Abstract
Background
Urogenital schistosomiasis due to Schistosoma hematobium infection is hypothesized to cause increased HIV-1 RNA shedding in semen in HIV co-infected men as result of chronic egg-induced inflammation in the prostate and the seminal vesicles. The effect of treatment with the antihelminthic agent praziquantel on seminal HIV-1 RNA load was assessed in this study.
Methods
HIV-1 RNA load was determined in blood plasma and semen at baseline and at 10-week follow-up. Praziquantel was administered at baseline and two weeks later.
Results
Eighteen HIV-positive men with S. haematobium co-infection were enrolled into the study. Status of antiretroviral therapy (ART): 6 ART-naïve and 12 ART-experienced. All participants became egg-negative in urine at follow-up. Among the ART-naïve men, the mean HIV-1 RNA load decreased by 0.32 log10 copies per mL (4.41 vs 4.09) in blood plasma from baseline to follow-up, and in semen by 1.06 log10 copies per mL (4.06 vs 3.00).
Conclusions
This study demonstrated a decline in seminal HIV-1 RNA load following praziquantel treatment of urogenital schistosomiasis infection in HIV-positive men. The finding needs further exploration in a larger randomized study targeting praziquantel as a supplementary preventive measure of sexual transmission of HIV-1 in S. haematobium endemic areas in sub-Saharan Africa.
Keywords: HIV, HIV-1 RNA, Schistosoma haematobium, semen, praziquantel, urogenital schistosomiasis
Urogenital schistosomiasis is caused by infection with the helminth parasite Schistosoma haematobium [1]. Transmission occurs when larval cercariae in the fresh water bodies penetrate the skin. Subsequently, the adult worm pairs migrate to the venous plexus of the pelvic organs, where eggs are produced for many years if the host is left without treatment. A proportion of those eggs are sequestered, giving rise to establishment of chronic inflammatory granulomatous lesions in the urinary bladder and the internal genitals in males and females living in S. haematobium endemic areas. Estimates show that about 140 million people are infected with S. haematobium [2]. About 85% of those live in sub-Saharan Africa, where the distribution overlaps with areas of high HIV-1 prevalence [3, 4].
The World Health Organization (WHO) has urged for increased research awareness toward the role of genital schistosomiasis in HIV transmission in sub-Saharan Africa [5]. Community-based studies from Zimbabwe, Tanzania, and Mozambique have consistently found increased HIV prevalence among women with female genital schistosomiasis (FGS) [6–8]. Presence of chronic egg-induced inflammatory lesions and increased vascularity as part of the FGS pathology is considered an underlying mechanism involved in facilitating entry of the HIV-1 RNA virus across the friable mucosa in the cervix and the vagina [9–11]. Presence of high densities of CD4+ T lymphocytes and macrophages surrounding schistosome eggs in the subepithelial tissue of the cervix may also contribute to the increased susceptibility to HIV infection [12].
Male genital schistosomiasis (MGS) has been hypothesized to constitute a risk factor for HIV transmission through increased HIV-1 RNA shedding in semen [13] in a similar manner as observed in men with gonococcal urethritis [14]. Postmortem studies have shown that the seminal vesicles and the prostate are affected by the egg-induced inflammation as frequently as the urinary bladder [15–17]. In support of the hypothesis, MGS has been found to be associated with leucocytospermia and increased seminal levels of pro-inflammatory cytokines [18, 19]. Those abnormalities tend to normalize after treatment with praziquantel, which takes effect by killing the adult worms, followed by resolution of the egg-induced inflammation of the affected urogenital organs within a few weeks [20]. In the HIV co-infected with urogenital schistosomiasis person, presence of HIV-1 hosting immune cells in the seminal vesicles and the prostate is expected to decline after praziquantel treatment, with diminished viral shedding in semen as a result.
To date, no studies have been conducted to clarify the impact of MGS on HIV transmission and the role of praziquantel treatment as a potential measure in the overall HIV control strategy in S. haematobium endemic areas in sub-Saharan Africa.
We conducted this study to assess the potential effect of praziquantel treatment on HIV-1 RNA load in blood plasma and semen in Zimbabwean HIV-positive men co-infected with urogenital schistosomiasis.
METHODS
Study Design and Area
The study design was a prospective, sequential comparison of 2 cohorts of HIV and S. haematobium co-infected men. The study was conducted in Ngundu area (Chivi district), Masvingo province in Zimbabwe from April to October 2015. Selection of the site was based on results from the recent national schistosomiasis survey that showed high prevalence of urogenital schistosomiasis among primary school–age children in the Ngundu area [21].
Study Participants
HIV-positive men age 18 to 49 years were recruited from the catchment area (population 21 000) of Ngundu Rural Health Centre, which serves as an opportunistic infection (OI) clinic among other essential health services for the community. The population of males of reproductive age (15–49 years) in the catchment area was 2825 (Figure 1). Of these, 1584 (78.6%) males accepted an invitation to the study. As the main aim was to screen as many males as possible in the community in order to identify males coinfected with urogenital schistosomiasis and HIV-1, the first strategy (A) involved screening of S. haematobium on day 1 among all males willing to join the study. Men who tested positive for S. haematobium were invited to Ngundu Rural Health Centre for a further informed consent form administration if they were willing to join the clinical studies, for which another urine sample would be collected on 2 successive days. Also, semen and blood would then be donated. This rather costly strategy (A) recruited 1090 males, but the yield of males coinfected with urogenital schistosomiasis and HIV-1 (n = 7; 0.6%) was discouragingly low (Figure 1). Thus, a second strategy B and, subsequently, a third strategy C were devised. In strategy B, women registered for antiretroviral therapy (ART) at 4 health facilities in the catchment area were asked to invite their male spouses to the study. The urine screening and recruitment procedure were repeated as in strategy A. Among 422 males invited, only 98 were recruited, and of those 5 (5.1%) were co-infected with S. haematobium and HIV. The third strategy C involved invitation of males registered for ART at the 4 health facilities within the catchment area. From the 72 HIV-positive males recruited, 10 (13.9%) participants were coinfected with S. haematobium. Finally, 22 males co-infected with S. haematobium and HIV-1 identified using the 3 strategies (A+B+C) were considered for the pilot study. Of those, 4 failed to donate semen and were excluded from the study. The remaining 18 participants were classified in accordance with antiretroviral therapy status: ART-naïve participants and ART-experienced participants on consistent ART in the last 6 months prior to enrollment in the study. ART-experienced participants were being treated with the following regimens: tenofovir + lamivudine and nevirapine or tenofovir + lamivudine and lopinavir/ritonavir.
Figure 1.
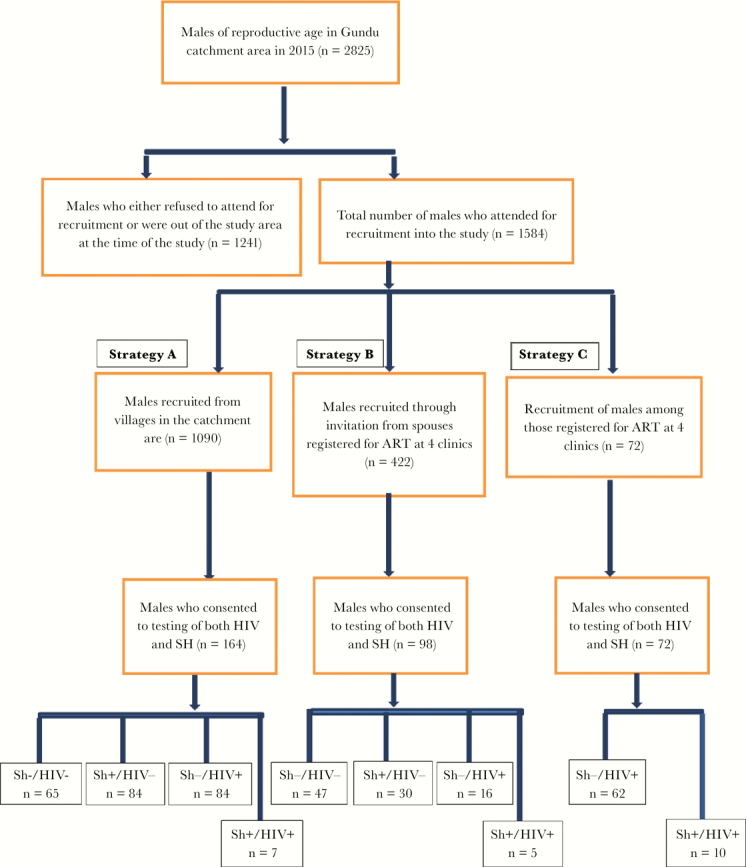
Flow chart showing the population of males of reproductive age in the Ngundu area and how these males were approached by different inclusion strategies (A, B, and C, respectively), leading to identification of 22 eligible candidates co-infected with HIV and schistosomiasis, among whom 18 took part in the final study. Abbreviations: ART, antiretroviral therapy; SH, Schistosoma haematobium.
Study Procedures
Screening for S. haematobum infection was performed by examination of urine samples collected on 3 consecutive days from each subject using the urine filtration technique [22]. Intensity of S. haematobium infection in each participant was determined by the mean egg count observed in 3 successive days: mild (1–9 eggs/10 mL urine), moderate (10–49 eggs/10 mL urine), and heavy (≥ 50 eggs/10 mL urine). A stool sample from each participant was examined for presence of S. mansoni eggs and soil-transmitted helminths by the whole stool formal ether concentration technique.
The measurement of CD4+ cell count was conducted in 5 mL of whole blood in a BD Vacutainer blood collection tube for CD4 stabilization, inverted 8 to 10 times, and stored at ambient temperature (2–30o C) prior to analysis by flow cytometry.
For measurement of HIV-1 viral load in blood, 5 mL of whole blood was collected using an EDTA tube. The specimen was centrifuged for 5 minutes at 3000 rpm to obtain plasma, 0.5 mL of which was then added to a NucliSENS Lysis Buffer tube, vortexed thoroughly, and stored at 2–8oC prior to HIV-1 RNA analysis. Semen samples were ejaculated into a medical condom (Male-FaktorPak) or a sterile container according to preference of the participant. The semen was allowed to liquefy for 1 hour at room temperature. The specimen was then centrifuged for 5 minutes at 5000 rpm; then 0.2 mL of the seminal plasma was added to the NucliSENS Lysis Buffer tube provided, vortexed thoroughly, and stored at 2–8oC. HIV-1 RNA analyses were carried out within 24 hours after receipt of blood and semen samples. NucliSENSEasyQ HIV-1 v2.0 assay was used for HIV-1 RNA quantification in blood and seminal plasma. The lower detection limit of the HIV-1 RNA concentration was 20 copies/mL (log10 1.3 copies/mL). The result of the HIV-1 measurement in blood and seminal plasma was categorized as quantifiable (≥20 copies/mL); detectable, but not quantifiable (<20 copies/mL); or nondetectable.
Sexually transmitted infection (STI) agents (Neisseria gonorrheae, Chlamydia trachomatis, Mycoplasma genitalium, and/or Trichomonas vaginalis) were assessed by the Nucliscense easyQ polymerase chain reaction (PCR) machine using the Nuclic acid sequencing base assay (Nasba) PCR real-time technique.
Participants were assigned treatment with PZQ (40 mg/kg bodyweight) at closure of the baseline study and 2 weeks later to increase efficacy of therapy.
Study Outcome Measures
The primary outcome measure was HIV-1 RNA viral load (log10 copies/mL) in plasma and in semen at follow-up in comparison with viral load at baseline prior to praziquantel treatment. Other outcome measures were urinary egg count (ova/10 mL urine) and CD4+ cell count (cells/mm3).
Ethical Consideration
The study was approved by the national ethical review board, Medical Research Council of Zimbabwe (MRCZ Ref: MRCZ/A/1853), and the community leadership (Provincial Medical Director, District Administrator, chiefs, village headmen, and councilors), and by the participants following signing of the informed consent.
Statistical Analysis
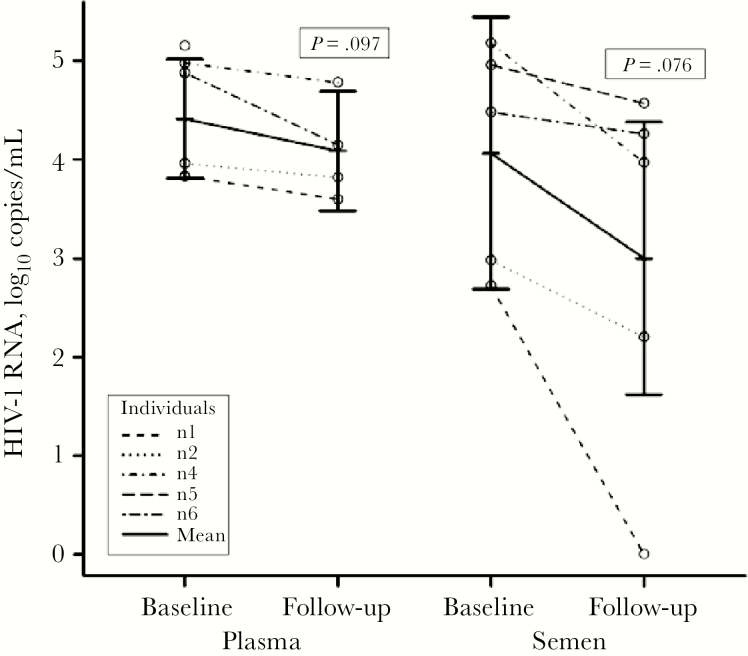
SPSS version 16 and R version 3.2.1 were used for data analysis [23]. S. haematobium infection intensity was expressed as number of eggs/10 mL urine. Mean blood plasma and semen HIV-1 RNA copies counted were log10 transformed. Calculation of the mean HIV-1 RNA load in plasma, or an HIV-1 RNA result of less <1.3 log10 copies in semen, was determined to be 1.28 log10 copies (19 copies)/mL and a negative result to be 0 log10 copies/mL. A paired t test was applied to test for changes in mean HIV-1 RNA load from baseline to follow-up. The confidence intervals in Figure 2 were obtained by use of a linear mixed model with random intercept.
Figure 2.
Comparison of paired HIV-1 RNA loads in plasma and in semen, respectively, in antiretroviral therapy (ART)–naïve patients at baseline and at follow-up after praziquantel treatment. SPSS version 16 and R version 3.2.1 were used for data analysis [23]. S. haematobium infection intensity was expressed as number of eggs/10 mL urine. Mean blood plasma and semen HIV-1 RNA copies counted were log10 transformed. Calculation of mean HIV-1 RNA load in plasma, or an HIV-1 RNA result of less <1.3 log10 copies in semen, was determined to be 1.28 log10 copies (19 copies)/mL, and a negative result to be 0 log10 copies /mL. A paired t test was applied to test for changes in mean HIV-1 RNA load from baseline to follow-up. The confidence intervals in Figure 2 were obtained by use of a linear mixed model with random intercept.
RESULTS
Baseline
Among the 18 study participants with HIV-1 and urogenital schistosomiasis co-infection, 6 were ART naïve and 12 ART experienced. The median age of the participants was 34 years (range, 29–38 years) in ART-naïve group and 35 years (range, 21–49 years) in the ART-experienced group (Table 1). Five of the 6 ART-naïve participants were found with moderate S. haematobium infection intensity, and 1 participant had mild infection intensity, whereas the distribution of infection intensity in the ART-experienced group was as follows: heavy (n = 2), moderate (n = 3), and mild (n = 7). None of the 18 participants were identified with S. mansoni eggs in feces, and they were all diagnosed negative for STI by PCR.
Table 1.
Urinary Egg Count, CD4 Count, and HIV-1 RNA Load in HIV-Positive Men Co-infected With Urogenital Schistosomiasis in Accordance With Antiretroviral Therapy Status: Naïve vs Experienced
| Individuals | Age | HIV-1 RNA, log10 copies/mLa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity of Schistosoma haematobium Infectionb | CD4+ Count, cells/mm3 | Plasma | Semen | ||||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | ||
| ART-naïve (n = 6) | |||||||||
| n1 | 38 | Moderate | Negative | 382 | 301 | 3.83 | 3.60 | 2.72 | ND |
| n2 | 32 | Mild | Negative | 412 | 621 | 3.96 | 3.82 | 2.98 | 2.20 |
| n3 | 29 | Moderate | NA | 340 | NA | 4.80 | NA | 1.94 | NA |
| n4 | 36 | Moderate | Negative | 237 | 255 | 4.98 | 4.78 | 5.18 | 3.97 |
| n5 | 34 | Moderate | Negative | 35 | NA | 5.15 | NA | 4.96 | 4.57 |
| n6 | 34 | Moderate | Negative | 172 | 176 | 4.88 | 4.15 | 4.48 | 4.26 |
| ART-experienced (n = 12) | |||||||||
| e1 | 21 | Mild | Negative | 230 | 311 | <1.3 | ND | <1.3 | ND |
| e2 | 43 | Mild | Negative | 316 | 343 | <1.3 | ND | <1.3 | NA |
| e3 | 49 | Mild | Negative | 222 | 164 | 2.14 | 1.83 | <1.3 | ND |
| e4 | 23 | Mild | Negative | 509 | 468 | <1.3 | ND | <1.3 | <1.3 |
| e5 | 36 | Mild | Negative | 251 | 246 | <1.3 | <1.3 | <1.3 | <1.3 |
| e6 | 42 | Mild | Negative | 521 | 660 | <1.3 | ND | <1.3 | ND |
| e7 | 33 | Mild | Negative | 359 | 474 | <1.3 | <1.3 | <1.3 | ND |
| e8 | 45 | Moderate | Negative | 348 | 249 | ND | ND | ND | 2.0 |
| e9 | 37 | Moderate | Negative | 76 | 114 | 2.65 | 2.43 | 3.20 | ND |
| e10 | 29 | Moderate | NA | 378 | NA | <1.3 | NA | 3.20 | NA |
| e11 | 33 | Heavy | Negative | 625 | 395 | 2.11 | 1.38 | ND | ND |
| e12 | 24 | Heavy | Negative | 631 | 468 | ND | NA | ND | ND |
Abbreviations: ART, antiretroviral therapy; NA, nonavailable; ND, nondetectable.
aLower detection limit was 1.3 log10 copies (20 copies)/mL.
bIntensity of Schistosoma haematobium infection by urine egg count (eggs/10 mL urine): mild (1–9), moderate (10–49), and heavy (≥50).
The mean CD4 count was 263 cells/ mm3 (range, 35–412 cells/mm3) in the ART-naïve group in comparison with 372 cells/mm3 (range, 76–631 cells/mm3) in the ART-experienced group (Table 1). Two participants in the ART-naïve group were observed with a CD4 count of less than 200 cells/mm3.
In the ART-naïve group, the mean blood plasma viral load was 4.59 log10 copies per mL (range, 3.83–5.15 log10 copies per mL) whereas mean seminal viral load was 3.71 log10 copies per mL (range, 1.94–5.18 log10 cps/mL). In the ART-experienced group, the mean plasma viral load was 1.31 log10 copies per mL (range, 0–2.65 log10 copies per mL), with the following distribution of viral load categories: negative (n = 2); <1.3 log10 copies/mL (n = 7) and ≥1.3 log10 copies/mL (n = 3). The mean seminal viral load was 1.28 log10 copies per mL (range, 0–3.20 log10 copies per mL), with the following distribution of viral load categories: negative (n = 3); <1.3 log10 copies/mL (n = 7) and ≥1.3 log10 copies/mL (n = 2). Of interest, in 2 ART-experienced participants (ID e9 and e10, respectively), the seminal viral load was 3.20 log10 copies per mL, which was a higher concentration than measured in blood plasma (Table 1).
Follow-up
One of the 6 ART-naïve participants and 1 of the 12 ART-experienced participants were absent at follow-up. No changes in ART status had occurred for any of the remaining 16 participants during the follow-up period, and none of them were detected with eggs in urine or in feces. STI PCR in urine was persistently negative for all participants.
No statistical difference in mean CD4+ count was observed in the 2 study groups when comparing baseline with follow-up results. For the 4 ART-naïve participants, the mean CD4+ count was 301 cells/mm3 at baseline vs 338 cells/mm3 at follow-up. For the 11 ART-experienced participants, the mean CD4+ count was 372 cells/mm3 at baseline vs 354 cells/mm3 at follow-up survey.
The results of HIV-1 RNA measurement in plasma were available in 4 of the 5 ART-naïve participants present at follow-up, whereas results in semen were available in all 5 participants (Table1). Figure 2 shows plasma and seminal HIV-1 RNA concentrations at baseline and at follow-up in the ART-naïve participants. The mean plasma viral load in ART-naïve participants decreased by 0.32 (CI, –0.76 to 0.11) log10 copies per mL from 4.41 log10 copies per mL (range, 3.83–4.98 log10 copies per mL) at baseline to 4.09 log10 copies per mL (range, 3.60–4.78 log10 copies per mL) at follow-up up (P = .097). The mean seminal viral load decreased by 1.06 (CI, –2.31 to 0.18) log10 copies per mL, from 4.06 log10 copies per mL (range, 2.72–5.18 log10 copies per mL) at baseline to 3.00 log10 copies per mL (range, 0–4.57 log10 copies per mL) at follow-up (P = .076).
The results of HIV-1 RNA measurement in both blood plasma and in semen were available in 9 ART-experienced participants at follow-up (Table 1). Among the 3 participants observed with detectable HIV-1 RNA in plasma above the lower detection limit (1.3 log10 copies/mL) at baseline, the mean viral load decreased by 0.42 (CI, –1.10 to 0.26) log10 copies per mL from 2.30 log10 copies per mL (range, 2.11–2.65 log10 copies per mL) at baseline to 1.88 log10 copies per mL (range, 1.38–2.43 log10 copies per mL) at follow-up (P = .12). In the remaining 6 ART-experienced participant, 1 was observed with nondetectable viremia at baseline vs 4 participants at follow-up. Two of the 9 ART-experienced participants with complete paired HIV-1 RNA measurement results were found with a nondetectable viral load in semen at baseline, as opposed to 6 at follow-up.
DISCUSSION
Despite the epidemiological overlap of urogenital schistosomiasis with HIV in sub-Saharan Africa [3, 4], no studies have been conducted to determine the effects of antischistosomal treatment with praziquantel on seminal HIV-1 RNA shedding in males co-infected with urogenital schistosomiasis. Literature on FGS and its public health implication, including its association with HIV transmission, is increasing [6, 7, 10, 24, 25], but only 2 community-based studies have generated data on MGS [13, 18, 19]. However, those studies did not seek to determine the role of MGS on HIV-1 transmission. Thus, to our knowledge, this is a first community-based scientific study reporting the results of the male genital schistosomiasis and HIV-1 in which the effect of praziquantel treatment of urogenital schistosomiasis on seminal HIV-1 RNA shedding was assessed.
The study has some limitations requiring cautious interpretation of the results due to (1) the small number of study participants, (2) the absence of a control group of HIV-positive men without urogenital schistosomiasis, and (3) the lack of diagnostic inflammatory markers in semen to support the study outcome.
In this setting, males were less forthcoming regarding visiting the health facilities for voluntary HIV counselling and testing (VCT). In general, males tend to seek health care late (to bed added) when HIV/AIDS is at advanced stage clinically. Some prefer to visit health facilities far away from their catchment areas in order to conceal their HIV status from family members including the wife. This made it more difficult for this study to yield a large number of HIV cases co-infected with urogenital schistosomiasis registered at local health facilities. On the other hand, cultural sensitivity toward donating semen by males and the voluntary nature of the study that allowed males to drop out of the study at any time imposed challenges. Several males opted out of the study during administration of informed consent form, which sought their autonomous decision to join the study following a full explanation about the study aims and methodology, which specified samples required from study participants.
It was shown in this study that praziquantel treatment of urogenital schistosomiasis in HIV co-infected men reduced semen viral load in all ART-naïve participants, but also to a minor extent in ART-experienced participants, a higher proportion of whom became HIV-1 RNA undetectable at follow-up in comparison with baseline. In addition, the seminal viral load of 3.20 log10 copies/mL was higher than the plasma viral load in 2 ART-experienced participants, even in 1 of them with a plasma viral load below the lower detection limit (<1.3 log10 copies/mL). Those findings may support the assumption that the seminal fluid–producing organs, primarily the prostate and the seminal vesicles, constitute a separate compartment containing an accumulated number of HIV-hosting immune cells as part of the egg-induced inflammatory environment in the pelvic organs. As a result, augmented numbers of HIV-1 RNA copies are shed into semen, presumably even when viral suppression in the blood is achieved by otherwise successful ART. In general, effective ART with an undetectable plasma viral load tends to limit the effect of urethritis on semen viral load, whereas when plasma viral load is poorly controlled, a high seminal viral load occurs, particularly in association with gonococcal urethritis [26]. However, the HIV RNA level in blood plasma is not reliable as an independent predictor of virus levels in semen, and the male genital tract is considered a distinct virological compartment from blood [27, 28]. Politch and colleagues have reported that STI and genital inflammation can override the suppressive effect of ART in seminal HIV shedding in HIV-infected men [29].
The ART-naïve participants in the study were diagnosed with light to moderate intensity of S. haematobium infection, defined as an egg count of 1 to 49 eggs/10 mL urine. The HIV-1 RNA load in semen may have been measured at an even higher level in ART-naïve participants with heavy intensity of infection (≥50 eggs/10 mL urine) due to an anticipated proportionally higher level of egg-induced inflammation in the urogenital organs, which contain a higher number in HIV-hosting immune cells, in concordance with findings in postmortem studies of S. haematobium–associated pathology in males [15, 16].
In a study by Dyer and colleagues, it was shown that HIV-1 RNA concentrations in blood and semen were significantly higher in HIV-positive men living in Malawi in comparison with US and Swiss HIV-positive men [30]. In a matched analysis of CD4+ cell counts between the 2 groups, a significant difference was also observed, with lower CD4+ cell counts in Malawian men. The findings may explain the high rates of HIV-1 sexual transmission and accelerated HIV-1 disease progression in sub-Saharan Africa. For further reflection, in Malawi, like in many countries in the southern Africa, schistosomiasis and other neglected tropical diseases are endemic, and there is growing evidence that schistosomiasis adversely impacts every phase of HIV/AIDS in co-infected individuals through inexpedient immune modulation [4, 31]. Individuals with Schistosoma have been found with higher cell surface densities of CCR5 and CXCR4 receptors on CD4+ T cells and monocytes in peripheral blood, which makes them more susceptible to HIV infection [32, 33].
Meta-analysis has shown that urethral infections due to STI pathogens are associated with significant increases in leukocyte concentrations and HIV-1 shedding in the genital tract [34]. These infections are likely to be particularly important in promoting the sexual transmission of HIV-1, and should therefore be the focus of HIV prevention strategies. Cohen and colleagues have shown that gonococcal and chlamydial urethritis in ART-naïve HIV-positive men in Malawi was associated with an increase in HIV-1 RNA concentrations in semen, which decreased significantly following treatment, in particular for gonococcal urethritis [14]. Similar observations have been made in ART-naïve HIV-positive men in a developed world setting being treated for urethritis, but not at the same significance level as the study in Malawi [35].
Urogenital schistosomiasis co-infection in HIV-positive men may constitute a neglected risk factor for HIV propagation in S. haematobium endemic areas in Africa due to increased HIV-1 RNA shedding in semen in an analogous way to the estimations in the probabilistic empiric model on high viral semen burden from inflammation in sub-Saharan Africa developed by Chakraborty and colleagues [36]. Because children and adults of both genders in those areas in most cases are chronically infected, the urogenital lesions therefore also tend to persist for decades, as opposed to lesions due to STIs, which are normally transient, with a few weeks’ duration in the clinical presentation. Hence, the contribution of urogenital schistosomiasis as risk factor for HIV transmission is more persistent than for STIs, which normally only occur sporadically and have short clinical duration. Both men and women as sexual partners living in S. haematobium endemic areas are dually affected by egg-induced genital lesions because both genders are exposed to the same water bodies containing the infective cercarial parasites. Hence, a neglected HIV transmission synergy may exist by which increased HIV-1 RNA shedding in semen due to the MGS-associated lesions in the prostate and seminal vesicles may act in a complementary way to the increased vulnerability to HIV infection in women with FGS due to the coexisting cervico-vaginal lesions. In addition, the effect of an occasional single-dose praziquantel treatment on the infection is commonly transient due to the re-exposure to the Schistosoma parasite in the local fresh water bodies. Therefore, repeated praziquantel treatment of the endemic populations by yearly intervals will be required in long-term control strategies targeting children as well as adults.
This study should be followed up by larger randomized controlled trials to assess the effect of praziquantel treatment in reduction of seminal HIV-1 RNA shedding in S. haematobium and HIV co-infected men. From an overall HIV control strategy perspective, such trials should also be designed to address the diagnosis and treatment of STIs as a commonly coexisting reproductive health condition in males living in S. haematobium endemic areas in sub-Saharan Africa.
Acknowledgments
We want to express our gratitude to community leaders for permission and support during project implementation. Acknowledgements are also due to the participants for taking part in the project and to the community in Chivi district, Masvingo Province, Zimbabwe, for the very kind hospitality provided to the field research team.
Author contributions. Conceived and designed the experiments: PDCL NM TM BM. Performed the experiments: NM BM TM. Analyzed the data: LF PDCL NM. Contributed reagents/materials/analysis tools: NM PDCL BM. Wrote the paper: NM PDCL BF LF. Edited the paper: PDCL NM LF TM BM
Financial support. This work was supported by the Department of Control of Neglected Tropical Diseases (NTD) at the World Health Organization (WHO).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet 2006; 368:1106–18. [DOI] [PubMed] [Google Scholar]
- 2. Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 2009; 3:e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014; 383:2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bustinduy A, King C, Scott J et al. . HIV and schistosomiasis co-infection in African children. Lancet Infect Dis 2014; 14:640–9. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Report of an informal working group on urogenital schistosomiasis and HIV transmission 2009. Available at: http://whqlibdoc.who.int/hq/2010/WHO_HTM_NTD_PCT_2010.5_eng.pdf. Accessed 15 February 2016.
- 6. Kjetland EF, Ndhlovu PD, Gomo E et al. . Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 2006; 20:593–600. [DOI] [PubMed] [Google Scholar]
- 7. Downs JA, van Dam GJ, Changalucha JM et al. . Association of Schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg 2012; 87:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brodish PH, Singh K. Association between Schistosoma haematobium exposure and human immunodeficiency virus Infection among females in Mozambique. Am J Trop Med Hyg 2016; 94(5):1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldmeier H, Krantz I, Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. Int J STD AIDS 1994; 5:368–72. [DOI] [PubMed] [Google Scholar]
- 10. Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol 2012; 28:58–65. [DOI] [PubMed] [Google Scholar]
- 11. Jourdan PM, Roald B, Poggensee G et al. . Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis 2011; 5:e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jourdan PM, Holmen SD, Gundersen SG et al. . HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am J Trop Med Hyg 2011; 85:1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leutscher P, Ramarokoto CE, Reimert C et al. . Community-based study of genital schistosomiasis in men from Madagascar. Lancet 2000; 355:117–8. [DOI] [PubMed] [Google Scholar]
- 14. Cohen MS, Hoffman IF, Royce RA et al. . Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997; 349:1868–73. [DOI] [PubMed] [Google Scholar]
- 15. Gelfand M, Ross CM, Blair DM et al. . Schistosomiasis of the male pelvic organs. Severity of infection as determined by digestion of tissue and histologic methods in 300 cadavers. Am J Trop Med Hyg 1970; 19:779–84. [PubMed] [Google Scholar]
- 16. Edington GM, Nwabuebo I, Junaid TA. The pathology of schistosomiasis in Ibadan, Nigeria with special reference to the appendix, brain, pancreas and genital organs. Trans R Soc Trop Med Hyg 1975; 69:153–6. [DOI] [PubMed] [Google Scholar]
- 17. Patil PS, Elem B. Schistosomiasis of the prostate and the seminal vesicles: observations in Zambia. J Trop Med Hyg 1988; 91:245–8. [PubMed] [Google Scholar]
- 18. Leutscher PD, Pedersen M, Raharisolo C et al. . Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium-infected individuals. J Infect Dis 2005; 191:1639–47. [DOI] [PubMed] [Google Scholar]
- 19. Leutscher PD, van Dam GT, Reimert CM et al. . Eosinophil cationic protein, soluble egg antigen, circulating anodic antigen, and egg excretion in male urogenital schistosomiasis. Am J Trop Med Hyg 2008; 79:422–6. [PubMed] [Google Scholar]
- 20. Vennervald BJ, Dunne DW. Morbidity in schistosomiasis: an update. Curr Opin Infect Dis 2004; 17:439–47. [DOI] [PubMed] [Google Scholar]
- 21. Midzi N, Mduluza T, Chimbari MJ et al. . Distribution of schistosomiasis and soil transmitted helminthiasis in Zimbabwe: towards a national plan of action for control and elimination. PLoS Negl Trop Dis 2014; 8:e3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mott KE, Baltes R, Bambagha J, Baldassini B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmed Parasitol 1982; 33:227–8. [PubMed] [Google Scholar]
- 23. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available at: http://www.R-project.org/. Accessed 1 March 2017. [Google Scholar]
- 24. Downs JA, Mguta C, Kaatano GM et al. . Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg 2011; 84:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Randrianasolo BS, Jourdan PM, Ravoniarimbinina P et al. . Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: a cross-sectional study in Madagascar. J Infect Dis 2015; 212:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadiq ST, Taylor S, Kaye S et al. . The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. AIDS 2002; 16:219–25. [DOI] [PubMed] [Google Scholar]
- 27. Ping LH, Cohen MS, Hoffman I et al. . Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol 2000; 74:8946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coombs RW, Lockhart D, Ross SO et al. . Lower genitourinary tract sources of seminal HIV. J Acquir Immune Defic Syndr 2006; 41:430–8. [DOI] [PubMed] [Google Scholar]
- 29. Politch JA, Mayer KH, Welles SL et al. . Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 2012; 26:1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Politch JA, Mayer KH, Welles SL et al. . High levels of human immunodeficiency virus type 1 in blood and semen of seropositive men in sub-Saharan Africa. J Infect Dis 1998; 177: 1742–6. [DOI] [PubMed] [Google Scholar]
- 31. Secor WE. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS 2012; 7:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Secor WE, Shah A, Mwinzi PM et al. . Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4(+) T cells and monocytes of patients with Schistosoma mansoni infection. Infect Immun 2003; 71:6668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleppa E, Ramsuran V, Zulu S et al. . Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS One 2014; 9:e98593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis 2008; 35:946–59. [DOI] [PubMed] [Google Scholar]
- 35. Sadiq ST, Taylor S, Copas AJ et al. . The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sex Transm Infect 2005; 81:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chakraborty H, Sen PK, Helms RW et al. . Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 2001; 15:621–7. [DOI] [PubMed] [Google Scholar]