Abstract
Cryptococcus gattii infections, especially including those with severe clinical manifestations, may be related to underlying host immunologic factors. We present 2 cases with autoantibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF), a key cytokine in macrophage function. Immunologic evaluation for anti-GM-CSF antibodies may be important to inform management and counseling.
Keywords: cryptococcus, GM-CSF autoantibodies, immune deficits, spinal epidural abscess
Cryptococcus gattii is an emerging infectious disease with expanding clinical manifestations and geographic distributions. Recently it has become clear that underlying host immunologic factors may explain some of these infections in previously healthy adults. We describe 2 cases with severe manifestations of C. gattii infection who were discovered to have autoantibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF). These cases exemplify the role of GM-CSF in the protection against C. gattii infection and highlight the role of anti-GM-CSF autoantibodies in cases with disseminated disease.
METHODS
Patients underwent immunologic work-up including complete blood counts, immunoglobulin levels, HIV testing, and CD4+ and CD8+ cell counts and percentages.
Additional immunologic testing was performed as previously described [1]. Specifically, plasma from each patient was screened for select anticytokine autoantibodies using a particle-based technology [2]. Fluorescing magnetic beads were conjugated to 2.5 mcg each of recombinant human GM-CSF, INF-gamma, and IL-17A. Beads were combined and incubated for 1 hour with subject or control plasma at 1:100 dilution, washed, and incubated with phycoerythrin-labeled antihuman IgG before being run on the Bio-Plex (Bio-Rad) instrument. Fluorescence intensity for each bead type was plotted as a function of antibody titer.
To demonstrate that patient plasma samples were able to block GM-CSF signaling, healthy control peripheral blood mononuclear cells (5 × 105 cells) were cultured in complete RPMI media containing control or patient plasma (10%) and left unstimulated or stimulated with GM-CSF (10 ng/mL; R&D) for 30 minutes at 37°C. Monocytes were identified by CD14 (BD Pharmingen) surface staining before being fixed and permeabilized for intracellular staining with phosphorylated signal transducer and activator of transcription 5 (pSTAT-5) (Y694) antibody (BD Pharmingen), as previously described [1]. Data were collected using FACSCalibur (BD Biosciences), analyzed using FlowJo (Treestar), and graphed with Prism5 (Graphpad).
RESULTS
Case 1
A 42-year-old Caucasian man presented with acute lower extremity paralysis over 24 hours. He reported a chronic cough and progressive constipation. He denied trauma or prior medical conditions. He lived in southern California and denied any recent travel or other specific exposures.
Examination revealed a temperature of 37.0oC, blood pressure of 133/72 mmHg, heart rate of 135/minute, respiratory rate of 16/minute, and oxygen saturation of 97% on room air. He had weakness of the lower extremities (1/5 strength of hip and knee flexors and extensors) and reduced sensation and deep tendon reflexes. Rectal tone was mildly diminished, and he had urinary retention. Cranial nerve examination was normal, and there was no papilledema. There was point tenderness over the second lumbar spine.
His white blood count was 14 000/mm3 with 83% neutrophils, hemoglobin 12.8 g/dL, platelets 305/mm3, erythrocyte sedimentation rate (ESR) 67 mm/h, and C-reactive protein 80.2 mg/L. Urinalysis and blood cultures were unremarkable. A magnetic resonance image (MRI) of the lumbar spine with and without gadolinium showed a L2 pathologic fracture with osteomyelitis and a large epidural abscess as well as left paraspinal abscess with extension into the psoas muscle (Figure 1A).
Figure 1.
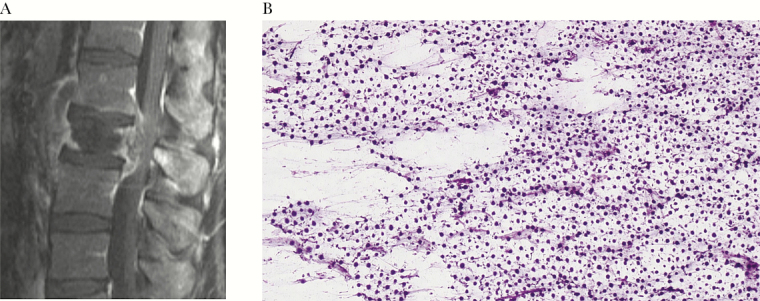
(A) Spinal epidural abscess with pathologic fracture of the second lumber vertebrae and osteomyelitis due to Cryptococcus gattii. (B)Cryptococcus gattii on Gomori methenamine silver stain of the surgical frozen specimens, 400× magnification.
Initial antimicrobial treatment included vancomycin, cefepime, and metronidazole for suspected bacterial spinal epidural abscess. Emergent decompressive laminectomies (L1-L3) with abscess drainage identified an encapsulated cavity within the epidural space with purulent material and severe destruction of the lumbar vertebrae. Frozen sections demonstrated sheets of encapsulated organisms consistent with Cryptococcus sp. (Figure 1B); 3 intraoperative specimens grew C. gattii, while bacterial and acid-fast bacilli cultures were negative. Cryptococcus serum antigen was positive at 1:5, and chest computed tomography (CT) scan revealed a 4.2-cm left upper lobe cavitary mass. MRI of the brain with and without gadolinium was unremarkable; lumbar puncture was deferred given the presence of the lumbar spinal infection at the location in which the procedure is typically performed and the lack of clinical or imaging findings of increased intracranial pressure.
Antimicrobials were changed to liposomal amphotericin B (AmBisome 4mg/kg iv daily) and flucytosine (1000 mg po every 6 hours). In addition, steroids (dexamethasone at 1 mg/kg/d, followed by a prednisone taper) were added to reduce spinal edema and inflammation. Molecular characterization of the C. gattii isolate was VGIII, and susceptibility testing showed minimum inhibitory concentrations (MICs) for amphotericin of 1 µg/mL, flucytosine of 16 µg/mL, fluconazole 8 of µg/mL, posaconazole of 0.5 µg/mL, and voriconazole of 0.12 µg/mL.
The patient’s immune system was evaluated. HIV and hepatitis panels were negative. CD4+ T cell count was 548 cells/mm3 and 29% (normal ranges, 365–1450 and 30%–60%), but CD8+ T cell count was low at 113 cells/ mm3 and 6% (normal ranges, 180–825 and 16%–40%). Autoantibodies to GM-CSF were identified, and the patient’s plasma blocked GM-CSF-induced STAT-5 phosphorylation in normal monocytes; autoantibodies to gamma-interferon and interleukin-17A were not detected (Figure 2).
Figure 2.
Laboratory evaluation of anti–granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies in patient plasmas. (A) Multiplex screen for anticytokine autoantibodies against GM-CSF, IFNg, and IL-17A in patient plasmas. Bars represent the mean and range values for healthy controls (n = 10). (B) Normal peripheral blood mononuclear cells were incubated in the presence of 10% normal or patient plasma and left unstimulated or stimulated with GM-CSF. CD14+ monocytes were analyzed for phosph-STAT5 by flow cytometry.
He had decompressive laminectomies on the day of admission, followed by an anterior corpectomy and fusion with cage placement for stabilization. Antifungal therapy consisted of 8 weeks of combination amphotericin B liposomal and flucytosine, followed by oral fluconazole 800 mg daily. The patient regained strength in his lower extremities during the 84-day hospitalization and ambulated without assistance at hospital discharge. He was advised to remain on fluconazole 400 mg daily as secondary prophylaxis.
Case 2
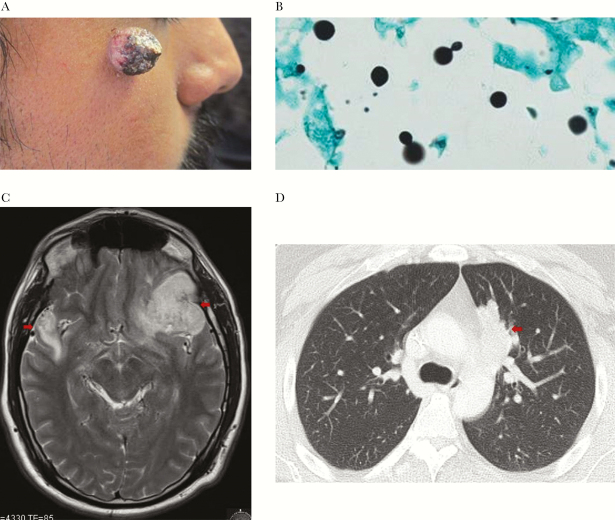
A 34-year-old Hispanic man presented with a 3-month history of a slowly enlarging, single facial lesion (Figure 3A). The patient also endorsed a 15-pound weight loss, night sweats, mild headaches, and bilateral visual acuity loss. He had no underlying medical problems and was born and resided in southern California.
Figure 3.
(A) Right face skin lesion due to cutaneous Cryptococcus gattii.(B) Skin biopsy, Gomori methenamine silver stain with 100× magnification with narrow based budding yeast consistent with Cryptococcus. (C) Magnetic resonance image of the brain showing irregular rim-enhancing intracranial masses involving the left frontal lobe and right temporal lobe (arrows). (D) Computed tomography scan of the chest with a 4.7-cm mass (arrow) in medial aspect of the left upper lobe.
Physical examination showed temperature of 36.9, blood pressure of 128/80, pulse of 95 beats per minute, respiratory rate of 16 breaths per minute, and an oxygen saturation of 98% on room air. Mild bilateral papilledema was seen of ophthalmoscopy with full extraocular motility. There were no other skin, neurologic, or other findings identified. Biopsy of his skin lesion showed yeast consistent with Cryptococcus sp. (Figure 3B).
Brain MRI with gadolinium showed rim-enhancing intracranial masses in the left frontal lobe, right temporal lobe, and left cerebellum with mass effect (Figure 3C). CT of the chest, abdomen, and pelvis revealed only a 4.7-cm left lung upper lobe mass (Figure 3D). Complete blood count, chemistry panel, and blood cultures were within normal limits. The cryptococcal serum antigen titer was weakly positive at 1:2. Amphotericin B liposomal (4 mg/kg/d), flucytosine (100mg/kg/d), and steroids were initiated.
Bronchoscopy was without lesions, and cultures were negative, but multiplex polymerase chain reaction (PCR; performed at the University of Washington) demonstrated C. gattii. Craniotomy with debridement of the frontal lesion confirmed the C. gattii diagnosis and reduced the size (≥3 cm) of the lesion. Respiratory and brain biopsy cultures were obtained after antifungal therapy had been initiated, likely accounting for the negative fungal culture results. Lumbar puncture revealed normal values for opening pressure (16 cm H2O), glucose (65mg/dL), and protein (48 mg/dL), but had an elevated white count of 17/µL comprised of 92% lymphocytes and 8% monocytes; cryptococcal antigen and stains for encapsulated yeasts were negative.
HIV and hepatitis panels were negative, CD4+ T cell count was normal at 675 cells/mm3 and 43%, and the CD8 count was normal at 424 cells/mm3 and 27%, respectively. Auto-antibodies to GM-CSF were identified, whereas autoantibodies to gamma-interferon and interleukin-17A were not detected (Figure 2).
After 10 weeks of amphotericin B liposomal and flucytosine, the patient was transitioned to oral fluconazole 800 mg daily and a steroid taper. The patient remained on this therapy for ~12 months, with gradual cessation of steroids. Given the identified underlying immune abnormality, the patient will remain on secondary prophylaxis with fluconazole 200–400 mg daily.
DISCUSSION
C. gattii diverged from C. neoformans several millions of years ago [3] and has become an increasingly recognized pathogen. C. gattii infections typically occur in tropical and subtropical areas and have been associated with exposure to eucalyptus trees as well as other types of vegetation. This organism has emerged as an important infection in an expanding number of areas including the southwestern United States and Australia as well as Spain, Italy, France, and the Netherlands [4]. Strains causing human disease have emerged in North America, likely via spread from South America [3]. As such, knowledge of this infectious agent and the potential for underlying immunologic factors predisposing to this emerging infection are important.
Life-threatening manifestations of C. gattii infections occur. While spinal granulomas/abscesses are rarely part of disseminated C. neoformans infection in immunosuppressed persons (eg, with AIDS, rheumatoid arthritis) [5, 6], this is the first known report of C. gattii spinal epidural abscess resulting in paraplegia. The second case exemplifies the cutaneous and brain manifestations of cryptococcal infections.
C. gattii often affects seemingly immunocompetent adults, but may also occur in those with cellular immunocompromise including HIV infection [7]. Among otherwise immunocompetent persons, newly recognized immune deficits have been recently described that may explain the underlying risks for developing cryptococcosis. The combination of environmental exposure to Cryptococcus after contact with vegetation/soil [4] and host susceptibility factors likely explains its occurrence in select persons. HIV testing and CD4 enumeration should be obtained to exclude HIV infection and idiopathic CD4 lymphopenia, respectively. Additional genetic variants may coexist with CD4 lymphopenia (“multi-hit model”) [8]. Autoantibodies to GM-CSF appear to predispose to severe C. gattii infections [1]. Similarly, autoantibodies to INF-gamma predispose to disseminated mycobacterial infections [9, 10], and anti-IL-17 autoantibodies are associated with chronic mucocutaneous candidiasis [11, 12].
In both of our cases, autoantibodies to GM-CSF were identified. Earlier studies showed a link between GM-CSF autoantibodies and acquired pulmonary alveolar proteinosis (PAP) [13]. In those cases, defects in alveolar macrophage function including reduced chemotaxis, phagocytosis, and microbicidal activity were noted [14, 15]. The subsequent identification of a patient with both PAP and cryptococcal meningitis led to an investigation of whether anti-GM-CSF autoantibodies were specifically associated with cryptococcal infections [1]. Four patients with cryptococcal meningitis (3 with C. neoformans and 1 with C. gattii) tested positive for anti-GM-CSF-autoantibodies [1]. Additional work included evaluating 67 archived blood and CSF samples of immunocompetent patients with cryptococcal meningitis; 3 (all with C. gattii) were found to have anti-GM-CSF autoantibodies, while none of the control patients had them [1]. In another study, plasma from 30 patients with cryptococcal meningoencephalitis had speciation of isolates to C. neoformans or C. gattii; 7 of the 9 patients with C. gattii were found to have GM-CSF autoantibodies, but no anti-GM-CSF autoantibodies were noted among those with C. neoformans [16].
GM-CSF plays a critical role in the host immune response to infection as a regulator of phagocyte and pulmonary macrophage function, which are important in the initial immune response after inhalation of Cryptococcus. Further, GM-CSF is a key factor for dendritic cell development under both steady-state and inflammatory conditions [17]. Autoantibodies to GM-CSF disrupt these important immunologic controls. Interestingly, C. gattii itself may cause a reduction in T-cell and natural killer cell production of GM-CSF, further hampering GM-CSF responses to fungi, as shown in a rat model [18]. Additionally, the fungus may inhibit production of TNF-alpha, an important cytokine in promoting dendritic cell maturation and T-cell-mediated immunity. Dendritic cells recognize and phagocytize C. gattii through a process that depends on maturation in the presence of TNF-alpha. Furthermore, C. gattii can evade immune response by failing to induce dendritic cell maturation and release of cytokines [19]. Overall, autoantibodies against GM-CSF and reduced production of GM-CSF and TNF-alpha likely impair cellular immunity against C. gattii infection and allow for fungal dissemination [16, 19]. Our patient plasmas prevented GM-CSF-induced STAT-5 phosphorylation in normal monocytes. The reasons for the development of autoantibodies against GM-CSF as well as the duration of antibodies prior to disease remain unknown.
Identifying GM-CSF autoantibodies may have clinical implications for understanding the pathogenesis of the infections as well as for primary treatment, secondary prophylaxis, and the risk for associated conditions such as PAP. In our cases, the detection of GM-CSF autoantibodies assisted in understanding our patients risk factors and management. C. gattii infections are typically managed similarly to C. neoformans [20], despite a paucity of specific literature. Disseminated disease is typically treated with amphotericin and flucytosine, followed by fluconazole. Severe C. gattii infections in the brain or spinal fluid may also benefit from transient suppression of host inflammatory responses (with corticosteroids) along with microbiological control [21].
Additional treatment considerations for patients with anti-GM-CSF autoantibodies may include exogenous GM-CSF [22] and/or B-cell-directed therapy (rituximab), especially among those with poor responses to standard therapies. Both inhaled and subcutaneous GM-CSF therapies have been trialed among patients with PAP and GM-CSF autoantibodies, with improvement of alveolar arterial gradients and symptomatic disease [23, 24]. Rituximab has also been utilized for PAP [25] and other autoantibody-related infections (eg, mycobacterial infections in the setting of anti-INF-gamma autoantibodies) [26], their role among patients with cryptococcal disease remains unclear.
After the successful treatment of the infection, those with persistent underlying immune deficits should be considered for prolonged secondary antifungal prophylaxis given the risk for recurrence. Among HIV-infected persons with low CD4 counts, maintenance therapy is advised per the Infectious Diseases Society of America guidelines [20]. Similarly, ongoing prophylaxis in the setting of persistent GM-CSF autoantibodies seems prudent, despite a lack of prospective studies. Both of our cases were advised to continue fluconazole indefinitely, as long as their titers of autoantibodies remain elevated. Preemptive counseling regarding the risk of other complications such as PAP [1] should also be considered. Neither of our cases had PAP on presentation, but each patient was counseled on its potential future occurrence. The timing and reasoning for why some but not all patients with GM-CSF autoantibodies develop 1 vs both conditions remain unknown. Further, whether the presence of GM-CSF autoantibodies predicts worse outcomes among those with C. gattii infection, mirroring the previously published link between other immune deficits (eg, CD4 lymphopenia) and poor outcomes [27], remains to be defined. GM-CSF autoantibodies have also been associated with severe extrapulmonary Nocardia infections [28], although so far no patients have been identified with both Nocardia and Cryptococcus disease.
The current cases illustrate the emerging infection C. gattii, which has an expanding geographic distribution and variable clinical manifestations. Underlying immunologic factors should be sought to inform management decisions. Testing for anti-GM-CSF autoantibodies, which predispose to disseminated C. gattii infections, should be considered. The triggers for development of anticytokine autoantibodies, their role in disease pathogenesis, and immune-directed treatment for these life-threatening infections are areas of future research.
Acknowledgements
Financial support. There is no funding for this work and none that applies to the manuscript.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rosen LB, Freeman AF, Yang LM et al. . Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 2013; 190:3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ding L, Mo A, Jutivorakool K et al. . Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol 2012; 32:238–45. [DOI] [PubMed] [Google Scholar]
- 3. Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev 2014; 27:980–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris J, Lockhart S, Chiller T. Cryptococcus gattii: where do we go from here? Med Mycol 2012; 50:113–29. [DOI] [PubMed] [Google Scholar]
- 5. Rambeloarisoa J, Batisse D, Thiebaut JB et al. . Intramedullary abscess resulting from disseminated cryptococcosis despite immune restoration in a patient with AIDS. J Infect 2002; 44:185–8. [DOI] [PubMed] [Google Scholar]
- 6. Zhou HX, Ning GZ, Feng SQ et al. . Cryptococcosis of lumbar vertebra in a patient with rheumatoid arthritis and scleroderma: case report and literature review. BMC Infect Dis 2013; 13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinel-Ingroff A, Kidd SE. Current trends in the prevalence of Cryptococcus gattii in the United States and Canada. Infect Drug Resist 2015; 8:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panackal AA, Rosen LB, Uzel G et al. . Susceptibility to cryptococcal meningoencephalitis associated with idiopathic CD4(+) lymphopenia and secondary germline or acquired defects. Open Forum Infect Dis 2017; 4: doi: 10.1093/ofid/ofx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel SY, Ding L, Brown MR et al. . Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol 2005; 175:4769–76. [DOI] [PubMed] [Google Scholar]
- 10. Höflich C, Sabat R, Rosseau S et al. . Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 2004; 103:673–5. [DOI] [PubMed] [Google Scholar]
- 11. Puel A, Döffinger R, Natividad A et al. . Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 2010; 207:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kisand K, Bøe Wolff AS, Podkrajsek KT et al. . Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 2010; 207:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uchida K, Beck DC, Yamamoto T et al. . GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 2007; 356:567–79. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka N, Watanabe J, Kitamura T et al. . Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte- macrophage colony stimulating factor. FEBS Lett 1999; 442:246–50. [DOI] [PubMed] [Google Scholar]
- 15. Kitamura T, Tanaka N, Watanabe J et al. . Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 1999; 190:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saijo T, Chen J, Chen SC et al. . Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. MBio 2014; 5:e00912–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012; 119:3383–93. [DOI] [PubMed] [Google Scholar]
- 18. Chen GH, Curtis JL, Mody CH et al. . Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol 1994; 152:724–34. [PubMed] [Google Scholar]
- 19. Huston SM, Li SS, Stack D et al. . Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J Immunol 2013; 191:249–61. [DOI] [PubMed] [Google Scholar]
- 20. Perfect JR, Dismukes WE, Dromer F et al. . Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta GU, Panackal AA, Murayi R et al. . Corticosteroids for shunted previously healthy patients with non-HIV cryptococcal meningoencephalitis. J Neurol Neurosurg Psychiatry 2017; doi: 10.1136/jnnp-2017-315830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tascini C, Vecchiarelli A, Preziosi R et al. . Granulocyte-macrophage colony- stimulating factor and fluconazole enhance anti-cryptococcal activity of monocytes from AIDS patients. AIDS 1999; 13:49–55. [DOI] [PubMed] [Google Scholar]
- 23. Venkateshiah SB, Yan TD, Bonfield TL et al. . An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest 2006; 130:227–37. [DOI] [PubMed] [Google Scholar]
- 24. Tazawa R, Trapnell BC, Inoue Y et al. . Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2010; 181:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borie R, Debray MP, Laine C et al. . Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J 2009; 33:1503–6. [DOI] [PubMed] [Google Scholar]
- 26. Browne SK, Zaman R, Sampaio EP et al. . Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012; 119:3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen SC, Slavin MA, Heath CH et al. ; Australia and New Zealand Mycoses Interest Group (ANZMIG)-Cryptococcus Study Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis 2012; 55:789–98. [DOI] [PubMed] [Google Scholar]
- 28. Rosen LB, Rocha Pereira N, Figueiredo C et al. . Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis 2015; 60:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]