Abstract
Background
Skin lesions are common in advanced HIV infection and are sometimes caused by serious diseases like systemic mycoses (SM). AIDS-related SM endemic to Western Cape, South Africa, include emergomycosis (formerly disseminated emmonsiosis), histoplasmosis, and sporotrichosis. We previously reported that 95% of patients with AIDS-related emergomycosis had skin lesions, although these were frequently overlooked or misdiagnosed clinically. Prospective studies are needed to characterize skin lesions of SM in South Africa and to help distinguish these from common HIV-related dermatoses.
Methods
We prospectively enrolled HIV-infected adult patients living in Western Cape, South Africa, with CD4 counts ≤100 cells/μL and widespread skin lesions present ≤6 months that were deemed clinically compatible with SM. We obtained skin biopsies for histopathology and fungal culture and collected epidemiological and clinical data.
Results
Of 34 patients enrolled and in whom a diagnosis could be made, 25 had proven SM: 14 had emergomycosis, and 3 each had histoplasmosis and sporotrichosis; for 5 additional patients, the fungal species could not be identified. Antiretroviral therapy (ART) had been initiated in the preceding 4 weeks for 11/25 (44%) patients with SM (vs no patients without SM). Plaques and scale crust occurred more frequently in patients with SM (96% vs 25%, P = .0002; and 67% vs 13%, P = .01, respectively).
Conclusions
Recent ART initiation and presence of plaques or scale crust should make clinicians consider SM in patients with advanced HIV infection in this geographic area. Clinical overlap between SM and other dermatoses makes early skin biopsy critical for timely diagnosis and treatment.
Keywords: Emergomyces africanus, emergomycosis, emmonsiosis, histoplasmosis, sporotrichosis
Cutaneous lesions are among the earliest and most common complications of HIV-1 infection, experienced at some point by 90% of HIV-infected persons [1]. The burden of HIV-associated skin disease may be even higher in resource-limited settings, influenced by poverty, climate, hygiene, and other poorly understood host factors [2]. Cutaneous lesions may also be early signs of serious and potentially fatal systemic diseases, and timely recognition of these lesions is a critical challenge for clinicians caring for patients with HIV [3].
In sub-Saharan Africa, the challenges of diagnosing and managing HIV-related skin diseases are compounded by high patient burdens, limited access to dermatologists [4], and a scarcity of published experience in these settings [2], specifically in patients with darker skin (ie, Fitzpatrick scale skin types IV to VI) [2, 5]. Moreover, geographically restricted diseases that are infrequently encountered elsewhere may exist in resource-limited settings [2]. Consequently, patients with treatable diseases may go undiagnosed and untreated.
The objectives for this study were to prospectively characterize HIV-infected patients with cutaneous lesions caused by systemic mycoses endemic to South Africa and to identify epidemiological and clinical findings that may help to distinguish these from other dermatoses.
METHODS
This study was performed at publicly funded health care facilities in the greater metropole of Cape Town, Western Cape Province, South Africa, from December 2014 to January 2016. We prospectively enrolled patients who met the following criteria: adults (age ≥ 18 years) with HIV-1 infection, CD4 lymphocyte count (CD4) ≤100 cells/μL, and widespread cutaneous lesions present for ≤6 months, which were considered by an attending or consulting clinician to be compatible with a systemic mycosis.
Demographic, historical, and clinical details were collected from patient interviews and chart reviews. Certain occupations including construction, forestry, agriculture, and horticulture/landscaping were designated a priori as likely to be associated with exposure to soil. Clinical photographs were taken and reviewed by an experienced dermatologist blinded to diagnosis. Skin lesion morphology and pattern of healing were recorded. Patients meeting all enrollment criteria provided informed written consent for enrollment as well as for obtaining potentially identifiable photographs for publications. Two skin biopsies were taken from each patient: 1 was fixed in formalin for histopathological examination (periodic acid-Schiff [PAS] and/or Grocott’s methenamine silver stain were performed on all cases), and the other was transferred in sterile saline for fungal culture. For the latter, Sabouraud agar was inoculated with pulverized tissue and incubated at 30°C for 6 weeks. All fungal growth was examined morphologically, and identification was confirmed using amplification and sequencing of the internal transcribed spacer (ITS) region of the rRNA gene using ITS2 and ITS4 primers [6]. In cases where yeasts were seen on histopathological examination but fungal cultures were negative, polymerase chain reaction (PCR) was performed on skin biopsy tissue (either unfixed or paraffin-embedded tissue) by amplification and sequencing of ITS. Blood was collected in BACTEC FAN aerobic bottles (Becton Dickinson, Franklin Lakes, New Jersey) and/or BACTEC Myco/F Lytic (Becton Dickinson) mycobacterial/fungal culture bottles for some cases, as determined by the attending clinicians. Urine was tested for Histoplasma antigen using an analyte-specific reagent enzyme immunoassay (EIA) targeting H. capsulatum galactomannan (Immuno-Mycologics [IMMY], Norman, Oklahoma). Cryptococcal antigen testing of serum used a lateral flow assay (IMMY). Other diagnostic tests were ordered by the treating doctors as clinically indicated.
A systemic mycosis was defined by the histological visualization of yeasts in the dermis, or isolation of a pathogenic fungus via culture from a normally sterile site. Patients were classified as having possible systemic mycoses if either of the antigen tests (Histoplasma antigenuria or cryptococcal antigenemia) were positive but the microbiological and histopathological tests were negative. Patients were considered to not have systemic mycoses if histopathological, microbiological, and antigen tests were all not suggestive of this diagnosis.
Statistical analysis included Fisher’s exact test for comparisons of categorical data and Mann-Whitney U test for comparisons of nonparametric variables using Social Science Statistics (http://www.socscistatistics.com/, accessed December 2016). Comparisons were made between patients with proven systemic mycoses and those with other diagnoses; in a sensitivity analysis, an additional patient with possible systemic mycosis was also included. We defined statistical significance as a P value ≤.05.
This study was approved by the human research ethics committees of University of Cape Town (HREC 138/2014), Stellenbosch University (N14/02/011), Institute of Tropical Medicine/University of Antwerp (ITG 926/14), and the institutional review boards of each hospital.
RESULTS
We screened 67 patients for enrollment during the study period. Twenty-eight patients were excluded: 4 patients declined participation, and 24 patients did not meet all enrollment criteria. Thirty-nine patients were thus enrolled in the study.
All patients had skin biopsies and clinical evaluations. In 5 patients, neither clinical nor histopathological diagnoses could be established, and they were excluded from further analysis. A diagnosis was made by histopathological examination of skin biopsy tissue in 31 patients; in 3 other patients, histopathological examination alone was insufficient to make the diagnoses; these were diagnosed by clinical or clinicopathological assessments.
Among the 34 patients included in the analysis, a diagnosis of systemic mycosis was proven by histopathological examination of skin biopsy in 25 patients. Of these, fungal species identity was proven by culture or molecular identification in 20 (80%). The fungal pathogens identified were E. africanus in 14 patients, and H. capsulatum and Sporothrix schenckii in 3 patients each. In 5 other patients with systemic mycoses proven by histopathological examination of skin, putative fungal pathogens could not be identified by culture (n = 4) and/or amplification and sequencing of ITS from either paraffin-embedded skin tissue (n = 3) or direct skin biopsy (n = 1). Histopathological examination was unable to reliably discern the causative pathogen (Figure 1).
Figure 1.
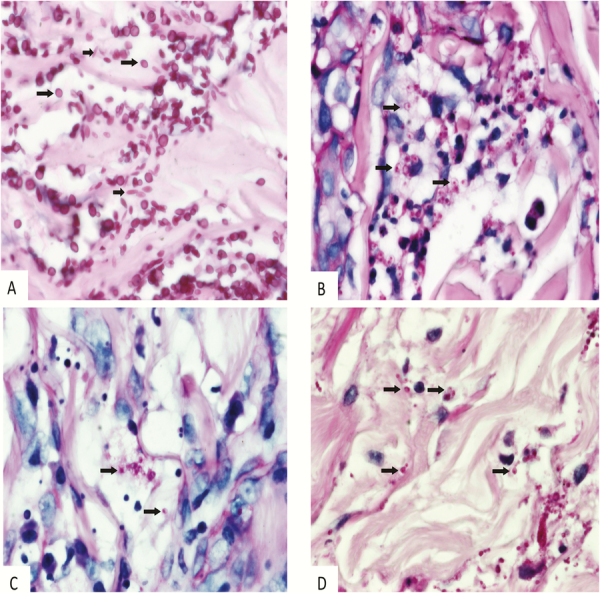
Histopathology of skin biopsies from patients with systemic mycoses. (A) Sporothrix schenckii. Ovoid yeast-like forms that measure 2–8 μm in size (long arrows), as well as elongated “cigar bodies” that vary in diameter from 2–4 and in length from 4–10 μm (short arrows). The larger size of the yeast-like forms and the presence of elongated forms are helpful to distinguish S. schenkii from Histoplasma capsulatum and Emergomyces africanus (Periodic Acid Schiff [PAS], ×1000). (B) H. capsulatum. Round to ovoid yeasts that vary in size from 2–3 to 3–5 μm with single budding nuclei and thin walls. Intra- and extracelluar oragnisms are present (arrows; PAS, ×1000). (C and D) E. africanus. Morphological features of fungal elements in tissue sections simulate the yeasts of H. capsulatum in particular (arrows; PAS, ×1000).
In 8 patients, a diagnosis other than systemic mycosis was made. In 4 patients, histopathological examination of skin biopsy tissue established alternative diagnoses including syphilis (n = 2), bacillary angiomatosis (n = 1), and cutaneous tuberculosis (n = 1). In 3 other patients, diagnoses were reached through clinicopathological correlation, including papular pruritic eruption (PPE, n = 2) and lichen planus (n = 1). In 1 patient, a clinical diagnosis was made of bacillary angiomatosis, supported by therapeutic response of classically appearing skin lesions to empiric doxycycline (despite a nondiagnostic skin biopsy); serology was not performed.
For 1 additional patient, a diagnosis of possible systemic mycosis was made. For all comparative analyses between patients with and without systemic mycoses, the inclusion of this patient did not significantly affect results. This patient is not considered in subsequent analyses or in the tables.
Epidemiological details and clinical histories of patients are presented in Table 1. Cutaneous lesions began ≤4 weeks after ART initiation in 11 (44%) patients with proven systemic mycoses; in contrast, in no patients without proven systemic mycoses did lesions begin ≤4 weeks after ART initiation. Preexisting skin lesions worsened (became larger or more numerous) following ART initiation in 5 patients with systemic mycoses, but not in any patients without systemic mycoses. Fever, cough, and recent weight loss were common among all patients enrolled and did not differ between patients with and without systemic mycoses. Comparing patients with and without systemic mycoses failed to reveal significant differences in duration of skin lesions or subjective features of skin lesions (ie, presence of pruritus or painfulness).
Table 1.
Epidemiological Features and Clinical Histories of Study Patients With Advanced HIV Infection and Recent-Onset, Widespread Skin Lesions Suspicious for Systemic Mycoses
| Epidemiological or Clinical Characteristics | Systemic Mycoses (n = 25) |
Other Diagnoses (n = 8)a |
P Value |
|---|---|---|---|
| Age, median (IQR), y | 35 (31–42) | 36 (35–45) | .5 |
| Male | 20 (75) | 5 (63) | .4 |
| Occupational risk for exposure to disturbed soil | 8 (32) | 0 (0) | .2 |
| Appearance of skin lesions ≤4 weeks after ART initiation | 11 (44) | 0 (0) | .03 |
| Preexisting skin lesions that worsened after ART initiation | 5 (20) | 0 (0) | .3 |
| Duration of skin lesions, median (IQR), wk | 4 (3–10) | 8 (4–12) | .6 |
| Skin lesions pruritic | 7 (28) | 2/6 (33) | 1.0 |
| Skin lesions painful | 5 (20) | 1/6 () | 1.0 |
| Fever | 15/22 (68) | 4/7 (57) | .7 |
| Weight loss | 23 (92) | 4/5 (80) | .4 |
| Cough | 17 (68) | 3/6 (50) | .6 |
| Upper respiratory tract complaint | 16 (64) | 2 (25) | .2 |
Data are number/total number (%) unless otherwise specified.
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
aOther diagnoses included tuberculosis (n = 1), syphilis (n = 2), papular pruritic eruption (n = 2), bacillary angiomatosis (n = 2), and lichen planus (n = 1).
Photographs of cutaneous lesions were collected for 33 patients (97%). Characteristics of cutaneous lesions by examination are presented in Table 2. In most patients, polymorphic skin lesions were noted on examination. Cutaneous plaques, scale crusts, and rims of erythema were more common in patients with systemic mycoses (P = .0002, P = .01, and P = .01, respectively), while papules, nodules, patches, and ulcers were common in both groups. Photographs of selected cutaneous lesions in patients diagnosed with systemic mycoses are shown in Figure 2.
Table 2.
Morphology of Skin Lesions in Patients With Advanced HIV Infection and Recent-Onset, Widespread Skin Lesions Suspicious for Systemic Mycoses
| Systemic Mycoses (n = 24) | Other Diagnoses (n = 8) a | P Value | |
|---|---|---|---|
| Plaques | 23 (96) | 2 (25) | .0002 |
| Papules | 19 (79) | 5 (63) | .4 |
| Patches | 6 (25) | 2 (25) | 1.0 |
| Nodules | 24 (100) | 8 (100) | 1.0 |
| Ulceration | 17 (71) | 5 (63) | .7 |
| Scale crust | 16 (67) | 1 (13) | .01 |
| Dry scale | 12 (50) | 3 (38) | .7 |
| Erythema | 22 (92) | 6 (75) | .3 |
| Rim of erythema | 16 (67) | 1 (13) | .01 |
Data are number/total number (%). Data missing for 1 patient with systemic mycosis.
aOther diagnoses included tuberculosis (n = 1), syphilis (n = 2), papular pruritic eruption (n = 2), bacillary angiomatosis (n = 2), and lichen planus (n = 1).
Figure 2.
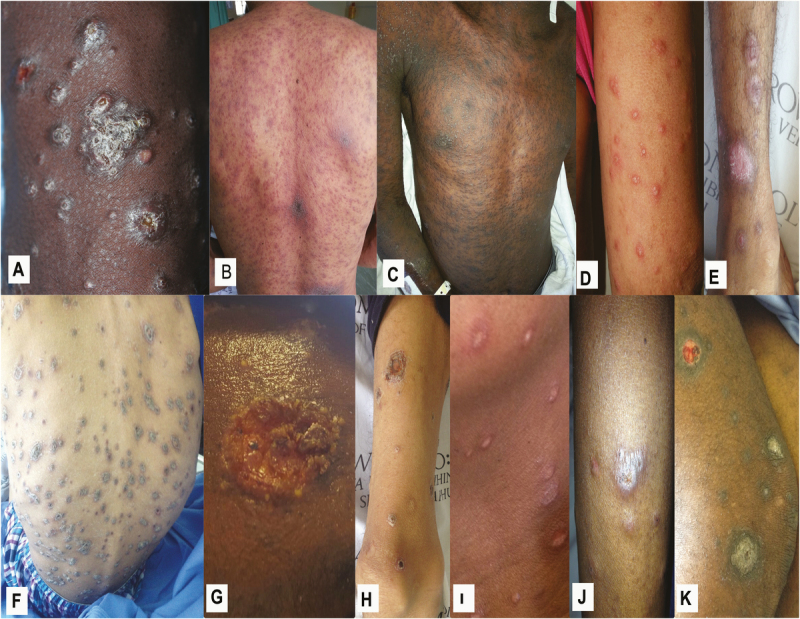
Selected cutaneous lesions of persons with advanced HIV infection and generalized, recent-onset skin lesions suspicious for systemic mycoses. Lesions shown are from patients with proven systemic mycoses caused by Emergomyces africanus (A–E), Sporothrix schenckii (F–H), and Histoplasma capsulatum (I–K).
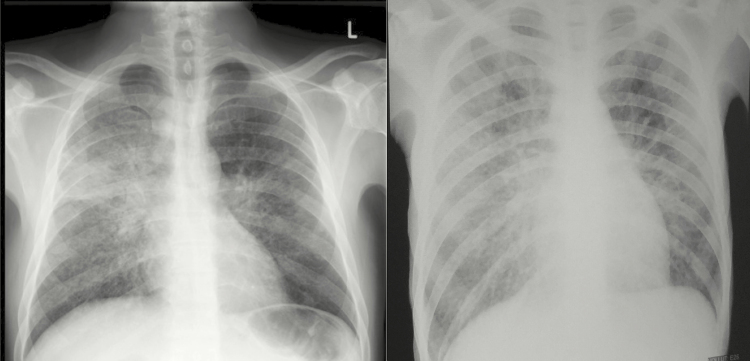
Among all patients enrolled, the median CD4 count was 28 cells/µL (interquartile range [IQR], 10–45 cells/µL), with no significant differences between patients with and without systemic mycoses. There were no significant differences in the proportion of patients with abnormal chest x-rays, anemia, or liver enzyme abnormalities according to whether systemic mycoses were diagnosed (data not shown). Examples of typical chest x-ray abnormalities in patients with HIV-associated emergomycosis are shown in Figure 3.
Figure 3.
Chest x-rays from 2 HIV-infected patients with E. africanus infection diagnosed by skin biopsy and in whom pulmonary tuberculosis was excluded. (A) Diffuse, bilateral reticulonodular infiltrates. (B) Bilateral reticulonodular infiltrates with multifocal airspace disease and bilateral hilar involvement.
Among patients diagnosed with systemic mycoses, fungal culture and PCR of skin biopsy tissue yielded the diagnosis in 17/22 (77%) and 2/7 (14%) of cases, respectively. Aerobic and mycobacterial/fungal blood cultures were positive in 0/11 and 4/17 patients, respectively. Urine Histoplasma antigen was detected in 4/17 (24%) of cases, including 3/10 patients with emergomycosis (23.99, 2.46, and 5.39 EIA units, respectively), 2/2 patients with histoplasmosis (54.97 and 89.43 EIA units, respectively), and none of 2 patients with sporotrichosis tested.
Of 25 patients who ultimately were diagnosed with systemic mycoses, 6 (24%) died, 17 (68%) survived, and 2 (8%) were lost to follow-up. For 17 survivors, the median follow-up duration was 12 months (IQR 10–15 months). One death occurred prior to diagnosis; skin biopsy histopathology and fungal cultures were only reported as positive after death. Treatment with amphotericin B deoxycholate (1 mg/kg) was started in 24. Among these, 1 patient died within 24 hours and another within 48 hours of initiating therapy. Twenty-two patients (88%) completed 10–14 days of amphotericin B; thereafter, 21 (84%) were switched to itraconazole, and another 1 patient left against medical advice without further therapy (and subsequently relapsed after 8 months). Three patients (12%) died during the itraconazole phase at 2, 8, and 10 weeks after completion of amphotericin B, respectively; systemic mycoses were implicated in the causes of death, with documented noncompliance to itraconazole and ART for 1 patient. Two patients (8%), both of whom survived, experienced skin biopsy culture-positive relapses despite reported compliance to itraconazole: 1 patient with sporotrichosis developed worsening cutaneous lesions after 2 weeks of itraconazole, and another with emergomycosis developed dense fleshy lesions on his nose 7 months into itraconazole therapy. Two patients (8%), both foreign nationals, were lost to follow-up at 1 month after discharge and could not be traced.
Antiretroviral therapy was an integral component of management for all patients. For patients with systemic mycoses who were ART-naïve, ART was initiated 14 days after starting amphotericin B (ie, usually corresponding with de-escalation to itraconazole). In patients who had defaulted ART, these were restarted with adherence counseling at 14 days after starting antifungal therapy. Where patients were already taking ART, this was continued or modified if there was evidence of virological failure or concern of drug-drug interactions. For both ART-naïve and -experienced patients, regimens with lopinavir/ritonavir were selected with itraconazole dose adjustment (to 200 mg daily) were selected over those with efavirenz due to drug-drug interactions known to increase exposure with the former and decrease exposure with the latter [7]. No cases of paradoxical immune reconstitution inflammatory syndrome (IRIS) were reported.
DISCUSSION
In this cross-sectional observational study, we characterized the clinicopathological spectrum and microbiological investigations of South African adults with advanced HIV infection and recent-onset, widespread cutaneous lesions. Features that were significantly associated with systemic mycoses in this cohort included recent initiation of ART and the presence of plaques and scale crust.
In the greater metropole of Cape Town, emergomycosis was the most common systemic mycosis diagnosed in this patient population: 14 patients were diagnosed with emergomycosis, versus 3 each with histoplasmosis and sporotrichosis over a 14-month enrollment period.
This study confirms prior reports from endemic areas that systemic mycoses frequently present soon after starting ART, suggesting an unmasking IRIS [8, 9]. Cutaneous lesions began within 4 weeks following ART initiation in 40% of patients with systemic mycoses. This is consistent with the findings of Nacher et al. from French Guyana, who reported that HIV-infected patients who were initiated on ART within the preceding 2 months were more likely to be diagnosed with disseminated histoplasmosis compared with untreated patients [9]. Although unmasking IRIS after ART initiation occurs with other diseases [10], our findings suggest that the appearance or worsening of cutaneous lesions after ART initiation should prompt consideration of systemic mycoses in endemic areas. In contrast with apparent episodes of unmasking IRIS, no episodes of paradoxical IRIS were observed [11].
A retrospective study of South African patients with proven emergomycosis reported on the yields of diagnostic investigations [8]. Whereas in the retrospective series 9 out of 37 patients (24%) had aerobic blood cultures positive for E. africanus [8], in our prospective series aerobic blood cultures were positive in none of the 11 patients with systemic mycoses, including 6 patients with emergomycosis. In contradistinction to the findings of the retrospective series, which reported the isolation of E. africanus from mycobacterial/fungal blood culture bottles in 15 of 25 (60%) patients with emergomycosis [8], we found that these samples were positive in just 4 of 17 patients (24%) with proven systemic mycoses, including 3 of 11 (27%) with emergomycosis. The reason for the lower yield of diagnostic tests in the present study is likely a reflection of ascertainment bias in the prior study; that is, the inclusion only of patients in whom E. africanus was successfully cultured inflated the yield of culture-based investigations.
Systemic mycoses carry poor prognoses in patients with advanced immunodeficiencies including HIV [12]. Among 52 immunocompromised South African patients reviewed with emergomycosis, the case-fatality rate was 50% [8]. Of 25 patients with proven systemic mycoses in the current series, 24% died and another 12% were lost to follow-up. Among 14 patients with proven emergomycosis, 21% died and 14% were lost to follow-up. The apparent lower case-fatality rate in the present study likely reflects earlier diagnosis of patients that resulted from performing skin biopsies: In the retrospective series of immunocompromised patients with emergomycosis, deaths occurred in 13 of 36 (39%) patients in whom skin biopsies were performed, compared with 12 of 16 (75%) patients in whom skin biopsies were not performed [8]. Of those 12 patients, only in 2 (17%) was an antemortem diagnosis of a systemic mycosis established [8]. Taken together, these data suggest that skin biopsies play a crucial role in the timely diagnosis and, ultimately, survival of immunocompromised patients with systemic mycoses.
Our study does have important limitations. Because we only enrolled patients clinically suspected by infectious disease physicians, dermatologists, and/or HIV practitioners of having systemic mycoses, these results are not generalizable to patients in whom systemic mycoses are not suspected. Moreover, the specific variables that guided these clinical assessments were left undefined, and the decision of whether a systemic mycosis was suspected instead relied on the clinical judgement of individual physicians. On the other hand, our retrospective data suggested protean clinical manifestations of emergomycosis [8], the most common AIDS-related systemic mycosis endemic to our setting, and use of rigid eligibility criteria (such as presence of fever and/or an abnormal chest x-ray, as examples) in the current study would likely have excluded some cases of systemic mycoses. Alternatively, the recruitment strategy may have inadvertently selected for confounders and/or introduced selection bias. For instance, recruitment may have been biased toward patients who were more ill or more likely to access specialist care. Another limitation of our study is the small sample size, which precluded multivariate analyses of factors associated with systemic mycoses. In addition, the small number of patients with histoplasmosis and sporotrichosis limits comparisons between mycoses. It is also important to note that not all investigations were performed in all patients, which may have resulted in misclassification of patients and limits our ability to evaluate some diagnostic tests. Finally, it is possible that patients were misclassified as not having systemic mycoses due to limitations in available diagnostic tests (eg, due to sampling error of skin biopsies).
Given the existence of overlap in the clinical presentations of systemic mycoses compared with other dermatoses in patients with advanced HIV infection, and the fact that AIDS-related systemic mycoses are universally fatal without treatment [8, 11, 13], it is critical that effective diagnostic tools are available for clinicians in endemic areas. Fortunately, skin biopsy for histological examination is an existing test with many favorable characteristics: It is simple to perform, relatively noninvasive, inexpensive, has the potential to provide results rapidly, and is sensitive for diagnosing systemic mycoses involving the skin. Nonetheless, our own experiences suggest that HIV-related cutaneous lesions are infrequently biopsied in South Africa, begging the question: Why not? Possible factors include high burdens of HIV-infected patients, failure of clinicians to recognize the significance of cutaneous lesions in systemically ill HIV-infected patients, limited access to dermatologists and dermatopathologists, lack of training in performing skin biopsies, and inadequate access to biopsy instruments. Attenuating the morbidity and mortality caused by AIDS-related mycoses will require addressing these challenges.
Acknowledgments
The authors gratefully acknowledge the participation of all patients. We also wish to acknowledge the referring clinicians. We specifically wish to acknowledge the following clinicians: Philip Botha, Tom Boyles, Tabie Greyling, Prue Ive, Nectarios Papavarnavas, Altaaf Parker, Prabash Sadhai, Katharina Starzacher, Susan Hugo, Marije Van Schalkwyck, and Zahira Ismail. We also wish to acknowledge the following microbiologists: Prenesh Naicker, Justina Wojno, Andrew Whitelaw, Colleen Bamford, Kim Hoek, Mae Newton-Foot, and Tsidiso Maphanga.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Coldiron BM, Bergstresser PR, Farthling CF et al. . Prevalence and clinical spectrum of skin disease in patients infected with human immunodeficiency virus. Arch Dermatol 1989; 125:357–61. [PubMed] [Google Scholar]
- 2. Amerson EH, Maurer TA. Dermatologic manifestations of HIV in Africa. Top HIV Med 2010; 18:16–22. [PubMed] [Google Scholar]
- 3. Tschachler E, Bergstresser PR, Stingl G. HIV-related skin diseases. Lancet 1996; 348:659–63. [DOI] [PubMed] [Google Scholar]
- 4. Tsang MW, Kovarik CL. Global access to dermatopathology services: physician survey of availability and needs in sub-Saharan Africa. J Am Acad Dermatol 2010; 63:346–8. [DOI] [PubMed] [Google Scholar]
- 5. Motswaledi MH, Visser W. The spectrum of HIV-associated infective and inflammatory dermatoses in pigmented skin. Dermatol Clin 2014; 32:211–25. [DOI] [PubMed] [Google Scholar]
- 6. White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR Protocols: A Guide to Methods and Applications. New York: Academic Press, Inc; 1990:315–22. [Google Scholar]
- 7. Vadlapatla RK, Patel M, Paturi DK et al. . Clinically relevant drug-drug interactions between antiretrovirals and antifungals. Expert Opin Drug Metab Toxicol 2014; 10:561–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwartz IS, Govender NP, Corcoran C et al. . Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis 2015; 61:1004–12. [DOI] [PubMed] [Google Scholar]
- 9. Nacher M, Sarazin F, El Guedj M et al. . Increased incidence of disseminated histoplasmosis following highly active antiretroviral therapy initiation. J Acquir Immune Defic Syndr 2006; 41:468–70. [DOI] [PubMed] [Google Scholar]
- 10. Osei-Sekyere B, Karstaedt AS. Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol 2010; 35:477–81. [DOI] [PubMed] [Google Scholar]
- 11. Wheat LJ, Freifeld AG, Kleiman MB et al. ; Infectious Diseases Society of America Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong-James D, Meintjes G, Brown GD. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol 2014; 22:120–7. [DOI] [PubMed] [Google Scholar]
- 13. Freitas DF, de Siqueira Hoagland B, do Valle AC et al. . Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med Mycol 2012; 50:170–8. [DOI] [PubMed] [Google Scholar]