Abstract
Type B insulin resistance is a rare syndrome characterized by fluctuating glucose levels (ranging from hyperglycemia with extreme insulin resistance to intractable hypoglycemia without exogenous insulin administration), high serum insulin levels, and insulin receptor autoantibodies. Most cases occur in the African American population in association with other underlying autoimmune systemic diseases. Treatments with high-dose steroids, immunosuppressants, and plasmapheresis have been used, with variable outcomes, in patients without spontaneous remission. We report the case of a 60-year-old African American woman with history of systemic lupus erythematosus presenting with extreme fluctuations in glucose levels, ranging from severe hyperglycemia to refractory hypoglycemia, with high serum concentration of insulin in both phases. Her presentation and phenotype were very similar to those seen in known cases of type B insulin resistance associated with insulin receptor antibodies. Treatment in other reported cases used a combination of high-dose steroids and immunosuppressants. We tried high-dose steroids, azathioprine, and intravenous immunoglobulins, which resulted in improvement and barely detectable insulin receptor antibody. We present a case of type B insulin resistance with abnormally low titers of insulin receptor antibodies despite a typical clinical course and response. Future research is needed to improve diagnosis and treatment in this rare disease.
Keywords: hypoglycemia, insulin receptor antibodies, insulin resistance, type B insulin resistance
A patient with type B insulin resistance who presented with severe hypoglycemia had a good response to immunosuppressive therapy and intravenous immunoglobulins.
Type B insulin resistance is a rare syndrome caused by autoantibodies to the cell surface insulin receptor [1]. This heterogenous metabolic syndrome is seen predominantly in African American women in the United States [2]. It almost always occurs concomitantly with an underlying autoimmune systemic disease such as systemic lupus erythematosus (SLE). It causes a spectrum of abnormalities ranging from severe hyperglycemia with extreme insulin resistance to intractable hypoglycemia. Other associated features are hyperandrogenism and acanthosis nigricans [3]. The exact prevalence and disease course are not well known because of the rarity of this syndrome. High-dose steroids, immunosuppressants (such as cyclophosphamide), cyclosporine A, azathioprine, rituximab, and plasmapheresis have been used in treatment, with variable effects [4–6]. We describe a patient whose clinical presentation and phenotype were consistent with type B insulin resistance and who responded to empiric treatment before the diagnosis could be completely established. This case demonstrates the difficulty in diagnosing and treating this condition.
1. Case Report
A 60-year-old African American woman with a history of SLE and Hashimoto hypothyroidism presented with substantial weight loss, nausea, and vomiting. She also noted frequent episodes of hypoglycemia over the preceding 3 months. She reported that her hypoglycemia could occur both overnight and a few hours after a meal. She had noticed dramatic weight loss of almost 100 pounds over a 12-month period. Physical examination revealed acanthosis nigricans on the face, neck, and upper back. She did not report any symptoms of hyperandrogenism.
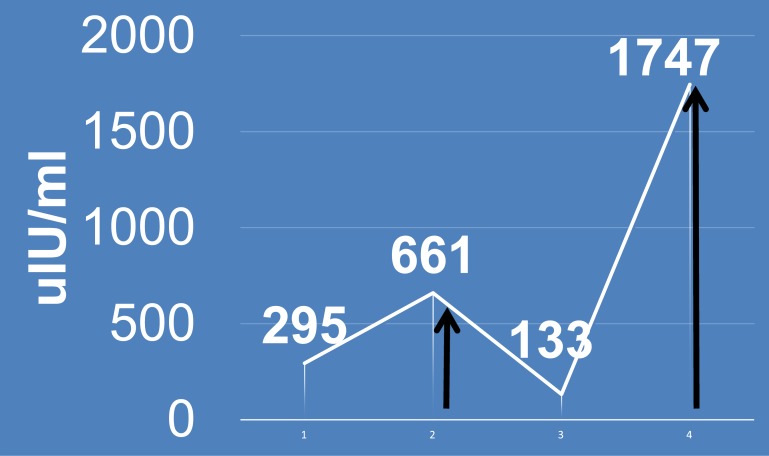
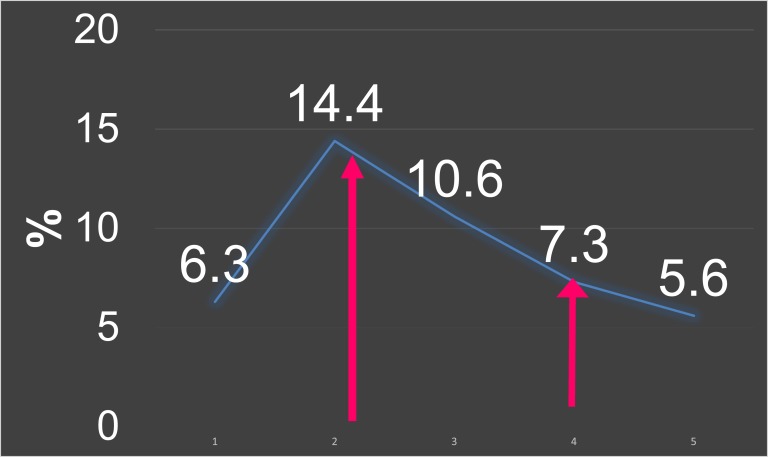
One year earlier, the patient was hospitalized for similar episodes of symptomatic and refractory hypoglycemia, with serum glucose values as low as 37 mg/dL. Investigations at that time indicated a hyperinsulinemic state (insulin, 661 μIU/mL [normal range, 2 to 19.6 μIU/mL]; proinsulin, 58.4 pmol/L [normal, <18.8 pmol/L]; C-peptide, 9.1 ng/mL [normal range, 0.8 to 3 ng/mL]; glycosylated hemoglobin, 7.3%) (Fig. 1 and 2) with blood glucose level of 49 mg/dL. A cosyntropin stimulation test yielded appropriate cortisol values of 9 μg/dL, 20 μg/dL, and 23 μg/dL at baseline, 30 minutes, and 60 minutes, respectively. Results on sulfonylurea screen and testing for serum insulin growth factor 2 were negative. Computed tomography of the abdomen with contrast showed a normal pancreas with no masses. She responded to treatment with intravenous (IV) immunoglobulin for three cycles, along with prednisone, 60 mg daily. The plan was to taper the prednisone dose gradually, but this resulted in hyperglycemia. She was diagnosed with type 2 diabetes and sent home on a basal bolus insulin regimen.
Figure 1.
Insulin trend. Arrows indicate treatment.
Figure 2.
HbA1c trend. Arrows indicate treatment points.
Because of the discrepancy in the proinsulin and insulin levels and her extensive history of autoimmune disorders, it was thought that she might have insulin receptor antibodies. Unfortunately, she was lost to follow-up and returned 1 year later. She did not have any hospitalizations elsewhere and had not seen another provider in the interim for this issue. In the interim she had discontinued insulin after she noticed blood glucose levels around 100 mg/dL at home.
During a hospital admission for small bowel obstruction in late October 2015, she had persistently low blood glucose levels (within 40 to 50 mg/dL) despite recovery of bowel function. She had continued hypoglycemia despite adequate nutrition and trial of IV dextrose, high-dose steroids, glucagon, and octreotide. During this admission, she also exhibited a hyperinsulinemic state during hypoglycemia (insulin, 1747 μIU/mL; C-peptide, 25 ng/mL) (Fig. 1 and 2). Insulin autoantibodies were negative (Table 1). She did not have any leukopenia during her hypoglycemic episodes. Her triglyceride level before development of hypoglycemia (in September 2013) was 69 mg/dL. A regimen of high-dose steroids (prednisone, 60 mg daily) did not stabilize her glucose levels. She then received a trial of IV immunoglobulin (1 mg/kg per day for 3 days), along with azathioprine, 100 mg daily, in addition to prednisone. This regimen has been used for treatment of type B insulin resistance with some success. She responded to this regimen within hours.
Table 1.
Comparison of Laboratory Results From Both Admissions
| Admission | Insulin (μIU/mL) | Proinsulin (pmol/L) | C-peptide (ng/mL) | HbA1c (%) | Glucose (mg/dL) |
|---|---|---|---|---|---|
| First | 661 | 58.4 | 9.125 | 7.3 | 49 |
| Second | 1747 | — | — | — | 34 |
Normal ranges are as follows: insulin, 2 to 19.6 μIU/mL; proinsulin, <18.8 pmol/L; C-peptide, 0.8 to 3 ng/mL.
Abbreviation: HbA1c, hemoglobin A1c.
Since then her glucose levels have improved, maintained at 70 to 110 mg/dL despite tapering of the steroid dose. Antibody levels were checked several months later when she was euglycemic; these levels were weakly positive, but no levels were available to quantify for assay. It has been almost 1 year since her last hospitalization, and she continues to have subjective episodes of hypoglycemia without any refractory hypoglycemia. She is currently receiving prednisone, 5 mg daily, as part of the treatment of SLE.
2. Discussion
Insulin receptor autoantibodies are thought to exert their effects by two mechanisms leading to insulin resistance. Autoantibodies bind to insulin receptors and competitively inhibit insulin action. In addition to binding inhibition, they accelerate the rate of receptor degradation. These antibodies also exhibit a clinical paradox between an insulin mimetic effect and their ability to cause insulin resistance, leading to a biphasic response of hyper- and hypoglycemia in the same patient [7]. Testing for these antibodies is usually by the immunoprecipitation method, preferably in the hyperglycemic phase. As seen in our patient, hypoglycemia can result at low titers of antibodies because it acts like a partial agonist. Insulin receptor antibodies were detected in our patient, albeit at a low level. However, given her clinical presentation, response to treatment, and the fact that these antibodies are not found in the general population, we feel that this case is consistent with type B insulin resistance.
We felt that factitious self-administration of insulin was extremely unlikely given that the patient was in a monitored setting when these hypoglycemic episodes occurred. Furthermore, with exogenous insulin-mediated hypoglycemia, C-peptide levels are typically undetectable (<0.2 ng/mL), whereas our patient had detectable levels. Although an insulinoma could give a similar presentation and may not be seen on computed tomography, we felt that an insulinoma was unlikely given her lack of improvement with empiric octreotide treatment, coupled with her dramatic and maintained clinical response to autoimmune therapy.
Literature review revealed three other case reports of spontaneous hypoglycemia and elevated antibody levels, but no clear identifiable pathogenic mechanism was identified [8]. Previous studies have associated hypoglycemia with a negative clinical prognosis for immediate outcome, but our patient has done well, with only subjective hypoglycemia.
Spontaneous remission occurs in about one third of patients. However, in those who do not achieve remission, the treatment has generally been unsatisfactory. Treatment in the hyperglycemic phase is usually with high doses of insulin (average, 5100 U/d in some studies), but managing the hypoglycemic phase becomes challenging. High-dose corticosteroids seem to have the most immediate effectiveness, but no studies suggest that long-term therapy has any benefit. Other strategies for treatment have been directed toward control of the underlying systemic disease, including immunosuppressive therapies, depending on its severity. Plasmapheresis, corticosteroids, or cytotoxic agents have only short-term benefits. Data regarding use of IV immunoglobulin for treatment are limited, but this therapy has been used in some cases. Long-term data are also limited because of the high mortality rate seen in this disease.
Our treatment of this patient was delayed by the variation in antibody titers, which precluded us from testing during the hypoglycemic phase. Because this condition can be fatal, it requires a high index of clinical suspicion and low threshold for empiric treatment. It is also important to identify these patients because they may be diagnosed with type 2 diabetes and treated with insulin; that treatment could be fatal if the patient later enters a hypoglycemic phase, as our patient did.
Although in this patient antibody titers are low, we still feel her clinical presentation and response to therapy are most consistent with type B insulin resistance. Future research is needed to improve diagnosis and treatment in this rare disease.
Acknowledgments
Current Affiliation: L. Viswanathan’s current affiliation is the Division of Endocrinology, Diabetes and Metabolism, Allegheny Health Network, Pittsburgh, PA 15212.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- IV
- intravenous
- SLE
- systemic lupus erythematosus.
References and Notes
- 1.Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976;294(14):739–745. [DOI] [PubMed] [Google Scholar]
- 2.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore). 2002;81(2):87–100. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SI, Accili D, Cama A, Imano E, Kadowaki H, Kadowaki T. Unusual forms of insulin resistance. Annu Rev Med. 1991;42(1):373–379. [DOI] [PubMed] [Google Scholar]
- 4.Malek R, Chong AY, Lupsa BC, Lungu AO, Cochran EK, Soos MA, Semple RK, Balow JE, Gorden P. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J Clin Endocrinol Metab. 2010;95(8):3641–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran HA, Reeves GE. Treatment of type B insulin resistance with immunoglobulin: novel use of an old therapy. Med J Aust. 2009;190(3):168. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson JW, Bremell T, Eliasson B, Fowelin J, Fredriksson L, Yu ZW. Successful treatment with plasmapheresis, cyclophosphamide, and cyclosporin A in type B syndrome of insulin resistance. Case report. Diabetes Care. 1998;21(8):1217–1220. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SI, Grunberger G, Marcus-Samuels B, Underhill LH, Dons RF, Ryan J, Roddam RF, Rupe CE, Gorden P. Hypoglycemia associated with antibodies to the insulin receptor. N Engl J Med. 1982;307(23):1422–1426. [DOI] [PubMed] [Google Scholar]
- 8.Chon S, Choi MC, Lee YJ, Hwang YC, Jeong IK, Oh S, Ahn KJ, Chung HY, Woo JT, Kim SW, Kim JW, Kim YS. Autoimmune hypoglycemia in a patient with characterization of insulin receptor autoantibodies. Diabetes Metab J. 2011;35(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]