Abstract
Background
Patients on chronic intermittent renal replacement therapy (RRT) are at risk for infection with carbapenem-resistant Enterobacteriaceae (CRE). However, the impact of RRT on outcomes after CRE infections remains to be defined. Here we perform a comparison of outcomes for CRE-infected patients with preserved renal function compared with CRE-infected patients on RRT.
Methods
Cases and controls were defined from a prospective cohort of CRE-infected patients from the Consortium on Resistance against Carbapenems in Klebsiella and other Enterobacteriaceae (CRACKLE). Cases were defined as CRE-infected patients on RRT at hospital admission, while controls were defined as CRE-infected patients with serum creatinine <2 mg/dL and not receiving RRT at admission. Risk factors for 28-day in-hospital mortality were assessed using multivariable logistic regression. An ordinal ranking of outcomes by desirability analysis was performed.
Results
Patients on RRT were more likely to have diabetes mellitus and cardiac disease than controls. Urinary sources of infection were less common in the RRT group. In RRT patients, 28-day in-hospital mortality was increased as compared with controls: 22/71 (31%) vs 33/295 (11%). RRT remained significantly associated with 28-day in-hospital mortality after adjustment for source of infection, prehospitalization origin, and severity of illness (adjusted odds ratio, 2.27; 95% confidence interval [CI], 1.09–4.68; P = .03). Using univariable desirability of outcome ranking analysis, RRT status was associated with a 68% (95% CI, 61%–74%) chance of a worse disposition outcome.
Conclusions
Chronic RRT in CRE-infected patients is associated with increased in-hospital mortality and worse disposition outcomes at 28 days.
Keywords: carbapenem-resistant Enterobacteriaceae, Klebsiella pneumoniae, mortality, renal failure, renal replacement therapy
Nearly 500000 Americans with end-stage renal disease (ESRD) required chronic intermittent renal replacement therapy (RRT) in 2013. Prevalence rates are rising, with more than 100 000 new patients initiating chronic RRT in 2013 [1]. RRT patients typically engage with the health care setting 3 times weekly and face more than 1.5 hospital admissions/person-year [2]. The majority (79%) of ESRD patients initiate hemodialysis via intravascular catheter, which increases the risk for line-related bloodstream infection [3]. According to a meta-analysis, RRT patients face intravenous antibiotic start rates ranging from 3.1–7.7 per 100 patient-months [4].
ESRD is associated with impaired innate and adaptive immune responses [5, 6]. In addition, ESRD may lead to bacterial overgrowth, particularly in the duodenum and jejunum [7]. Beyond increased bacterial counts in the gut, an increase in abundance of potentially pathogenic bacterial families such as Enterobacteriaceae is observed. This occurs at the expense of Lactobacillaceae and Prevotellaceae, both normal components of healthy gut flora [8].
These increased opportunities for acquisition in ESRD patients translate into higher incidence of infection with multidrug-resistant organisms than the general population, particularly methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) [9]. There are no published studies of incidence of CRE infections in the ESRD population. Outcomes of CRE infections in ESRD patients on RRT are also poorly studied. In a sample in which gram- negative bacteria comprised 52% of the cases, 30-day mortality of 15.1% was observed among patients with ESRD on RRT [10]. Compared with patients not on RRT, ESRD patients on RRT face mortality rates more than 2 times higher for sepsis, pneumonia, and endocarditis [11–13]. ESRD patients on RRT are also more likely to die from antibiotic-resistant organisms such as MRSA (adjusted odds ratio [aOR], 5.4; 95% confidence interval [CI], 1.5–18.7) and Clostridium difficile (aOR, 2.15; 95% CI, 2.07–2.24) [14, 15]. For CRE, 1 study identified a median survival of 1 month for CRE-infected patients with ESRD on RRT compared with more than 24 months for the control group of ESRD patients on RRT without infection [16]. The aim of this study was to examine outcomes in CRE-infected patients on RRT compared with CRE-infected patients with normal renal function in a large prospective, multicenter cohort of CRE-infected patients.
METHODS
Patients
The Consortium on Resistance against Carbapenems in Klebsiella and other Enterobacteriaceae (CRACKLE) is a prospective, multicenter, observational study of hospitalized patients with CRE in the Great Lakes Region of the United States [17–20]. The study period for the current study was December 2011 to July 2016. Two nonoverlapping nested cohorts of patients with CRE infection were constructed. Patients who did not meet criteria for infection and were deemed to have CRE colonization were excluded. Standardized, a priori definitions of infection were used, as previously described [21]. The first cohort (“RRT patients”) contained all patients who were on RRT at the time of admission for the index hospitalization. The second cohort (“control patients”) consisted of all patients who did not have renal failure upon or during hospital admission. Renal failure was defined as either a need for RRT and/or a serum creatinine >2 mg/dL. In both cohorts, unique patients were included only once, at the time of their first CRE infection. All the health systems involved in this study had approval from their respective institutional review boards.
Microbiology
CRE was defined as Enterobacteriaceae isolates with nonsusceptibility to any of the following carbapenems: meropenem, imipenem, or ertapenem, as outlined by the Clinical and Laboratory Standards Institute (CLSI) [22]. Bacterial identification and routine antimicrobial susceptibility testing was performed with MicroScan (Siemens Healthcare Diagnostics) or Vitek2 (bioMérieux) at the clinical sites. Additional susceptibilities were obtained by GN4F Sensititre tray (Thermo Fisher) or Etest (bioMérieux), as indicated. In more than 90% of tested isolates, carbapenem resistance was mediated through blaKPC-2 or blaKPC-3, as previously described [18, 19].
Clinical Data
Clinical data from the electronic medical record were entered into a centralized database. The index hospitalization was defined as the first hospital stay within the study period during which a CRE infection occurred. Critical illness was determined as a Pitt bacteremia score ≥4 points on the day of the index culture [23]. The Pitt bacteremia score has previously been validated for nonbacteremic infections [24]. The Charlson comorbidity index was calculated at hospital admission, as described [25]. Outcomes at 28 days after the date of the index culture were categorized as follows: discharged home, discharged to a skilled nursing facility, remains admitted, discharged to long-term acute care hospital (LTACH), dead or discharged to hospice. For this purpose, the status at hospital discharge was carried forward, and patients who were transferred to another hospital prior to the 28-day mark were considered “remains admitted.”
Statistics
Differences between groups were analyzed using the Wilcoxon rank sum test for continuous variables. Fisher’s exact test and Pearson testing were used for categorical variables where appropriate. Nominal logistic regression was performed to determine the univariable association of each variable of interest and 28-day in-hospital mortality. All variables that were associated at a level of P <.1 were included in a multivariable model. An ordinal outcome was constructed based on the 28-day outcomes, with the following order of categories: “discharged home” (best), “discharged to skilled nursing facility,” “remains admitted,” “discharged to long-term acute care hospital,” “dead or discharged to hospice” (worst) [26]. P values of ≤.05 were considered statistically significant. JMP 10.0.1 software (SAS, Inc, Cary, NC) was used for all analyses.
RESULTS
Patient Characteristics
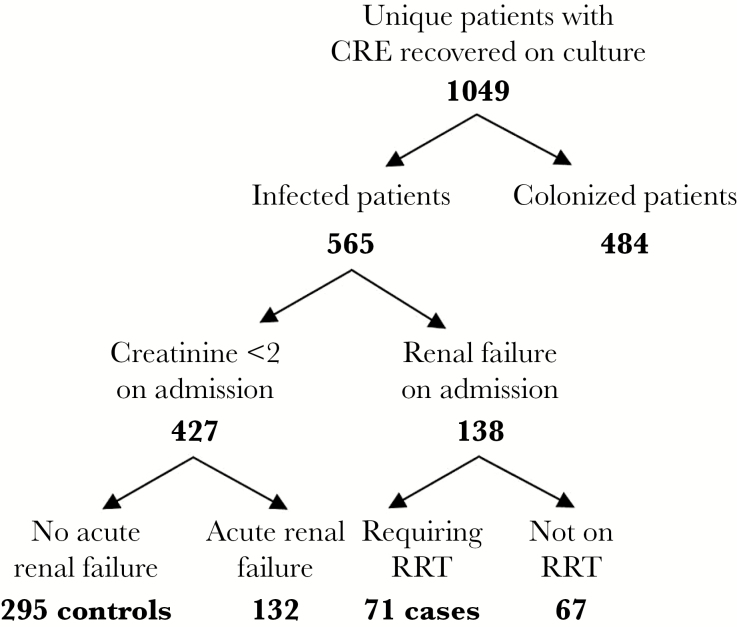
The study population included 71 case patients with end-stage renal disease (ESRD) requiring RRT and 295 control patients without renal failure, selected as shown in Figure 1. Carbapenem-resistant Klebsiella pneumoniae comprised 90% of infections (n = 330). In the remaining 10% of patients, Enterobacter spp. (n = 20; 5%), Morganella morgannii (n = 6; 2%), Proteus mirabilis (n = 3; 1%), Providencia stuartii (n = 3; 1%), Escherichia coli (n = 2; 1%), and Citrobacter spp. (n = 2; 1%) were isolated. The characteristics of the study population are shown in Table 1. The distributions of age, sex, and race did not differ significantly between the RRT and control groups. Besides renal failure, other comorbid conditions were also more common in RRT patients. Specifically, diabetes mellitus (DM) and cardiac disease were associated with the RRT group; the rates of DM and cardiac disease were 62% and 63% in RRT patients, respectively, compared with 37% and 34%, respectively, in the control group (P < .0001 for both). The distribution of infections also differed between the groups (P = .01), which was primarily driven by a higher proportion of urinary tract infections in the control group (34%) compared with the RRT population (14%). Furthermore, the 2 groups varied significantly by origin at time of admission, with 40% of control patients admitted from home compared with 30% of RRT patients and 5% of control patients admitted from LTACH compared with 20% of RRT patients.
Figure 1.
Selection of isolates. Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; RRT, renal replacement therapy.
Table 1.
Clinical Characteristics
| All | RRT | Control | P a | |
|---|---|---|---|---|
| n | 366 | 71 | 295 | |
| Age, median (IQR), y | 62 (50–74) | 62 (53–71) | 62 (49–75) | .99 |
| Female | 189 (52) | 36 (51) | 153 (52) | .90 |
| Race | .10 | |||
| White | 198 (54) | 32 (45) | 166 (56) | |
| Black | 140 (38) | 35 (49) | 105 (36) | |
| Other | 28 (8) | 4 (6) | 24 (8) | |
| Charlson comorbidity index, median (IQR) | 3 (1–5) | 6 (4–7) | 2 (1–4) | <.0001 |
| Diabetes mellitus | 152 (42) | 44 (62) | 108 (37) | <.0001 |
| Cardiac diseaseb | 144 (39) | 45 (63) | 99 (34) | <.0001 |
| Type of infection | .01 | |||
| Bloodstream infection | 92 (25) | 21 (30) | 71 (24) | |
| Pneumonia | 80 (22) | 16 (23) | 64 (22) | |
| Urinary tract infection | 109 (30) | 10 (14) | 99 (34) | |
| Wound infection | 40 (11) | 11 (15) | 29 (10) | |
| Other infection | 45 (12) | 13 (18) | 32 (11) | |
| Origin | <.01 | |||
| Home | 138 (38) | 21 (30) | 117 (40) | |
| Skilled nursing facility | 137 (37) | 25 (35) | 112 (38) | |
| Hospital transfer | 63 (17) | 11 (15) | 52 (18) | |
| Long-term acute care hospital | 28 (8) | 14 (20) | 14 (5) | |
| Critical illnessc | 147 (40) | 39 (55) | 108 (37) | <.01 |
| Length of stay, median (IQR), d | 12 (7–24) | 13 (9–24) | 11 (6–25) | .24 |
All data expressed as n (%), unless otherwise indicated.
Abbreviations: IQR, interquartile range; RRT, renal replacement therapy.
aUnivariable relationship between variable of interest and RRT.
bCardiac disease defined as the presence of coronary artery disease and/or congestive heart failure.
cCritical illness defined as Pitt bacteremia score ≥4 at the time of index culture.
Antibacterial Treatment
Directed anti-CRE treatment given within 14 days after index culture is summarized in Table 2. Upon comparison of the usage of specific antibiotics, colistin use was more common in the RRT group, with 23/71 (32%) receiving colistin as compared with 25/295 (8%) in the control patients (P < .0001). Other specific antibiotic usage was not different between groups. Of note, critical illness was more common in patients treated with colistin; 28/48 (58%) patients who received colistin were critically ill, as compared with 119/318 (37%) patients who did not receive colistin (P < .01). Furthermore, colistin use was more common in patients with bloodstream infections (22/92; 24%), compared with patients with other types of infection (26/274; 9%; P = .001). Similar proportions of cases and controls received agents without in vitro susceptibility to CRE.
Table 2.
Treatment Characteristics
| All | RRT | Control | P a | |
|---|---|---|---|---|
| n | 366 | 71 | 295 | |
| Colistin | 48 (13) | 23 (32) | 25 (8) | <.0001 |
| Tigecycline | 117 (32) | 22 (30) | 95 (32) | .88 |
| Amikacin | 58 (16) | 13 (18) | 45 (15) | .59 |
| Gentamicin | 65 (18) | 12 (17) | 53 (18) | 1.0 |
| Trimethoprim/sulfamethoxazole | 37 (10) | 9 (13) | 28 (9) | .39 |
| Carbapenem | 130(36) | 31 (44) | 99 (34) | .13 |
| Fosfomycinb | 16 (4) | 1 (1) | 15 (5) | .33 |
| Ceftazidime/avibactam | 28 (8) | 3 (4) | 25 (8) | .32 |
| Antibiotics without in vitro anti-CRE activity | 62 (17) | 11 (15) | 51 (17) | .86 |
| None of above | 22 (7) | 2 (3) | 20 (7) | .59 |
All data expressed as n (%), unless otherwise indicated. Shown is the number of patients who received a given antibiotic in the 14 days following index culture. Percentages accumulated exceed 100% as patients may have received more than 1 antibiotic.
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; RRT, renal replacement therapy.
aUnivariable relationship between variable of interest and RRT.
bFosfomycin use was only recorded in patients with urinary tract infections.
Outcomes
Total length of stay of the index hospitalization was not different between RRT patients and control patients. The RRT group had illness of significantly higher severity at the time of first positive CRE culture. The impact of risk factors on 28-day mortality is shown in Table 3. The all-cause 28-day in-hospital mortality rates were 22/71 (31%) in RRT patients, compared with 33/295 (11%) in control patients (P < .0001). Risk factors associated with increased mortality on multivariable analysis include bloodstream infection, transfer from hospital or LTACH, high levels of critical illness, and RRT. Notably, RRT remained significantly associated with 28-day in-hospital mortality after adjustment for source of infection, prehospitalization origin, and critical illness (aOR, 2.27; 95% CI, 1.09–4.68; P = .03).
Table 3.
Risk Factors for 28-Day In-Hospital Mortality
| Survivor | Nonsurvivor | P a | aOR | 95% CI | P b | |
|---|---|---|---|---|---|---|
| n | 311 | 55 | ||||
| Age, median (IQR) | 62 (49–74) | 63 (53–74) | .85 | |||
| Female | 161 (52) | 28 (51) | 1.0 | |||
| Race | .31 | |||||
| White | 173 (56) | 25 (45) | ||||
| Black | 114 (37) | 26 (47) | ||||
| Other | 24 (8) | 4 (7) | ||||
| Charlson comorbidity index ≥3 | 160 (51) | 31 (56) | .56 | |||
| Diabetes mellitus | 128 (41) | 24 (44) | .77 | |||
| Cardiac diseasec | 120 (39) | 24 (44) | .54 | |||
| Source | <.0001 | .001 | ||||
| Blood (ref.) | 66 (21) | 26 (47) | - | - | ||
| Urine | 105 (34) | 4 (7) | 0.16 | 0.05–0.51 | ||
| Respiratory | 66 (21) | 14 (25) | 0.27 | 0.12–0.65 | ||
| Other | 74 (24) | 11 (20) | 0.39 | 0.16–0.92 | ||
| Origin | .001 | |||||
| Home (ref.) | 122 (39) | 15 (27) | - | - | ||
| Skilled nursing facility | 125 (40) | 12 (22) | 0.63 | 0.26–1.48 | ||
| Hospital transfer | 49 (16) | 15 (27) | 1.93 | 0.81–4.66 | ||
| Long-term acute care hospital | 15 (5) | 13 (24) | 4.53 | 1.60–12.97 | ||
| Critical illnessd | 107 (34) | 40 (73) | <.0001 | 4.28 | 2.15–8.93 | <.0001 |
| RRT | 49 (16) | 22 (40) | <.001 | 2.27 | 1.09–4.68 | .03 |
All data expressed as n (% of survivors and nonsurvivors, respectively), unless otherwise indicated.
Abbbreviations: aOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range; RRT, renal replacement therapy.
a P value for univariable relationship with 28-day in-hospital mortality.
b P value for multivariable relationship with 28-day in-hospital mortality.
cCardiac disease defined as the presence of coronary artery disease and/or congestive heart failure.
dCritical illness defined as Pitt bacteremia score ≥4 at the time of index culture.
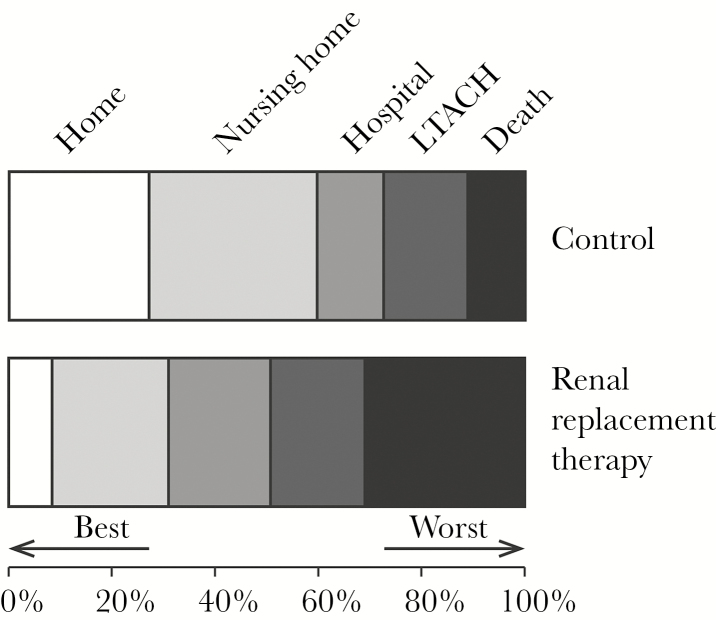
The ordinal outcome of disposition at 28 days by desirability of outcome ranking (DOOR) analysis (dead/hospice, LTACH, admitted, nursing home, and home) is shown as a mosaic plot in Figure 2. The probability of a worse outcome in the RRT patient group was 68% (95% CI, 61%–74%) when compared with the control group.
Figure 2.
Mosaic plot of outcomes at 28 days. If a patient was discharged prior to 28 days, the last observation was carried forward. Using desirability of outcome ranking analysis, renal replacement therapy patients had a 68% (95% confidence interval, 61%–74%) likelihood of a worse outcome at 28 days as compared with controls. Abbreviation: LTACH: long-term acute care hospital.
DISCUSSION
In our cohort, CRE-infected patients receiving RRT have a higher likelihood of death and increased in-hospital mortality compared with controls, even after adjusting for source of infection, level of care at admission, infection type, and level of critical illness. RRT patients tended to have more comorbid conditions, which likely contributed to the observed increased hospital mortality. RRT patients with CRE infections were more likely to be admitted from higher levels of care than control patients and less likely than controls to be admitted from home. In addition, they are more acutely ill at the time of their CRE infection when compared with control patients with normal renal function. RRT patients also have a lower proportion of low-mortality urinary tract infections and higher proportions of bloodstream infections.
In addition, a DOOR analysis was performed to rank patients based on the range of possible dispositions following treatment of CRE infection [26]. In a DOOR analysis, when comparing outcomes between 2 groups, a likelihood of 50% of a worse outcome is indicative of no numerical difference between the groups. Similarly, if the 95% confidence interval crosses 50%, there is no statistically significant difference between the groups. In our study, the likelihood of a worse outcome for a patient in the RRT group as compared with a patient in the control group was 68%. DOOR analysis is a novel method of analyzing outcomes on a spectrum of patient experiences. We have recently used DOOR analysis to compare patients treated with colistin vs ceftazidime-avibactam [27]. DOOR analysis provides an opportunity to analyze several states between alive and dead that are important to patients. In this study, we have used DOOR analysis to give a visual and statistical association between the exposure of interest (RRT in this case) and outcomes after CRE infection. While this is an association that does not imply causality, the robust observation of poor outcomes in this vulnerable patient population and the quantification of this effect add to the existing literature of impact of infections on patients with chronic renal failure. Furthermore, these findings add to existing studies to emphasize the clear need for infection control measures and antibiotic stewardship in patients on RRT [28].
RRT patients have been shown to have a higher risk for the acquisition of multidrug-resistant (MDR) organisms than patients with normal renal function. For instance, receipt of RRT was associated with an aOR of 2.34 for infection with ampC-carrying organisms [29]. More data exist for higher rates of infection with MRSA and VRE in the RRT population when compared with patients with normal renal function [30, 31]. Outcomes in resistant Enterobacteriaceae infections in the chronic RRT population are rare in the literature. A 42-month study of French RRT patients showed that 42% developed any bloodstream infection during the study period, 32/93 experienced hospitalization or death, and isolation of an MDR pathogen was associated with an increased chance of hospitalization or death (OR, 2.75; 95% CI, 1.01–7.48) [32]. An Israeli study found higher mortality in CRE-infected patients than those infected with carbapenem-susceptible strains (OR, 1.9; 95% CI, 1.2–3.1). Thirty-day mortality was associated with several comorbid conditions, including dialysis (OR, 5.6; 95% CI, 2.7–11.5) and chronic renal failure (OR, 3.1; 95% CI, 1.9–5.1), though they did not compare RRT and non-RRT patients with CRE infections [33]. Bacteremic RRT patients in the United Kingdom experienced worse outcomes than bacteremic renal transplant patients in a setting where Enterobacteriaceae comprised the majority (57/77; 74%) of the infecting strains; of which 15/57 (26%) were ESBL-producing Enterobacteriaceae [34].
The difference in distribution of infections between the 2 groups was driven primarily by the lower share of urinary tract infections in the RRT group; this finding is expected as 72% of RRT patients are anuric at 1 year from RRT initiation [35]. We have previously shown that within the various sources of CRE infection in hospitalized patients, bacteremia and pneumonia are associated with the highest mortality risk, and urinary tract infection the lowest [21]. Similarly, proportions of in-hospital mortality observed in patients with CRE urinary tract infections were similar to controls with CRE urinary tract colonization [21]. Adjustment for source was, therefore, an important part of our multivariable analysis. Even after this adjustment, there was a large excess risk of death associated with RRT.
The increased use of colistin in the RRT population may have several explanations that are not mutually exclusive. First, it may reflect the acuity of illness at the time of index culture. An association between critical illness and colistin use was noted. In 1 study of colistin use in a 2200-bed health system, patients receiving colistin had high proportions of sepsis/severe sepsis/septic shock (80%), mechanical ventilation (62%), and baseline renal insufficiency (25%), suggesting that colistin is used principally in a critically ill population [36]. Second, a lack of concern for nephrotoxicity in RRT patients may play a role. This is difficult to assess from the literature as no studies have specifically surveyed providers’ use of colistin. One study of antibiotic choice in health care–associated pneumonia treatment identified only duration of admission >5 days and Acinetobacter baumanii prevalence >10% in respiratory cultures as predictors of colistin use; renal replacement therapy was not a studied variable; however, prevalence of chronic renal failure in the study population was 15% [37]. Furthermore, patients requiring any kind of renal replacement therapy including RRT are typically excluded from studies of colistin use. Third, colistin may be used more for specific infection types. We observed an association between colistin use and bloodstream infections but not pneumonia, which may reflect a lower propensity for CRE to cause pneumonia as compared with Pseudomonas aeruginosa and Acinetobacter baumannii.
The study had several limitations as a result of its observational design and geographical origin. The etiology of carbapenem resistance in the hospitals studied is predominantly blaKPC; therefore, these results may not be generalizable to infecting isolates with other mechanisms of carbapenem resistance. Also, the study was conducted in the Great Lakes region of the United States, and other patterns may be observed elsewhere. Our choice of a serum creatinine <2 mg/dL for inclusion in the control group does not ensure normal renal function. However, this level would be unusual in a patient with moderate to severe renal failure.
Previous studies have identified both carbapenem resistance and chronic RRT as risk factors for poor outcomes during infection episodes. Gram-negative multidrug resistance has been previously associated with increased mortality in bacteremic episodes, and chronic RRT has been associated with increased risk of acquiring MDR infection and an increased risk of death. Here we show that chronic RRT is associated with higher mortality in CRE infections compared with control CRE-infected patients with serum creatinine <2 mg/dL. Furthermore, by DOOR analysis, we show that RRT patients have a 68% likelihood of an overall worse outcome. Beyond the human and financial costs of increased levels of care at discharge, this analysis highlights the impact of spread of CRE to nursing homes, LTACH, and, in chronic RRT patients, RRT centers. Because CRE infections result in such high levels of morbidity and mortality, further attention is needed to prevent infection by CRE, particularly in the RRT population and in the RRT centers they frequent.
Acknowledgments
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number UM1AI104681 and number R21AI114508, and by funding to DVD and FP from the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research. BE was supported by SUNY Downstate Medical Center Start-up funding. VGF was supported by Mid-Career Mentoring Award K24-AI093969 from the NIH. In addition, research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R21AI114508 (DvD and RAB), R01AI100560 (RAB), R01AI063517 (RAB), and R01AI072219 (RAB). This study was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01BX001974 to RAB, from the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 (RAB). Further support came from the Research Program Committees of the Cleveland Clinic (DVD), an unrestricted research grant from the STERIS Corporation (DVD). YD was supported by research awards R01AI104895 and R21AI123747 from the NIH. KSK is supported by the National Institute of Allergy and Infectious Diseases (Division of Microbiology and Infectious Diseases protocol 10-0065 and R01AI119446-01).
Potential conflicts of interest. No potential conflicts: BE, EC, FP, RAS, RCK, JD, BK, and SE. Potential conflicts of interest: SSR: research support from bioMerieux, BD Diagnostics, BioFire, OpGen, Forest Laboratories, Achaogen, Nanosphere and Pocared; honorarium from bioMerieux. YD: grant support from the NIH; advisory boards for Meiji, Allergan, The Medicines Company, Curetis, Roche. KK: consultant and grant investigator for Allergan; consulting fee, grant recipient, and speaker honorarium from Speaker’s Bureau; grant recipient and consultant for Merck; consultant for Xellia and Achaogen. RRW: grant support from Allergan. RAB: grant investigator and grant recipient for AstraZeneca, Merck, Melinta, Steris, NIH, VA Merit Review. VGF: grant/research support from Advanced Liquid Logic, Cubist, Cerexa, MedImmune, Merck, NIH, Novartis, Pfizer, Theravance; paid consultant for Affinium, Baxter, Cerexa, Cubist, Debiopharm, Durata, Merck, Novartis, NovaDigm, The Medicines Company, MedImmune, Pfizer, Theravance, Trius; honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, Vertex Pharmaceuticals; Merck Co-Chair V710 Vaccine. DvD: advisory board for Allergan, Achaogen, Shionogi, Tetraphase, Sanofi-Pasteur, MedImmune, Astellas; research funding from Steris Inc., Scynexis. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.USRDS. Annual Data Report. 2015. Available at: https://www.usrds.org/adr.aspx. Accessed 20 October 2017. [Google Scholar]
- 2. Dalrymple LS, Johansen KL, Romano PS et al. . Comparison of hospitalization rates among for-profit and nonprofit dialysis facilities. Clin J Am Soc Nephrol 2014; 9:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zarkowsky DS, Arhuidese IJ, Hicks CW et al. . Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg 2015; 150:529–36. [DOI] [PubMed] [Google Scholar]
- 4. D’Agata EM. Antimicrobial use and stewardship programs among dialysis centers. Semin Dial 2013; 26:457–64. [DOI] [PubMed] [Google Scholar]
- 5. Kato S, Chmielewski M, Honda H et al. . Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kallen AJ, Arduino MJ, Patel PR. Preventing infections in patients undergoing hemodialysis. Expert Rev Anti Infect Ther 2010; 8:643–55. [DOI] [PubMed] [Google Scholar]
- 7. Simenhoff ML, Dunn SR, Zollner GP et al. . Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab 1996; 22:92–6. [PubMed] [Google Scholar]
- 8. Vaziri ND, Wong J, Pahl M et al. . Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013; 83:308–15. [DOI] [PubMed] [Google Scholar]
- 9. Calfee DP. Multidrug-resistant organisms in dialysis patients. Semin Dial 2013; 26:447–56. [DOI] [PubMed] [Google Scholar]
- 10. Rojas L, Muñoz P, Kestler M et al. . Bloodstream infections in patients with kidney disease: risk factors for poor outcome and mortality. J Hosp Infect 2013; 85:196–205. [DOI] [PubMed] [Google Scholar]
- 11. Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int 2000; 58:1758–64. [DOI] [PubMed] [Google Scholar]
- 12. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest 2001; 120:1883–7. [DOI] [PubMed] [Google Scholar]
- 13. Bhatia N, Agrawal S, Garg A et al. . Trends and outcomes of infective endocarditis in patients on dialysis. Clin Cardiol 2017; 40:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reed SD, Friedman JY, Engemann JJ et al. . Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 2005; 26:175–83. [DOI] [PubMed] [Google Scholar]
- 15. Pant C, Deshpande A, Anderson MP, Sferra TJ. Clostridium difficile infection is associated with poor outcomes in end-stage renal disease. J Investig Med 2012; 60:529–32. [DOI] [PubMed] [Google Scholar]
- 16. Bleumin D, Cohen MJ, Moranne O et al. . Carbapenem-resistant Klebsiella pneumoniae is associated with poor outcome in hemodialysis patients. J Infect 2012; 65:318–25. [DOI] [PubMed] [Google Scholar]
- 17. van Duin D, Cober E, Richter SS et al. . Residence in skilled nursing facilities is associated with tigecycline nonsusceptibility in carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2015; 36:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Duin D, Cober E, Richter SS et al. . Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother 2015; 70:1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Duin D, Perez F, Rudin SD et al. . Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58:4035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Duin D, Cober ED, Richter SS et al. . Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clin Microbiol Infect 2014; 20:O1117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hauck C, Cober E, Richter SS et al. ; Antibacterial Resistance Leadership Group Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 2016; 22:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 23. Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 1999; 11:7–12. [DOI] [PubMed] [Google Scholar]
- 24. Feldman C, Alanee S, Yu VL et al. ; International Pneumococcal Study Group Severity of illness scoring systems in patients with bacteraemic pneumococcal pneumonia: implications for the intensive care unit care. Clin Microbiol Infect 2009; 15:850–7. [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 26. Evans SR, Rubin D, Follmann D et al. . Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015; 61:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Duin D, Lok JJ, Earley M, et al. Colistin vs. ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snyder GM, Patel PR, Kallen AJ et al. . Factors associated with the receipt of antimicrobials among chronic hemodialysis patients. Am J Infect Control 2016; 44:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammer KL, Stoessel A, Justo JA et al. . Association between chronic hemodialysis and bloodstream infections caused by chromosomally mediated AmpC-producing Enterobacteriaceae. Am J Infect Control 2016; 44:1611–6. [DOI] [PubMed] [Google Scholar]
- 30. Nielsen LH, Jensen-Fangel S, Benfield T et al. . Risk and prognosis of Staphylococcus aureus bacteremia among individuals with and without end-stage renal disease: a Danish, population-based cohort study. BMC Infect Dis 2015; 15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zacharioudakis IM, Zervou FN, Ziakas PD et al. . Vancomycin-resistant enterococci colonization among dialysis patients: a meta-analysis of prevalence, risk factors, and significance. Am J Kidney Dis 2015; 65:88–97. [DOI] [PubMed] [Google Scholar]
- 32. Fram D, Taminato M, Ponzio V et al. . Risk factors for morbidity and mortality of bloodstream infection in patients undergoing hemodialysis: a nested case-control study. BMC Res Notes 2014; 7:882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussein K, Raz-Pasteur A, Finkelstein R et al. . Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect 2013; 83:307–13. [DOI] [PubMed] [Google Scholar]
- 34. Melzer M, Santhakumaran T, Welch C. The characteristics and outcome of bacteraemia in renal transplant recipients and non-transplant renal patients. Infection 2016; 44:617–22. [DOI] [PubMed] [Google Scholar]
- 35. Shafi T, Jaar BG, Plantinga LC et al. . Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 2010; 56:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pogue JM, Lee J, Marchaim D et al. . Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–84. [DOI] [PubMed] [Google Scholar]
- 37. Rello J, Ulldemolins M, Lisboa T et al. ; EU-VAP/CAP Study Group Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J 2011; 37:1332–9. [DOI] [PubMed] [Google Scholar]