomol. 1977;18:381–420. doi: 10.1146/annurev.en.18.010173.002121. [DOI] [PubMed] [Google Scholar]
- 6.Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo D, Greenbaum P, Pfeiffer D, Wheelock G, Flanagan TR. Post emergence growth of the ovarian follicles of Aedes aegypti. J Insect Physiol. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- 7.Klowden MJ. Endocrine aspects of mosquito reproduction. Arch Insect Biochem Physiol. 1997;35:491–512. [Google Scholar]
- 8.Merrit RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- 9.Kevan PG, Baker HG. Insects as flower visitors and pollinators. Annu Rev Entomol. 1983;28:407–453. [Google Scholar]
- 10.Roy S, Smykal V, Johnson L, Saha TT, Zou Z, Raikhel AS. Regulation of reproductive processes in female mosquitoes. Adv Insect Physiol. 2016;51:145–188. [Google Scholar]
- 11.Noriega FG. Juvenile hormone biosynthesis in insects: What is new, what do we know, what questions remain?. ISRN; 2014. org/10.1155/2014/967361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Briegel H. Mosquito reproduction: incomplete utilization of the blood meal protein for oogenesis. J Insect Physiol. 1985;31:15–21. This paper explores the essential role of isoleucine in mosquito reproduction. [Google Scholar]
- 13.Zhou G, Miesfeld RL. Differential utilization of blood meal amino acids in mosquitoes. Open Access Insect Physiol. 2009;1:1–12. [Google Scholar]
- 14.Chang Y, Judson C. Amino-acid composition of human and guinea pig blood proteins, and ovarian proteins of the yellow-fever mosquito Aedes aegypti and their effects on the mosquito egg-production. Comp Biochem Phys A. 1979;62:753–755. [Google Scholar]
- 15**.Caroci A, Noriega FG. Free amino acids are important for the retention of protein and non-protein meals by the midgut of Aedes aegypti females. J Insect Physiol. 2003;49:839–844. doi: 10.1016/S0022-1910(03)00134-3. This study highlights the importance of a rise in free AA during the early phases of blood meal digestion as a signal that prevents early defecation of the meal components. [DOI] [PubMed] [Google Scholar]
- 16**.Barillas-Mury C, Noriega FG, Wells MA. Early trypsin activity is part of the signal transduction that activates transcription of late trypsin in the midgut of the mosquito Aedes aegypti. Insect Biochem Mol Biol. 1995;25:241–246. doi: 10.1016/0965-1748(94)00061-l. This study reveals the significance of increases in midgut free AA during the early phases of blood meal digestion as a signal that induces the synthesis of digestive enzymes. [DOI] [PubMed] [Google Scholar]
- 17.Noriega FG, Wells MA. A molecular view of protein-meal digestion in the yellow fever mosquito Aedes aegypti. J Insect Physiol. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 18.Brandon MC, Pennington JE, Isoe J, Zamora J, Schillinger AS, Miesfeld RL. TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2008;38:916–22. doi: 10.1016/j.ibmb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Hansen IA, Attardo R, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J Biol Chem. 2005;21:20565–20572. doi: 10.1074/jbc.M500712200. This study describes how amino acid signaling via the target of rapamycin (TOR) pathway is a key requirement for the activation of egg development after a blood meal. [DOI] [PubMed] [Google Scholar]
- 20*.Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol. 2014;5:103. doi: 10.3389/fphys.2014.00103. This review reviews the interplay of juvenile hormone, ecdysone, nutrients and insulin peptides pathways in mosquitoes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Scaraffia PY, Wells MA. Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J Insect Physiol. 2003;49:591–601. doi: 10.1016/s0022-1910(03)00031-3. A classical study that described the critical role of proline connecting metabolic pathways in energy production, flight physiology and ammonia detoxification. [DOI] [PubMed] [Google Scholar]
- 22**.Scaraffia PY, Isoe J, Murillo A, Wells MA. Ammonia metabolism in Aedes aegypti. Insect Biochem Mol Biol. 2005;35:491–503. doi: 10.1016/j.ibmb.2005.01.012. This study investigated the mechanisms by which Ae. aegypti mosquitoes are able to metabolize ammonia. [DOI] [PubMed] [Google Scholar]
- 23.Scaraffia PY, Tan G, Isoe J, Wysocki VH, Wells MA, Miesfeld RL. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2008;105:518–523. doi: 10.1073/pnas.0708098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isoe J, Petchampai N, Isoe YE, Mazzalupo S, Scaraffia PY. Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding–induced adulticidal activity. FASEB J. 2017:fj-201601185R. doi: 10.1096/fj.201601185R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennington JE, Goldstrohm DA, Wells MA. The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti. J Insect Physiol. 2003;49:115–121. doi: 10.1016/s0022-1910(02)00267-6. [DOI] [PubMed] [Google Scholar]
- 26.Mazzalupo S, Isoe J, Belloni V, Scaraffia PY. Effective disposal of nitrogen waste in blood-fed Aedes aegypti mosquitoes requires alanine aminotransferase. FASEB J. 2016;30:111–120. doi: 10.1096/fj.15-277087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belloni V, Scaraffia PY. Exposure to L-cycloserine incurs survival costs and behavioral alterations in Aedes aegypti females. Parasites Vectors. 2014;7:373. doi: 10.1186/1756-3305-7-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price DP, Schilkey FD, Ulanov A, Hansen IA. Small mosquitoes, large implications: crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasites Vectors. 2015;8:252. doi: 10.1186/s13071-015-0863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett MA, Bradley TJ. Extracellular accumulation of proline, serine and trehalose in the haemolymph of osmoconforming brackish-water mosquitoes. J Exp Biol. 1987;129:231–238. doi: 10.1242/jeb.129.1.231. [DOI] [PubMed] [Google Scholar]
- 30.Zhou G, Kohlhepp P, Geiser D, Frasquillo MC, Vazquez-Moreno L, Winzerling JJ. Fate of blood meal iron in mosquitos. J Insect Physiol. 2007;53:1169–1178. doi: 10.1016/j.jinsphys.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitts RJ. A blood-free protein meal supporting oogenesis in the Asian tiger mosquito, Aedes albopictus (Skuse) J Insect Physiol. 2014;64:1–6. doi: 10.1016/j.jinsphys.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira JH, Goncalves RL, Lara FA, Gandara AC, Menna-Barreto RFS, Edwards MC, Laurindo FRM, Silva-Neto MAC, Sorgine MHF, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GRC, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. This paper provides a nice review of the mechanisms to detoxify heme. [DOI] [PubMed] [Google Scholar]
- 34.Lima VLA, Dias F, Nunes RD, Pereira LI, Santos TSR, Chiarini LB, Ramos TD, Silva-Mendes BJ, Perales J, Valente RH, Oliveira PL. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS One. 2012;7:e38349. doi: 10.1371/journal.pone.0038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Bottino-Rojas V, Talyuli OAC, Jupatanakul N, Sim S, Dimopoulos G, Venancio TM, Bahia AC, Sorgine MH, Oliveira PL, Paiva-Silva GO. Heme signaling impacts global gene expression, immunity and dengue virus infectivity in Aedes aegypti. PLoS One. 2015;10:e0135985. doi: 10.1371/journal.pone.0135985. This study describe that increases of heme causes changes in the expression of genes associated to antioxidant activities, energy metabolism, cell cycle, cellular signaling, immunity and blood meal digestion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunkov BC, Georgieva T, Yoshiga T, Hall M, Law JH. Aedes aegypti ferritin heavy chain homologue feeding of iron or blood influences message levels, lengths and subunit abundance. J Insect Sci. 2002:7. doi: 10.1093/jis/2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geiser DL, Conley JL, Elliott JL, Mayo JJ, Winzerling JJ. Characterization of Anopheles gambiae (African malaria mosquito) ferritin and the effect of iron on intracellular localization in mosquito cells. J Insect Sci. 2015;15:68. doi: 10.1093/jisesa/iev049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeaue NP, Morales N, Komalamisra RE, Morales-Vargas RE. Antioxidative systems defense against oxidative stress induced by blood meal in Aedes aegypti. J Trop Med Public Health. 2011;42:542–549. [PubMed] [Google Scholar]
- 39.Marinotti O, Calvo E, Nguyen QK, Dissanayake S, Ribeiro JM, James AA. Genome-wide analysis gene expression in adult Anopheles gambiae. Insect Biochem Mol Biol. 2006;15:1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 40.Arosio P, Levi S. Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta. 2010;1800:783–792. doi: 10.1016/j.bbagen.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan JH, Maryon EB. How mammalian cells acquire copper: an essential but potentially toxic metal. Biophysical J. 2016;1:7–13. doi: 10.1016/j.bpj.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez M, Noriega FG. Aedes aegypti pharate 1st instar quiescence affects larval fitness and metal tolerance. J Insect Physiol. 2012;58:824–829. doi: 10.1016/j.jinsphys.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez M, Noriega FG. Aedes aegypti pharate 1st instar quiescence: A case for anticipatory reproductive plasticity. J Insect Physiol. 2013;59:318–324. doi: 10.1016/j.jinsphys.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez M, Noriega FG. The sub-lethal larval metal stress response of the Dengue Fever Mosquito. Physiological Entomol. 2014;39:111–119. doi: 10.1111/phen.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies SA, Terhzaz S. Organellar calcium signalling mechanisms in Drosophila epithelial function. J Exp Biol. 2009;212:387–400. doi: 10.1242/jeb.024513. [DOI] [PubMed] [Google Scholar]
- 46.Davies SA, Cabrero P, Overend G, Aitchison L, Sebastian S, Terhzaz T, Dow JAT. Cell signalling mechanisms for insect stress tolerance. J Exp Biol. 2014;217:119–128. doi: 10.1242/jeb.090571. [DOI] [PubMed] [Google Scholar]
- 47.Miller J, Chi T, Kapahi P, Kahn AJ, Kim MS, Hirata T, Romero MF, Dow JAT, Stolle ML. Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urology. 2013;190:1648–1656. doi: 10.1016/j.juro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Søvik E, LaMora A, Seehra G, Barron AB, Duncan JG, Ben-Shahar Y. Drosophila divalent metal ion transporter Malvolio is required in dopaminergic neurons for feeding decisions. Genes, Brain Behaviour. 2017 doi: 10.1111/gbb.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mühlenhoff U, Hoffmann B, Ritcher N, Rietzschel N, Spantgar F, Stehling O, Uzarska MA, Lill R. Compartmentalization of iron between mitochondria and the cytosol and its regulation. Europ J Cell Biol. 2015;94:292–308. doi: 10.1016/j.ejcb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 50**.Singh KRP, Brown AWA. Nutritional requirements of Aedes aegypti. J Insect Physiol. 1957;1:199–220. This paper thoroughly investigate all the nutritional requirements of Ae aegypti larvae. [Google Scholar]
- 51.Lichtenstein EP. Growth of Culex molestus under sterile conditions. Nature. 1948;4110:227. doi: 10.1038/162227b0. [DOI] [PubMed] [Google Scholar]
- 52.Donini A, Gaidhu MP, Strasberg DR, O’Donnell MJ. Changing salinity induces alterations in hemolymph ion concentrations and Na+ and Cl− transport kinetics of the anal papillae in the larval mosquito, Aedes aegypti. J Exp Biol. 2007;210:983–992. doi: 10.1242/jeb.02732. [DOI] [PubMed] [Google Scholar]
- 53.Ignell R, Okawa S, Englund JE, Hill S. Assessment of diet choice by the yellow fever mosquito Aedes aegypti. Physiol Entomol. 2010;35:274–286. [Google Scholar]
- 54.Akhter H, Misyura L, Bui P, Doninin A. Salinity responsive aquaporins in the anal papillae of the larval mosquito, Aedes aegypti. Comp Biochem Physiol A. 2017;203:144–151. doi: 10.1016/j.cbpa.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Brammer JD, White RH. Vitamin A deficiency: Effect on mosquito eye ultrastructure. Science. 1969;163:821–823. doi: 10.1126/science.163.3869.821. [DOI] [PubMed] [Google Scholar]
- 56*.Tan SB, Nazni WA, Misni S, Zuranini Z, Lee HL. Effects of vitamin B fortified sucrose solution on the longevity and reproductive potentials of laboratory-bred Culex quinquefasciatus Say adult. Tropical Biomed. 2016;33:141–148. This study provides evidence of the effect of vitamin B on reproductive potential of mosquitoes. [PubMed] [Google Scholar]
- 57*.Behmner ST, Nes WD. Insect Sterol Nutrition and Physiology: A global overview. Adv Insect Physiol. 2003;31:1–72. This review paper summarizes sterol homeostasis studies in insects. [Google Scholar]
- 58.Feldlaufer MF, Weirich GF, Imberski RB, Svoboda JA. Ecdysteroid production in Drosophila melanogaster reared on defined diets. Insect Biochem Molec Biol. 1995;25:709–712. doi: 10.1016/0965-1748(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 59.Talyuli OAC, Bottino-Rojas V, Taracena ML, Macedo Soares AL, Oliverira JHM, Oliveira PL. The use of a chemically defined artificial diet as a tool to study Aedes aegypti physiology. J Insect Physiol. 2015;83:1–7. doi: 10.1016/j.jinsphys.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 61.Ponton F, Wilson K, Holmes A, Raubenheimer D, Robinson KL, Simpson SJ. Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proc R Soc B. 2014;282:2014–2029. doi: 10.1098/rspb.2014.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricci I, Damiani C, Capone A, DeFreece C, Rossi P, Favia G. Mosquito/microbiota interactions: from complex relationships to biotechnological perspectives. Current Opinion Microb. 2012;15:278–284. doi: 10.1016/j.mib.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Cirimotich CM, Ramirez JL, Dimopoulos G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microb. 2011;10:307–310. doi: 10.1016/j.chom.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gendrin M, Rodgers FH, Yerbanga RS, Ouedraogo JB, Basañez MG, Cohuet A, Christophides GK. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nature Commun. 2015;6:5921. doi: 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw WR, Attardo GM, Aksoy S, Catteruccia FA. Comparative analysis of reproductive biology of insect vectors of human disease. Curr Opin Insect Sci. 2015;10:148–148. doi: 10.1016/j.cois.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobson SL, Rattanadechakul W, Marsland EJ. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity. 2004;93:135–142. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- 67*.Caragata EP, Rancès E, O’Neill SL, McGraw EA. Competition for amino acids between Wolbachia and the mosquito host. Aedes aegypti Microbial Ecology. 2014;67:205–218. doi: 10.1007/s00248-013-0339-4. This study provides evidence of competition between mosquitoes and symbionts for micronutrient resources. [DOI] [PubMed] [Google Scholar]
- 68.Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, O’Neill SL, McGraw EA. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013;9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caragata EP, Dutra HLC, Moreira LA. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microbial Cell. 2016;3:293–295. doi: 10.15698/mic2016.07.513. [DOI] [PMC free article] [PubMed] [Google Scholar]