Abstract
Background
Type 2 diabetes has been associated with higher levels of depression and depressive symptoms. Few longitudinal studies have been conducted of comorbid depression and diabetes, especially among Latinos.
Objectives
To determine whether diabetes increased the progression to elevated depressive symptoms among older Latinos in a population-based cohort.
Design
Prospective cohort study.
Participants
Individuals from the Sacramento Latino Study on Aging, aged ≥60 years in 1998–1999 and followed annually until 2008 (n=1586).
Main Measures
We defined diabetes by self-report, fasting blood glucose ≥126 mg/dL or HbA1c ≥6.5%, diabetic medication use. Elevated depressive symptoms were defined as Center for Epidemiological Studies-Depression (CES-D) score ≥16, or use of antidepressant medication.
Multi-state Markov modeling was used to assess the effects of time-dependent diabetes on transitions between 3 states over time: 1) low CES-D score (“Normal”), 2) elevated CES-D score/Treated (“Depressed”), and 3) Death. Bivariate analyses identified covariates significantly associated with any transition. These included gender and baseline measures of age, education, body mass index, hypertension, and stroke.
Results
In a fully adjusted model, compared to non-diabetics, diabetics had a 35% higher rate of developing elevated depressive symptoms or starting treatment with an antidepressant (HR 1.35, 95% CI 1.13, 1.62). Time-dependent diabetes was associated with a lower rate of regression from Depressed to Normal (HR 0.72, 95% CI 0.59, 0.88) and 2.3 fold increase in progression from a Depressed state to Death (HR 2.31, 95% CI 1.57, 3.40).
Conclusion
Diabetes increased the risk of developing elevated depressive symptoms among older Mexican-Americans. Older Latinos with diabetes should be screened for depressive symptoms and prioritized for closer follow-up, potentially through increased reliance on team-based models of care.
Keywords: Diabetes, Depression, Hispanic health, geriatrics, vulnerable populations
INTRODUCTION
Latinos aged 65 and older represent one of the fastest growing segments of the U. S. population. They are projected to be the largest racial/ethnic minority in this age group by 2019 (1). Older Latinos are disproportionately affected by both depression and diabetes. It is estimated that Latino adults have a 66% increased risk of type 2 diabetes compared to non-Latino adults (2). Similarly, Latinos have a higher lifetime prevalence of depression (3). One study of Mexican-Americans reported a lifetime prevalence of 46% among women and 19.6% among men (in comparison to national lifetime prevalence of depression of 16.1% for all adults in the U.S.) (3, 4). Depression among individuals with diabetes is associated with lower adherence to diabetes medications and self-care (5, 6), increased health care costs (7), higher odds of functional disability (8), increased rates of dementia (6), poorer health outcomes (9), and increased risk of cardiovascular and all-cause mortality compared to the general population (6, 10).
Despite the high prevalence of both diabetes and depression in older adults, there have been few longitudinal or population-based studies that examine the development of depressive symptoms among Latinos with Type 2 diabetes compared to those without diabetes. Many prior studies of the association between diabetes and depression have not delineated the race/ethnicity of participants (11–15). Those studies that report race/ethnicity have low numbers of Latino participants (16, 17). In addition, many studies have been clinic-based (12, 14, 16), raising the question of whether the increased diagnosis of depression among individuals with diabetes stems from increased contact with the medical system due to the diabetes diagnosis and closer follow-up of these patients. The few population-based longitudinal studies of Mexican-Americans have primarily focused on diabetes outcomes among participants with diabetes and depressive symptoms rather than on the risk of depressive symptoms among individuals with diabetes (18, 19).
The purpose of this study was to determine whether Type 2 diabetes was associated with a higher incidence of depressive symptoms among older Latinos in a population-based cohort. Given that depressive symptoms may vary over time, we used a Markov Transitions Model to characterize the effect of diabetes on transitions to and from elevated depressive symptoms and to death over time.
METHODS
Study Design and Population
We analyzed data from the Sacramento Area Latino Study on Aging (SALSA), a population-based prospective cohort of Latino individuals aged 60 years and older. A full description of the design and methods for this study has been published elsewhere (20). Briefly, 1789 individuals were recruited from within the Census tracts of Sacramento, Yolo, Sutter, Solano, Yuba, and Placer counties with proportional densities of Latinos greater than 10% based on updated 1990 Census data. Recruitment occurred from March 1998 through June 1999, with an overall response rate of 82.2%. Interviews and clinical assessments were conducted in participants’ homes by bilingual/bicultural technicians and information was obtained regarding demographic, health, and functional status every 12 to 15 months. Participants chose English or Spanish for survey administration. There were a total of seven examinations in the home and six semi-annual phone interviews. Annual loss-to-follow-up was about 5%, primarily due to mortality.
Measures
Type 2 diabetes status and depressive symptoms were measured during seven annual home visits. Individuals were classified as having diabetes if they fulfilled any of five criteria: 1) were on anti-hyperglycemic medications (insulin or oral medications, as ascertained by medicine cabinet inventory performed during annual home visits), 2) self-reported a physician’s diagnosis, 3) had fasting blood glucose ≥126 milligrams/deciliter, 4) had HgbA1c ≥6.5% (which was measured starting at the third follow-up visit), or 5) death certificate listed diabetes as a cause of death (21).
The Center for Epidemiological Studies-Depression (CES-D) scale was administered to all SALSA participants at baseline and every 12–15 months. This scale has been widely used and validated in Latino and geriatric populations (22). A CES-D score of ≥16 was used to define depressive symptoms (23). This definition is commonly used in clinical studies, and a study of primary care outpatients found that this cut-off was associated with a 95% sensitivity and 70% specificity (23). Individuals with CES-D score < 16 and who were not using antidepressants were classified as “Normal. ” Individuals were classified as being “Depressed” if they: 1) had depressive symptoms with a CES-D score ≥16, or 2) used an antidepressant drug as derived from a medicine cabinet inventory (24). Antidepressant drugs included predominantly selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs), with smaller numbers of individuals on atypical antipsychotics, noradrenergic and specific serotonergic antidepressants (NaSSAs), norepinephrine-dopamine reuptake inhibitors (NDRIS), and serotonin-norepinephrine reuptake inhibitors (SNRIs).
Socio-demographic variables included participant age, gender, education, marital status, place of birth, preferred language, and monthly household income level. Participants were also asked to self-report medical history. Hypertension was classified as self-report of a doctor’s diagnosis, an elevated systolic and diastolic blood pressure (>140/90) or use of an anti-hypertensive medication. Blood pressure, height and weight were measured at home visit and body mass index (BMI) was calculated as (kg/m2).
Statistical Analysis
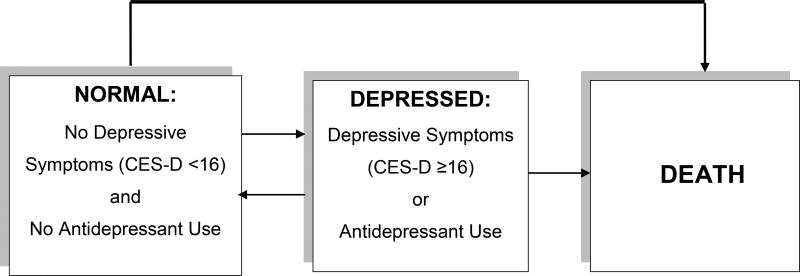
To capture the variable nature of depressive symptoms over time, we used Multi-state Markov models (25) to model subject transitions between three possible states: 1) “Normal”: CES-D score < 16 and no antidepressant use), 2) “Depressed”: CES-D score ≥16 or antidepressant use, and 3) “Death. ” Multi-state Markov models describe the associations of covariates with probabilities of state transitions using hazard rates, assuming that hazard rates depend only on the currently occupied state and not on previously occupied states (25). We checked this assumption by fitting a multinomial logistic model for the current state that included the states occupied at one and two visits prior as covariates and the results indicated that the assumption was reasonable. The magnitudes of the regression coefficients associated with the states lagged by one visit were noticeably larger than the magnitudes of the regression coefficients of the states lagged by two visits, indicating that the multi-state Markov models described the data reasonably well. Our models allowed transitions from “Normal” to “Depressed”, “Normal” to Death, “Depressed” to “Normal”, and “Depressed” to Death (Figure). The model also allowed for no transitions, for example remaining “Normal. ” We included only participants who had at least 2 visits with a depressive symptoms score. Bivariate analyses identified covariates that were significantly associated with at least one transition. These covariates included gender and baseline measures of age, education, BMI, hypertension, and stroke. Age, education, and body mass index were categorized into 3 groups respectively for the purposes of this analysis (Table 2). We then fit a series of Multi-state Markov models to the data. Model 1 included only the main predictor of interest, time-dependent diabetes. Model 2 included adjustment for baseline age and gender. Model 3 added baseline covariates that were significantly associated with at least one transition: years of education, BMI, hypertension, and history of stroke. Nativity, income, having a primary care provider, and having insurance were not included in the final model as they were not statistically significantly associated with any of the transitions. Hazard ratios for each possible transition were estimated for each covariate effect. Because of concerns of misclassification of depression by including those on antidepressants who may have been on them for other reasons, we conducted a sensitivity analysis in which we examined the CES-D scores among those taking antidepressants and repeated bivariate analysis of the association of diabetes with depressive symptoms (CES-D score ≥16) with no inclusion of antidepressants in the outcomes. The number of observations with an antidepressant treatment was not sufficient to permit separate analyses due to power considerations.
FIGURE 1.
Conceptual Model of Transitions between Depression States and Death in a Community-based Cohort of Older Latinos. Individuals may also remain in the same state (Normal or Depressed) throughout the study.
Table 2.
Unadjusted and Adjusted Hazard Ratios for Time-dependent Diabetes as Predictor of Transitions between Normal, High Depressive Symptoms and Death, n=1586.
| Multivariate Modelsa | ||||
|---|---|---|---|---|
|
| ||||
| States | Normal → Depressed HR (95% CI) |
Normal → Death HR (95% CI) |
Depressed → Normal HR (95% CI) |
Depressed → Death HR (95% CI) |
| Model 1b | 1.32 (1.11, 1.56) | 1.94 (1.11, 3.38) | 0.77 (0.64, 0.93) | 1.53 (1.04, 2.23) |
| Model 2c | 1.34 (1.10, 1.62) | 1.94 (0.80, 4.66) | 0.73 (0.61, 0.88) | 1.77 (1.08, 2.88) |
| Model 3d | 1.35 (1.13, 1.62) | 0.99 (0.51, 1.92) | 0.72 (0.59, 0.88) | 2.31 (1.57, 3.40) |
| Bivariate adjustments: Age: in years | ||||
| 60–69 | reference | reference | reference | reference |
| 70–79 | 1.36 (1.15, 1.62) | 1.89 (1.04, 3.42) | 1.07 (0.89, 1.28) | 2.19 (1.38, 3.48) |
| 80+ | 1.78 (1.30, 2.43) | 4.09 (1.73, 9.67) | 0.81 (0.56, 1.18) | 4.89 (2.94, 8.13) |
| Female gender | 1.20 (1.01, 1.44) | 1.14 (0.57, 2.27) | 0.62 (0.51, 0.75) | 0.34 (0.23, 0.49) |
| Education: in years | ||||
| <9 | reference | reference | reference | reference |
| 9–12 | 0.72 (0.59, 0.88) | 0.55 (0.27, 1.12) | 1.11 (0.90, 1.37) | 0.84 (0.52, 1.34) |
| >12 | 0.49 (0.39, 0.62) | 0.53 (0.26, 1.09) | 0.94 (0.74, 1.20) | 0.75 (0.41, 1.37) |
| Body Mass Index | ||||
| <25 | reference | reference | reference | reference |
| 25≤30 | 0.95 (0.75, 1.21) | 0.58 (0.24, 1.40) | 1.23 (0.96, 1.59) | 1.03 (0.63, 1.66) |
| >30 | 0.97 (0.77, 1.22) | 0.87 (0.42, 1.77) | 0.99 (0.78, 1.27) | 0.50 (0.30, 0.85) |
| Hypertension | 1.27 (1.06, 1.51) | 2.44 (1.16, 5.14) | 1.02 (0.84, 1.23) | 1.31 (0.85, 2.02) |
| Stroke | 1.73 (1.29, 2.32) | 3.88 (2.02, 7.45) | 0.84 (0.62, 1.15) | 1.07 (0.59, 1.92) |
Abbreviations (in alphabetical order): CES-D=Center for Epidemiological Studies-Depression scale.
Normal= CES-D score <16 and not using an antidepressant, Depressed= CES-D score ≥16 or using an antidepressant
Unadjusted
Adjusting for age and gender
Adjusted for age, gender, years of education, BMI, history of hypertension and stroke
All analyses were performed using the msm package in R version 3.1.1 (2011). This uses a continuous time model, while properly accounting for the fact that states other than death are only observed at intermittent follow-up times.
Ethical considerations
The SALSA study was approved by the Institutional Review Boards at the University of Michigan, the University of California, Davis, and the University of California, San Francisco.
ESULTS
Study analyses included 1586 older Latinos (94% Mexican or Mexican-American, 6% self-identified as ‘Other’ Latino and were Central or South American). Individuals were followed during 10 years (mean follow-up period 6.32 years, median 7.63 years, range 0–9.42 years). During this time period, there were 740 (47%) total cases of diabetes (including 514 individuals with diabetes at baseline and 226 incident cases) and 423 (27%) deaths. Less than 1% of new diabetes cases were diagnosed through review of death certificates. There were a total of 6,564 transitions between states from baseline to follow-up (from 1998 until 2008). Of these, 56% remained in a normal state, 15.7% remained in a depressed state, 11% moved from normal to depressed, 10% moved from depressed to normal and 6.0% died. Baseline characteristics of cohort participants by baseline diabetes status are shown in Table 1 (n=1583; 3 individuals missing baseline diabetes status are not included in the table but were included in analysis and models). More than half of study participants were female (58%) and 46% were 60–69 years old at baseline. SALSA was evenly split between U. S. and foreign-born participants (50%) and most spoke primarily Spanish (56%). Almost all participants reported having some form of medical insurance (91%) and a primary care provider (89%), regardless of diabetes status. At baseline, participants with diabetes were more likely to have been born in the U. S. (58% vs 47%, p-value <0.001) than participants without diabetes. Marital status, years of education completed, retirement, and monthly household income were similar among participants with and without baseline diabetes. Individuals without diabetes were more likely to be currently employed. Among individuals with diabetes at baseline, the proportion of individuals with depressive symptoms (CES-D ≥16) was slightly greater (28% vs 24%, p-value= 0.06) than among those without diabetes, though this did not reach statistical significance.
Table 1.
Baseline Characteristics of the Study Population by Presence vs Absence of Type 2 Diabetes Mellitus,a n=1583.b
| Diabetes N=514 |
No Diabetes N=1069 |
p-value | |
|---|---|---|---|
|
| |||
| N (%) | N (%) | ||
| Male sex | 229 (45) | 441 (41) | 0.21 |
| Age (in years) | 0.48 | ||
| 60–69 | 244 (48) | 491 (46) | |
| 70–79 | 218 (42) | 448 (42) | |
| 80+ | 52 (10) | 130 (12) | |
| Primary Language Spanish | 286 (56) | 598 (56) | 0.91 |
| Immigrant (Mexican or Other Latin | 217 (42) | 567 (53) | <0.0001 |
| American) | |||
| Marital status | 0.43 | ||
| Single | 12 (2) | 35 (3) | |
| Married/Living with someone | 313 (61) | 624 (58) | |
| Widowed | 113 (22) | 264 (25) | |
| Divorced/Separated | 75 (15) | 145 (14) | |
| Retired | 397 (90) | 799 (91) | 0.65 |
| Currently employedc | 61 (12) | 202 (19) | 0.001 |
| Monthly household income (in $) | 0.90 | ||
| <1000 | 218 (43) | 450 (43) | |
| 1000–1499 | 108 (21) | 217 (21) | |
| 1500–1999 | 61 (12) | 116 (11) | |
| 2000–2499 | 49 (10) | 110 (11) | |
| ≥2500 | 70 (14) | 160 (15) | |
| Have primary care physician | 475 (93) | 938 (88) | 0.005 |
| Have health Insurance | 479 (93) | 972 (91) | 0.17 |
| Hypertension | 413 (80) | 668 (63) | <0.0001 |
| History of Stroke | 74 (14) | 66 (6) | <0.0001 |
| CES-D score ≥16 | 144 (28) | 253 (24) | 0.06 |
| Mean (SD) | Mean (SD) | ||
| Years of education completed | 7.3 (5.4) | 7.5 (5.3) | 0.47 |
| Hemoglobin A1c (%) | 7.3 (1.3) | 5.7 (0.6) | <0.0001 |
| Fasting blood sugar (FBS, in mg/dL) | 156.3 (57.8) | 93.9 (12.0) | <0.0001 |
| Body mass index (BMI, in kg/m2) | 31.0 (6.3) | 29.2 (5.7) | <0.0001 |
Baseline diabetes
Table does not include 3 individuals with missing baseline diabetes status but who were included in the final analysis and models.
‘Employed’ and ‘Retired’ are not mutually exclusive categories. Individuals who were retired could be employed in a different position after retirement; percentages do not add up to 100%.Percentages may not equal 100% due to rounding.
Abbreviations (in alphabetical order): BMI= Body Mass Index. CES-D=Center for Epidemiological Studies-Depression scale. FBS= Fasting Blood Sugar.
Over the course of up to 10 years of follow-up, a total of 329 participants (20.7%) used antidepressants; 13.1% used more than one type of antidepressant at a time. The majority were on SSRIs (64%) or TCAs (40%), with smaller numbers on anti-psychotics (14%), NaSSAs (5%), NDRIs (5%), and SNRIs (4%). Eighty-one percent of the sample never used an antidepressant and 7% transitioned from no use to use and 7% transitioned from use to no use. As expected, those on antidepressants had higher mean CES-D scores (15 vs 9.6, p<0.001, full results not shown). In sensitivity analyses, exclusion of antidepressant use from the outcome definition did not substantively change the associations between diabetes and transitions in the bivariate analysis. Use of antidepressants was kept in the definition of “Depressed” in further analyses.
In bivariate analyses, (Table 2), older age, presence or history of hypertension or stroke were significantly associated with an increased hazard of progression from “Normal” to “Depressed” or Death. Higher education was associated with decreased progression from “Normal” to “Depressed” though the transitions between other states did not reach statistical significance.
In the unadjusted Model 1 (Table 2), time-dependent diabetes was associated with increased rates (hazards) of progression from “Normal” to “Depressed” or Death. Compared to those without diabetes, those with diabetes had a 23% lower rate of transition from “Depressed” to “Normal. ” The rate of progression from “Depressed” to Death was increased in those with diabetes.
After adjustment for gender and baseline age (Model 2), time-dependent diabetes remained a significant predictor of progression from “Normal” to “Depressed. ” The association with progression to Death was no longer statistically significant. The association between diabetes and increased hazard of progression from “Normal” to “Depressed” remained statistically significant. After further adjustment for years of education, BMI, hypertension, and stroke (Model 3), these associations remained similar in magnitude. However, the effect of diabetes on transition from “Normal” to Death was no longer statistically significant in Models 2 and 3 and the confidence interval included 1.0. In Model 3, an individual with diabetes had a 35% higher rate (hazard) of developing depressive symptoms or starting treatment with an antidepressant, compared to non-diabetics. Diabetes was associated with a 28% lower rate of regression to “Normal” from “Depressed. ” Diabetes was associated with a 2.3 fold increase in the rate of progression from ‘Depressed’ to Death.
Conclusion
In this large population-based cohort of older Latinos, diabetics had higher rates of developing elevated depressive symptoms or initiating antidepressant therapies that were more likely to persist over ten years of follow-up. We also found a high prevalence of diabetes with almost half (47%) of the participants meeting diagnostic criteria for diabetes by the end of the study. The associations we found between diabetes and elevated depressive symptoms and antidepressant therapy use were not explained by other important medical conditions or socioeconomic factors. This study adds to prior research that individuals with diabetes are at higher risk for depressive symptoms, and provides more information about older Latinos, a rapidly growing segment of the U. S. population.
Most prior research among Mexican-origin individuals has focused on diabetes outcomes among participants with concomitant diabetes and depressive symptoms (18, 19). Few have examined the risk of depressive symptoms in individuals with diabetes compared to those without diabetes (16, 17). A national longitudinal study of older Mexican-Americans with diabetes demonstrated greater incidence of microvascular and macrovascular complications, disability and mortality among individuals with concomitant depressive symptoms (measured at baseline using the CES-D, and once during 7 years of follow-up using the Composite International Diagnostic Interview, CIDI) (18). In a randomized controlled trial of a diabetes management intervention among Mexican-Americans in San Diego County, California, lower depression scores on the Patient Health Questionnaire-9 (PHQ-9) were associated with lower hemoglobin A1c levels, greater social and environmental support for disease management, better diabetes self-management, and lower BMI and triglyceride levels in two years of follow-up (19).
Previous studies reporting on the incidence of depression among diabetics in the general population have questioned whether increased contact with the medical system (and the diabetes diagnosis itself) led to increased diagnosis and medical labeling of depression (14, 15). This population-based study has the advantage of systematic and direct ascertainment of depressive symptoms and medication information, regardless of frequency of contact with the medical system and whether the individual was being treated for diabetes or not. Further, the long length of follow-up allows us to examine dynamic changes in depressive symptoms over time using the Markov Transitions Model. The increased rate of transition from “Depressed” to Death further suggests a synergistic effect between diabetes and depressive symptoms on mortality.
Several limitations should be considered in interpreting our study findings. While it has been postulated that there may be a bidirectional association between diabetes and depressive symptoms (17, 26), our analysis approach was only designed to evaluate the association between diabetes and depressive symptoms. Other studies have used different measures to assess for depressive symptoms (such as the PHQ-9 or Beck Depression Inventory) rather than the CES-D, limiting the ability to compare results. Furthermore, the CES-D is a commonly used and well-validated measure of depressive symptoms, with high sensitivity and specificity for depression (23), but given it was not accompanied by a full diagnostic interview for depression we cannot speak to the incidence of clinical depression among our participants. A psychiatric consult may not be routinely feasible in primary care. Increasingly, primary care physicians are relying on validated depression screening tools, such as the PHQ-9, to diagnose and prompt further evaluation and monitoring of patients. We did not collect information on self-reported depression diagnosis, so it is possible that individuals were taking antidepressant medications for other conditions (such as for neuropathic pain, insomnia or other mood disorders). However, similar to prior longitudinal studies, we decided to include antidepressant use as part of the outcome measure to avoid misclassification of individuals with adequately treated depression and thus low CES-D scores while taking an antidepressant medication (17, 27). Sensitivity analyses demonstrated that participants on antidepressants had higher mean CES-D scores compared to those who were not taking those medications. This suggests that inclusion of antidepressant use in our outcome definition does not lead to differential misclassification of the outcome.
Our study has several implications for primary and mental health care providers serving older Mexican-origin Latinos. There is evidence that in routine clinical practice there is under-diagnosis of depression among individuals with diabetes, and that this is particularly true in older adults, despite recognition that depressive symptoms worsen diabetes outcomes and lead to increased dementia and all-cause mortality (6, 9, 10, 28). Our study supports the need to more actively screen older Latino adults with diabetes for depressive symptoms and to evaluate them for treatment. Additionally, given the association between diabetes and obstructive sleep apnea (OSA), and the increased incidence of depression among individuals with OSA, it is important to screen for treatable sleep disorders among individuals with diabetes (29). Treatment of depression has been shown to improve depressive symptoms, and studies on collaborative care suggest that such models for treatment of depression can have favorable effects on cardiovascular risk factors and be cost-effective (6). Furthermore, individuals with diabetes and sub-threshold symptoms of depression may benefit from early culturally-appropriate interventions to prevent depression (30). Culturally-sensitive models of care, with a reliance on team-based models, need to be further explored in the treatment and prevention of depression among older Mexican-Americans and other ethnic minorities. A study of Latinos in a safety net setting also emphasized the high rates of relapse for depressive symptoms, so individuals previously treated for depression must undergo continued monitoring even after remission (6). Future research is needed to identify factors that influence vulnerability or resiliency to depression and diabetes, as well as into programs aimed at preventing the development of depression in individuals with diabetes.
In older Latinos, we found that diabetes and depressive symptoms interact in clinically significant ways over 10 years of follow-up. Specifically, diabetes increases risk of depressive symptoms and the transition to death is increased among individuals with diabetes and depressive symptoms. Older Latinos with diabetes should be actively screened for depressive symptoms and prioritized with closer follow-up and a team-based approach. Given increasing concurrent mental health conditions and chronic diseases, new models to integrate culturally appropriate mental health services into primary care are needed.
Acknowledgments
Funding source: MG is funded by the NIH Grant D55HP23202 (UCSF Primary Care Research Fellowship), Faculty Development in Primary Care. The Sacramento Area Latino Study on Aging (SALSA) was funded through NIH grant AG12975 and DK60753.
Sponsor’s Role: None.
Footnotes
Prior presentations: Early results of this work were presented at the annual Society of General Internal Medicine meeting in Toronto Canada, on April 22nd, 2015, as a poster presentation.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions:
All authors had full access to all of the data in the study. Dr. Haan takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Garcia, Haan. Acquisition of the data: Haan, Lee. Analysis and interpretation of the data: Garcia, Lee, Neuhaus, Gonzalez, To, Haan. Drafting of the manuscript: Garcia, Haan. Critical revision of manuscript for important intellectual content: Garcia, To, Gonzalez, Haan. Statistical analysis: Lee, Neuhaus.
References
- 1.Department of Health and Human Services AoA. [on April 14 2015];2010 Pages http://www.aoa.aclgov/Aging_Statistics/minority_aging/Facts-on-Hispanic-Elderly.aspx.
- 2.Center for Disease Control. [on 4/14/2015 2015]; Pages http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 3.Vega WA, Kolody B, Aguilar-Gaxiola S, et al. Lifetime prevalence of DSM-III-R psychiatric disorders among urban and rural Mexican Americans in California. Arch Gen Psychiatry. 1998;55:771–778. doi: 10.1001/archpsyc.55.9.771. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control. [on June 18, 2015];2011 Pages http://www.cdc.gov/mmwr/preview/mmwrhtml/su6003a1.htm.
- 5.Sumlin LL, Garcia TJ, Brown SA, et al. Depression and adherence to lifestyle changes in type 2 diabetes: A systematic review. Diabetes Educ. 2014;40:731–744. doi: 10.1177/0145721714538925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davydow DS, Katon JG, Rollman BL, et al. Improving mental and physical health outcomes in general healthcare settings: A Gedenkschrift in honor of Wayne Katon, MD (1950–2015) Gen Hosp Psychiatry. 2015;37:375–386. doi: 10.1016/j.genhosppsych.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–470. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- 8.Egede LE. Diabetes, major depression, and functional disability among U.S. adults. Diabetes Care. 2004;27:421–428. doi: 10.2337/diacare.27.2.421. [DOI] [PubMed] [Google Scholar]
- 9.de Groot M, Anderson R, Freedland KE, et al. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann M, Kohler B, Leichsenring F, et al. Depression as a risk factor for mortality in individuals with diabetes: A meta-analysis of prospective studies. PLoS One. 2013;8:e79809. doi: 10.1371/journal.pone.0079809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palinkas LA, Lee PP, Barrett-Connor E. A prospective study of Type 2 diabetes and depressive symptoms in the elderly: The Rancho Bernardo Study. Diabet Med. 2004;21:1185–1191. doi: 10.1111/j.1464-5491.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 12.Brown LC, Majumdar SR, Newman SC, et al. Type 2 diabetes does not increase risk of depression. CMAJ. 2006;175:42–46. doi: 10.1503/cmaj.051429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maraldi C, Volpato S, Penninx BW, et al. Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: The health, aging, and body composition study. Arch Intern Med. 2007;167:1137–1144. doi: 10.1001/archinte.167.11.1137. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor PJ, Crain AL, Rush WA, et al. Does diabetes double the risk of depression? Ann Fam Med. 2009;7:328–335. doi: 10.1370/afm.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nouwen A, Winkley K, Twisk J, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia. 2010;53:2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med. 2005;165:1260–1266. doi: 10.1001/archinte.165.11.1260. [DOI] [PubMed] [Google Scholar]
- 17.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 19.Fortmann AL, Gallo LC, Walker C, et al. Support for disease management, depression, self-care, and clinical indicators among Hispanics with type 2 diabetes in San Diego County, United States of America. Rev Panam Salud Publica. 2010;28:230–234. doi: 10.1590/s1020-49892010000900014. [DOI] [PubMed] [Google Scholar]
- 20.Wu JH, Haan MN, Liang J. Impact of diabetes on cognitive function among older Latinos: A population-based cohort study. J Clin Epidemiol. 2003;56:686–693. doi: 10.1016/s0895-4356(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 21.Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarnaccia PJ, Angel R, Worobey JL. The factor structure of the CES-D in the Hispanic Health and Nutrition Examination Survey: The influences of ethnicity, gender and language. Soc Sci Med. 1989;29:85–94. doi: 10.1016/0277-9536(89)90131-7. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JL, Jones GN, Scarinci IC, et al. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. The Center for Epidemiologic Studies-Depression. Int J Psychiatry Med. 2001;31:25–40. doi: 10.2190/FUFR-PK9F-6U10-JXRK. [DOI] [PubMed] [Google Scholar]
- 24.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Kalbfleisch J, Lawless JF. The analysis of panel data under a Markov assumption. J Am Stat Asso. 1985;80:863–871. [Google Scholar]
- 26.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3:450–460. doi: 10.1016/S2213-8587(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 27.Rubin RR, Knowler WC, Ma Y, et al. Depression symptoms and antidepressant medicine use in Diabetes Prevention Program participants. Diabetes Care. 2005;28:830–837. doi: 10.2337/diacare.28.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrak F, Baumeister H, Skinner TC, et al. Depression and diabetes: treatment and health-care delivery. Lancet Diabetes Endocrinol. 2015;3:472–485. doi: 10.1016/S2213-8587(15)00045-5. [DOI] [PubMed] [Google Scholar]
- 29.Hirshkowitz M. The clinical consequences of obstructive sleep apnea and associated excessive sleepiness. J Fam Pract. 2008;57(8 Suppl):S9–16. [PubMed] [Google Scholar]
- 30.Reynolds CF, 3rd, Thomas SB, Morse JQ, et al. Early intervention to preempt major depression among older black and white adults. Psychiatr Serv. 2014;65:765–773. doi: 10.1176/appi.ps.201300216. [DOI] [PMC free article] [PubMed] [Google Scholar]