Abstract
An individual's microbiome is likely to be an important contributor to certain health disparity diseases and conditions. We present a framework to study the role of the microbiome and the multiple factors that are likely to influence differences in disease predisposition, onset, and progression at the individual and population level.
The contemporary understanding of disease highlights its complex and multifactorial etiology. Effectively reducing disease burden requires better understanding of its determinants in order to address the relevant social, behavioral, cultural, biological, economic, and institutional factors that work in concert to influence the onset, progression, and severity of disease over an individual's life course. The mutable environment in which we live further complicates efforts to solve the mysteries of human health and disease [1]. One area of intense investigation is the human microbiome and its role in health and disease. Humans are host to a multitude of microorganisms that modulate human health and disease. Much of microbiome research has focused on characterizing not only the healthy microbiome but also the microbiome in common chronic conditions such as diabetes and obesity [2]. These studies have yielded valuable insight into the triggers responsible for shifts in microbial communities across many disease states. Multiple factors, such as the host immune system, environment, host lifestyle and hygienic factors, and genetic variation, have been implicated in the reported shifts observed in the human microbiome.
Health disparities are defined as avoidable health differences in the incidence, prevalence, mortality, and burden of diseases and other adverse health conditions that exist among specific population groups in the United States. There are large racial and ethnic differences in health, and researchers have not fully explored the extent to which variation in the microbiome can possibly contribute to our understanding of racial and ethnic health disparities [1]. Racial and ethnic group membership reflects, in part, differences in socioeconomic status (SES) which are strong predictors of variation in health. However, racial disparities persist at every level of SES, and we need to better understand how health is shaped by differential exposure to risk factors and resources, over the life course, in psychosocial, physical, and chemical environments linked to ethnicity and SES and biological adaptation to these exposures [1]. Thus, factors such as diet, lifestyle, and other health-related behaviors likely influence ethnic differences in health and combine with other exposures to potentially modify the microbiome over time, resulting in poorer health outcomes. Inclusion of individuals from diverse ancestral, cultural, and social backgrounds in microbiome studies is a key step in advancing our understanding of health disparities. This is especially true in cases where investigators are able to link prevalence differences in a specific health condition or disease with identifiable population groups. Uncovering the role of the microbiome in health disparities could enhance our understanding of why some populations have poorer survival rates, greater severity of disease, and overall elevated disease risks compared to others. Furthermore, exploring the microbiome and the differences therein is likely to be important in efforts to reduce and eliminate health disparities while shedding light on how social and environmental exposures interact with biology to affect disease risk and outcome [1]. For example, skin pigmentation and exposure to sunlight and ultraviolet radiation are likely to impact skin microbial communities, yet have not been systematically explored in the context of skin health or disease.
One commentary by Fortenberry challenges the use of race and ethnicity categories in microbiome research [3]. Fortenberry, in our opinion, appropriately cautions the scientific community to be far more critical of the use of racial and ethnic categories as proxies for the true causes of microbial diversity. Microbiome research has the potential to translate its insights into better understanding of health disparities while precluding attribution of causal inference to specific racial and ethnic population groups [4].
Researchers studying the microbiome have captured limited information on socioeconomic, psychosocial, cultural, and behavioral factors as well as diet in ancestrally diverse study populations [4,5]. For example, previous studies have shown that black and Hispanic women in the United States of reproductive age tend to have higher rates of adverse pregnancy outcomes (i.e., pre-term births and miscarriage), sexually transmitted infections (STIs), bacterial vaginosis, and yeast infections. The vaginal microbiome was characterized in a cohort of women of European and African ancestry, revealing ethnic differences in vaginal pH and the microbiome. European women were more likely than the African American women to harbor a Lactobacillus-dominated microbiome. Lactobacillus and other related organisms appear to help maintain vaginal health [5]. Another study analyzed the gut microbiome in European and rural African children from two distinct geographical locations and cultures [6]. Significant differences were observed in the gut microbiome of two groups and were heavily influenced by geography and diet, one high in fiber, and the other high in fat [4].
Although the above studies reveal interesting features of the microbiome in ethnically diverse populations, limited environmental, social, and behavioral data on study participants continue to be an important limitation. For example, interest is emerging in understanding the role of the early feeding environment and its potential effect on the intestinal microbiome and immune responses. Researchers speculate that early feeding practices can shape weight loss/gain, alter the gut microbiota, and increase risk for developing chronic conditions such as obesity later in life. Moreover, feeding practices correlate with biological and behavioral factors such as maternal weight, eating habits, sleep patterns, socioeconomic status, and other health-related behaviors (e.g., smoking and alcohol use). Basic science researchers who study the role of the immune system and the microbiome in health and disease should incorporate a comprehensive examination of an individual's environmental context, including cultural practices, diet, chemical exposures, stress, and the effects that these may have on the microbiome.
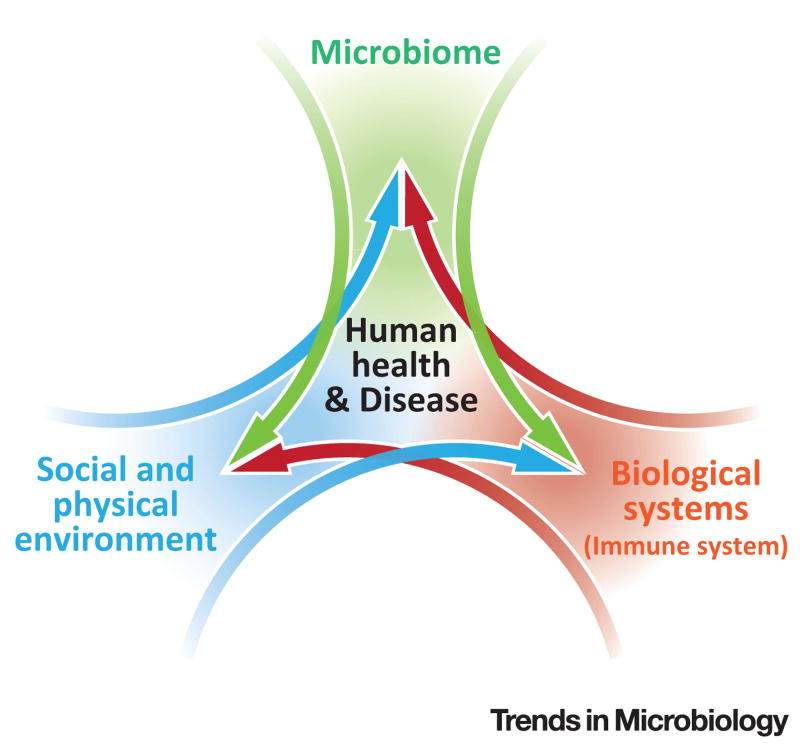
A model developed to examine infectious disease, the epidemiologic triad, highlights the influence of the agent, environment, and host in disease onset [7]. The goal of such studies is to prevent disease by modifying one or more of the factors in the triad. The biopsychosocial model, which complements the epidemiologic triad, maintains that biological, psychological, and social processes must be considered in health and disease [7]. We present the immune system as an exemplar biological process for understanding the interaction between the microbiome and the social and physical environment in disease outcomes (Figure 1).
Figure 1. Framework for Human Health and Disease.
This conceptual framework explores three overlapping but distinct and complex areas of health research–the microbiome, biological processes (e.g., immune system), and the social and physical environment. This framework provides an integrative approach to redefine our current understanding of how these areas shape human health and disease.
Exposures from the environment are numerous, ubiquitous, hard to measure, and temporally dynamic over our lifetime. Environmental exposures such as chemicals, tobacco use, residential pets, pests, and mold can alter health status. Social factors such as segregation, violence, poverty, poor education, health practices, and limited access to healthy food options and medical care can contribute to poor environmental health, all of which are likely major contributors to the patterns of health disparities and health inequities. More thorough and in-depth studies, combining the microbiome with detailed and comprehensive delineation of environmental exposures, are required to fully capitalize on the ways in which the microbiome can inform our understanding of the causes and consequences of disease risks and enhance our ability to effectively combat disease.
Stress is an important psychosocial factor that can increase disease risk. Stress impairs multiple physiological systems, including the immune system, and increases the likelihood of risky health behaviors that can adversely affect health outcomes [8]. Physiologic responses to stress include an increase in cortisol levels and reduction in glucocorticoid sensitivity. We speculate that a number of factors which include but are not limited to discrimination, stigma, depression, and poor environmental health conditions likely play a major role in this framework through an interaction with the immune system.
The host immune system is extremely sensitive to changes in the environment and the microbiome. Consequently, perturbations of any kind may result in an aberrant immune response and increased susceptibility to chronic disease. We speculate that a bidirectional interaction exists between the microbiome and psychosocial indicators, and both change in response to the health status of the individual. We recognize that the microbiome may possibly change in response to the immune system, and conversely, the immune system may respond to changes in the microbiome. Furthermore, the same bidirectional relationship observed between the microbiome and psychosocial indicators exists between overall health status and psychosocial indicators. A detailed examination of the impact of participant-reported stress, stigma, discrimination, and anxiety on one's health is essential in unraveling the contribution of each factor in disease onset and progression.
This framework investigates three distinct but overlapping areas of health research – the microbiome, biological processes, and social and physical environmental factors – to offer an integrative theory to our current understanding of human health and disease (Figure 1). It is important to note that this model is not limited to the information presented here but may include other chronic disorders (e.g., metabolic or mental health) that influence changes in immune status over time. Variation in disease phenotype is multifactorial, including differences in access to health care, immunity, environment, and the host microbiome. Researchers have begun exploring the role of the microbiome in certain health conditions for which there are disparities, including asthma, diabetes, sickle-cell disease, colon cancer, pre-term birth, and bacterial vaginosis which we reference in Table 1. Other examples, not discussed here, include obesity, and periodontal and cardiovascular disease. We contend that this model will have broad utility in the investigation of prevention strategies, interventions, and improved and/or novel research methods for understanding health disparities.
Table 1. The Role of the Microbiome in Examples of Health Conditions with a Health Disparity.
| Health condition | Contributing factors | Disparity in incidence | Relevant studies | Refs |
|---|---|---|---|---|
| Asthma | Genomics, lifestyle, health behaviors, pollutants, and environment | Black, Hispanic | Early-life microbial (and allergen) exposures may offer protection against asthma:
|
[10] |
| Diabetes | Genomics, diet, lifestyle, health behaviors, environment, and physical inactivity | American Indian, Alaska Native, Black, Hispanic | Gut microbiota characterized:
|
[11] |
| Sickle-cell disease | Single gene mutation influenced by genomic variation and environment | Black, Hispanic, Southern European, Middle Eastern, and Indian | Neutrophil ageing and micro-biome in sickle-cell disease:
|
[12] |
| Colorectal cancer | Genomics, diet, health status, lifestyle, health behaviors, and environment | Black | Gut micro-biota characterized:
|
[13] |
| Pre-term birth | Genomics, health status, lifestyle, health behaviors | Black, Hispanic, and others | Vaginal micro-biome characterization during pregnancy:
|
[14] |
| Bacterial vaginosis | Genomics, diet, health status, lifestyle, health behaviors, and environment | Black, Hispanic | Vaginal micro-biome characterized in four ethnic groups:
|
[15] |
This integrated health disparities science research approach will require the collaboration of investigators from multiple disciplines, including basic and computational scientists, clinicians, social and behavioral scientists, and epidemiologists. Each discipline plays a vital role in delineating the relevant factors that accumulate over the life course to influence disease risk and differences in health outcomes. One challenge to this approach is the need to modify or generate novel analytic tools to integrate social and biological data to investigate disparities in health outcomes. The ability to understand and translate the findings from these studies to a broader audience will be an audacious challenge, but one the scientific field should prepare to tackle. Thus, the future of health disparities research science should include integration of multiple disciplines focusing on the whole person – including the microbiome – to uncover unknown disease etiology and better understand human health and disease [9].
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. The opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Human Genome Research Institute, the National Institutes of Health, or the Department of Health and Human Services.
References
- 1.Williams DR, et al. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartstra AV, et al. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 3.Fortenberry JD. The uses of race and ethnicity in human microbiome research. Trends Microbiol. 2013;21:165–166. doi: 10.1016/j.tim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Ou J, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fettweis JM, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160:2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen IH, Syme SL. The social environment and health: a discussion of the epidemiologic literature. Annu Rev Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 9.Jackson FL, et al. Conceptual shifts needed to understand the dynamic interactions of genes, environment, epigenetics, social processes, and behavioral choices. Am J Public Health. 2013;103(Suppl. 1):S33–S42. doi: 10.2105/AJPH.2013.301221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dannemiller KC, et al. Indoor microbial communities: Influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol. 2016;138:76–83. doi: 10.1016/j.jaci.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross MC, et al. 16S gut community of the Cameron County Hispanic Cohort. Microbiome. 2015;3:7. doi: 10.1186/s40168-015-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai V, et al. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutrition J. 2009;8:49. doi: 10.1186/1475-2891-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman RW, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravel J, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl. 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]