Abstract
Objective
To examine the relationship between objectively-assessed moderate-to-vigorous intensity physical activity (MVPA) and 4-year weight loss (WL) and WL maintenance among individuals with diabetes enrolled in the Look AHEAD trial.
Methods
MVPA was measured on a subgroup of lifestyle intervention participants with accelerometry data at baseline, 1 and 4 years (n=553; age: 59.7±6.8yrs; BMI:35.5±5.9kg/m2). Minutes/week of bout-related MVPA was calculated (≥3 METs, ≥10-min bouts) and adherence to the national PA recommendation for WL maintenance (≥250 min/week) was assessed.
Results
Independent of 1-year WL, 4-year MVPA (β=-0.003, SE=0.002, p=0.006), but not 1-year MVPA (β=0.0001, SE=0.001, p=0.50) was significantly associated with 4-year WL. Compared to ‘Non-maintainers’ (≥10% WL at Year 1, but <10% at Year 4; n=132), WL Maintainers (≥10% WL at Years 1 and 4; n=103) had higher MVPA at Year 1 (253.4±251.8 vs. 163.9±158.2 min/wk, p=0.002) and Year 4 (155.3±180.6 vs. 111.4±154.5 min/wk, p=0.046). While 38.8% and 22.3% of WL Maintainers engaged in ≥250 min/week at years 1 and 4 respectively, many engaged in <150 min/week (Year 1: 41% and Year 4: 61%).
Conclusions
Higher weekly MVPA is associated with greater long-term WL and weight maintenance; however many individuals are able to maintain ≥10% WL while engaging in little MVPA.
Keywords: weight control, exercise, type 2 diabetes, accelerometery
Introduction
Physical activity (PA) has been shown to have a modest effect on initial weight loss (WL); however PA appears to play a more prominent role in WL maintenance (1-4). The American College of Sports Medicine (ACSM) recommends that adults participate in ≥150 minutes/week of MVPA to prevent significant weight gain and reduce chronic disease risk. However, for the prevention of weight regain following WL, ≥250 minutes/week of MVPA is recommended (1). While these guidelines are evidence-based, they were derived largely from studies that relied on self-report measures of PA which are often prone to participant biases, including social desirability influence and imprecise recall (5). Findings from one study confirm previous self-report PA findings, demonstrating that 200-300 minutes/week of objectively-assessed MVPA, accumulated in bouts of >10 minutes, was associated with improved WL at 18 months. However, the proportion of participants meeting the recommended 250 min/week threshold was not examined (6). Given that stronger associations between objective PA, rather than subjective PA, and various health indictors have been observed, (7, 8), it is imperative that the role of objectively-assessed PA continue to be examined within the context of long-term WL and weight maintenance.
The optimal level of PA needed for long-term WL and WL maintenance is not entirely understood. While long-term WL is often examined by determining the proportion of participants meeting a clinically significant WL threshold at a distant time point (e.g., ≥10% at 18 months), WL maintenance requires an individual to both achieve and maintain a WL ≥10% both initially and at follow-up (9, 10). Few studies have examined the quantity of objectively-measured MVPA required for successful WL maintenance within the context of behavioral WL intervention trials longer than 18 months. Further, it is unclear whether the level of MVPA needed to maintain WL is similar in older adults, and specifically those with diabetes, given that those individuals typically have lower than average levels of MVPA (11, 12).
The Look AHEAD trial provides an excellent opportunity to address these questions. Look AHEAD is a multi-center, randomized trial examining the effect of an intensive lifestyle intervention on the primary and secondary prevention of cardiovascular disease in overweight and obese adults with Type 2 diabetes. We have previously reported on changes in weight (13) and objectively-assessed PA in Look AHEAD (14) and found that those randomized to the intensive lifestyle intervention (ILI) arm had significantly greater WL and MVPA minutes at 1 and 4 years, compared to Diabetes Support and Education (DSE; control condition). Further, the percent of participants losing and maintaining ≥10% weight loss was far greater in ILI than DSE. In this paper we examine the relationship between: 1) PA and WL at 1 year (short-term WL), 2) PA and WL at 4 years (long-term WL), and 3) PA and WL maintenance between years 1 and 4. Specifically, we capitalize on the large number of individuals randomized to ILI who achieved ≥10% WL at Year 1 (n=235; 43% of sample) and examine whether there are differences in MVPA between those who maintain and do not maintain this magnitude of WL. Further, we examine individual level MVPA data and identify the percentage of WL maintainers achieving the PA guidelines for weight control (1).
Methods
Participants
Accelerometry data in Look AHEAD was collected at 8 of the 16 sites of this trial (n=2,622). Full descriptive data for ILI participants (n=1309) enrolled in the accelerometer sub-study have been reported previously (15). Given that the aim of this paper was to examine changes in PA and WL between years 1 and 4 (i.e., WL maintenance), we studied only ILI participants who had weight and ‘valid’ accelerometry data (see definition below) at baseline, year 1, and year 4 (n=573). Further, participants who underwent bariatric surgery at any time point were excluded (n=20); thus the present analyses focus on 553 participants. Participants included in these analyses were similar to the entire accelerometer sub-study cohort on all demographics measures, except for BMI, which was lower in the current subgroup of participants (35.5±5.9 vs. 36.4±6.0 kg/m2). In short, participants included in these analyses were at baseline 59.7±6.8 years of age, 55% were female, and 76% were Caucasian. All participants provided written informed consent, and study procedures were approved by each center's institutional review board.
Outcome measures
Weight change
Body weight was assessed at baseline, Year 1, and Year 4 using a digital scale (model BWB-800; Tanita, Willowbrook, IL) by a staff member masked to the intervention assignment. The change in weight from baseline to 1 year and baseline to 4 years was calculated.
Objective assessment of physical activity
The RT3 triaxial accelerometer (StayHealthy, Monrovia, CA) was used to provide an objective measure of PA at baseline, Year 1, and Year 4. Participants were instructed to wear this waist-mounted device for seven consecutive days during waking hours, removing it only for periods of bathing, showering, or other water-based activities. Participants were also instructed not to alter their typical PA pattern while wearing this device. Data collection and quality control procedures have been reported previously (15). In short, accelerometer wear time was calculated by taking all possible daily minutes (1440 min) and subtracting periods of time that the device was not worn (defined as ≥30 continuous minutes of zero activity counts). A ‘valid’ day was defined as ≥10 hours of wear time and participants needed ≥4 ‘valid’ days to be included in the analyses.
Physical activity intensity was determined for each minute that the device was worn. Metabolic equivalent (MET) values were calculated by dividing the estimated energy expenditure for a given minute by the estimated resting energy expenditure, both of which were provided by the StayHealthy software that accompanied the accelerometer. Using ACSM's criteria, MVPA was defined as any activity ≥ 3 METs (16). Consistent with the national PA guidelines which recommend that MVPA bouts be ≥10 minutes in duration, the accelerometry data were analyzed to determine ‘bout-related MVPA’, which was defined as any activity ≥3 METs and ≥10 minutes in duration, allowing for a 1-minute interruption in MVPA (i.e., 1 minute <3.0 METs). MET-minutes/week spent in bout-related MVPA was calculated by multiplying the number of MVPA minutes by the mean MET value.
Treatment conditions
Look AHEAD participants were randomly assigned to an intensive lifestyle intervention or Diabetes Support and Education, which served as the comparison group. Full descriptions of the ILI and DSE conditions have been provided previously (13, 17). The analyses presented focuses only on ILI participants.
Intensive Lifestyle Intervention
During Months 1-6, ILI participants attended 3 weekly group sessions and one individual counseling session per month, which was reduced to 2 group and one individual session/month in Months 7-12. During Years 2-4, participants had one, in-person, individual meeting (20-30 min) with their interventionist each month, with a second individual contact by telephone (10-15 min) or email, 2 weeks later. Further, in Years 2-4, monthly group sessions were offered, in which participants listened to a presentation on a new topic on lifestyle modification which included information on food intake, PA, or behavior change.
In Year 1, ILI participants were prescribed a calorie goal of 1200-1800 kcal/day depending upon initial body weight, were instructed to consume <30% of total calories from dietary fat, and meal replacements were provided. Participants were given a home-based PA regimen designed to gradually increase structured activity to ≥175 min/week within the first 6 months, with a further increase to ≥200 min/week for those who met this goal. Effective behavioral strategies such as regular self-weighing, daily self-monitoring, and stimulus control techniques were discussed. In Years 2-4, participants continued with individualized calorie goals and were encouraged to continue to exercise at least 175 min/week.
Statistical Analyses
Change in PA and weight over the 3 time points was assessed using general linear models (GLM) for continuous variables and chi-square tests for categorical variables. Post-hoc comparisons across time points and outcomes utilized a Bonferroni correction. Correlations were computed to examine the relationship between both 1-year PA and 1-year change in PA with 1-year WL, while also controlling for baseline PA and Year 1 accelerometer wear time and other demographic variables, including age, gender, and race. Exploratory analyses examined the relationship between 1-year or 4-year PA and weight change, by stratifying participants into one of four PA categories: <50, 50 to <150, 150 to <250, and ≥250 min/week. GLM was used to compare these PA categories on 1-year WL or 4-year WL and post-hoc group comparisons utilized a Bonferroni adjustment. Linear models were used to examine whether 1 or 4-year PA was most strongly associated with 4-year WL and to determine whether 4-year WL was predominately driven by changes in 1-year WL or PA engagement. Linear models were also used to compare WL maintainers and non-maintainers on PA at each time point. Exploratory analyses, examining whether the distribution of WL Maintainers falling into various PA categories at Years 1 and 4 differed from Non-maintainers, were performed using Chi-square analyses. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and statistical significance was set at p<0.05.
Results
Table 1 presents weight change and PA data at baseline, 1 and 4 years in these participants. Mean WL was 9.7% at Year 1 and 5.0% at Year 4. Forty-three percent achieved a clinically significant WL (i.e., ≥10%) at Year 1 and 44% of these participants maintained a WL of at least 10% through Year 4. On average, bout-related MVPA increased by 63 min/week at Year 1 but returned to near baseline at Year 4. Only 21% and 12% of participants achieved or exceeded the national PA goal for WL maintenance (≥250 min/week) at Years 1 and 4 respectively.
Table 1. Descriptive physical activity and body weight data stratified by assessment time point in ILI participants (n=553).
| Baseline | Year 1 | Year 4 | Overall p-value over time | |
|---|---|---|---|---|
| Physical activity | ||||
| Accelerometer wear time (hours/day) | 13.1 (12.9, 13.3) | 12.9 (12.7, 13.1) | 12.7 (12.4, 12.9) | 0.05 |
| Bout-related MVPA (min/week) | 98.7 (86.8, 110.6)a | 161.6 (142.3, 180.8)b | 110.5 (92.1, 128.9)a | <0.0001 |
| MET-min/week | 564.3 (492.9, 635.7)a | 924.1 (811.3, 1037)b | 613.7 (502.6, 724.8)a | <0.0001 |
| % achieving ≥150 min/week of MVPA | 122 (22.1%)a | 198 (35.8%)b | 128 (23.2%)c | <0.0001 |
| % achieving ≥250 min/week of MVPA | 61 (11.0%)a | 115 (20.8%)b | 68 (12.3%)c | <0.0001 |
| Body weight | ||||
| Weight (kg) | 100.9 (99.3, 102.5)a | 91.0 (89.5, 92.5)b | 95.8 (94.2, 97.4)c | <0.0001 |
| Weight change from baseline (%) | -9.7 (-10.2, -9.1)a | -5.0 (-5.6, -4.4)b | <.0001 | |
| % achieving ≥5% weight loss | 421 (76.1%)a | 269 (48.6%)b | <.0001 | |
| % achieving ≥10% weight loss | 235 (42.0%)a | 129 (23.3%)b | <.0001 | |
Mean (95% CI) or N (%). Values with different superscripts across columns are significantly different from one another after Bonferroni adjustment
Aim 1: Physical activity and weight loss at year 1
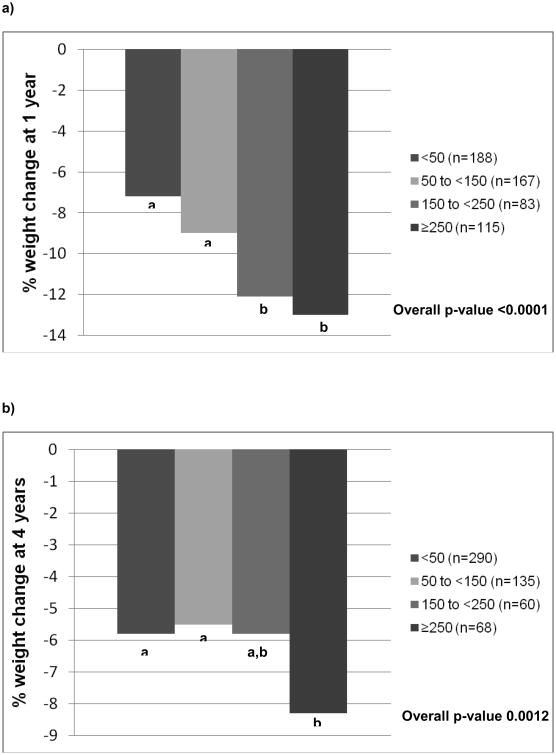
The relationship between PA and WL at Year 1 was examined both continuously and categorically (n=553). Year 1 PA was significantly associated with 1-year weight change (r=0.23, p<0.0001), and this relationship was further strengthened after controlling for baseline PA and demographic factors (r=0.34, p<0.0001). To illustrate this association, we stratified participants by Year 1 MVPA and examined 1-year weight change (Figure 1a). The PA groups were as follows: <50 min/week (n=188), 50 to <150 min/week (n=167), 150 to <250 min/week (n=83), and ≥250 min/week (n=115). Participants engaging in 150 to <250 or ≥250 min/week of MVPA had significantly greater WL at 1-year compared to those engaging in <50 or 50 to <150 min/week. Similar findings were observed when the change in PA from baseline to 1-year was examined – participants with greater improvements in PA also had better WL at Year 1 (adjusted r=0.34, p<0.001).
Figure 1.
a: Percent weight change at 1 year, stratified by 1-year MVPA levels (Aim 1)
b: Percent weight change at 4 years, stratified by 4-year MVPA levels (Aim 2)
Physical activity categories based upon minutes/week of bout-related MVPA at Year 1 (a) and Year 4 (b). Overall p-value represents a group effect for categorized MVPA. Values with different superscripts indicate that groups are significantly different from one another. a. Percent weight change at 1 year, stratified by 1-year MVPA levels (Aim 1), b. Percent weight change at 4 years, stratified by 4-year MVPA levels (Aim 2)
Aim 2: Physical activity and long-term weight loss (year 4)
Similar to Year 1 analyses, participants were grouped into 1 of 4 categories based upon 4-year MVPA, and were compared on 4-year WL. As shown in Figure 1b, participants engaging in ≥250 minutes/week at year 4 (n=68) had a mean 4-year WL of 8.26% (95% CI: 6.08-10.43), which was significantly greater than those engaging in 50 to <150 minutes/week (mean=5.54%, 95% CI=3.66-7.44, n=135) and <50 minutes/week (mean=5.80%, 95% CI=4.02-7.59, n=290). There were no significant differences between the other MVPA groups.
We also examined whether 4-year MVPA predicted percent weight change at year 4, independent of 1-year PA or 1-year WL. Findings reveal that 1-year weight change was most strongly associated with percent weight change at 4 years (β=0.638, SE=0.04, 95% CI: 0.56-0.71,p<0.001). However, 4-year MVPA was also a significant predictor of 4-year WL (β=-0.003, SE=0.001, 95% CI: -0.0001 to -0.006, p=0.006), while 1-year MVPA was not (β=0.001, SE=0.001, 95% CI: -0.002, 0.003, p=0.50). Translated clinically, these findings suggest that for every additional 1 kg WL at Year 1, 4-year WL was increased by 0.61 kg and for every additional 30 minutes of MVPA at Year 4, 4-year WL increased by 0.12 kg.
Aim 3: Physical activity and weight maintenance
Weight maintenance (i.e., weight change from Year 1 to Year 4) was examined only in those 235 participants achieving ≥10% WL at Year 1 and participants were stratified into one of two categories based upon their Year 1 and Year 4 WL: 1) Maintain WL (n=103): 1-year and 4-year WL ≥10% and 2) Non-maintain (n=132): 1-year WL ≥10% and 4-year WL <10%. By definition, 4-year WL was significantly greater in Maintainers (mean=15.1%, 95% CI 14.23-15.98) compared to Non-maintainers (mean=4.0%, 95% CI 3.27-4.78; p<0.001). On average, Maintain and Non-maintain groups regained 11% and 72% of their initial WL respectively between Years 1 and 4.
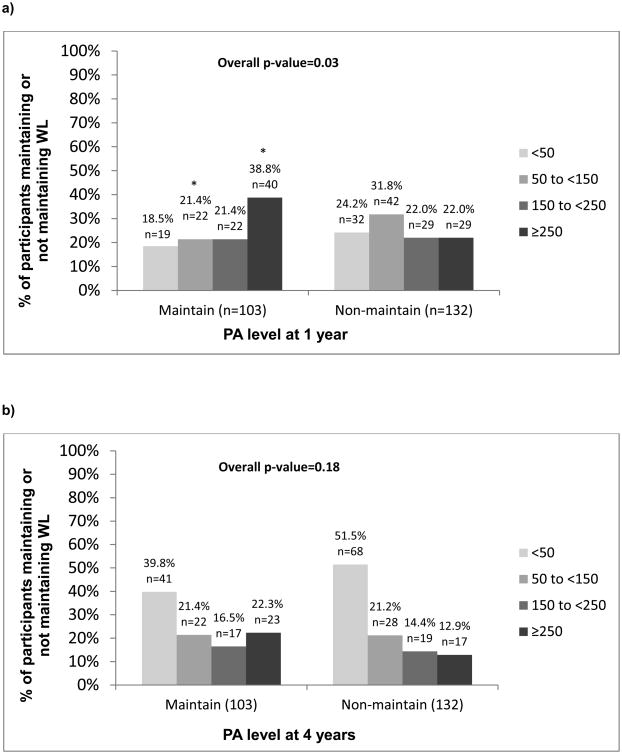
Table 2 compares the activity level of Maintainers and Non-maintainers. While baseline MVPA levels did not differ between groups, the Maintain WL group engaged in significantly more activity at both Year 1 and Year 4. Exploratory analyses were conducted to examine whether the distribution of participants falling into the various PA categories differed between WL Maintainers and Non-maintainers at Year 1 and Year 4 (Figure 2). Compared to the Non-maintain group, a greater percentage of Maintainers were engaging in ≥250 min/week at Year 1 (38.8% vs. 22.0%; p<0.05). At Year 4, 22.3% of Maintainers and 12.9% of Non-maintainers were engaging in ≥250 minutes/week (p=0.06). Of note, 18% and 40% of those maintaining ≥10% WL between Years 1 and 4 were engaging in <50 min/week of bout-related MVPA at Years 1 and 4 respectively.
Table 2. Mean bout-related MVPA at several time points stratified by weight maintenance categories (Aim 3).
| Maintain WL (n=103) | Non-Maintain WL (n=132) | P-value for difference between groups | |
|---|---|---|---|
| Baseline | 112.12 (85.58, 138.66) | 129.49 (95.61, 163.37) | 0.425 |
| Year 1 | 253.42 (204.22, 302.62) | 163.86 (136.62, 191.10) | 0.002 |
| Year 4 | 155.34 (120.04, 190.64) | 111.40 (84.79, 138.01) | 0.046 |
| Year 1 to Year 4 change | -98.08 (-141.86, -54.30) | -52.46 (-80.83, -24.09) | 0.073 |
Mean (95% CI)
Figure 2.
a: Aim 3: Percentage of participants maintaining or not maintaining weight loss from years 1-4, stratified by MVPA levels at year 1
b: Aim 3: Percentage of participants maintaining or not maintaining weight loss from years 1-4, stratified by MVPA levels at year 4
Maintain: ≥10% weight loss at Year 1 and Year 4; Non-maintain: ≥10% weight loss at Year 1 and <10% weight loss at Year 4. Overall p-value represents whether the distribution of participants falling into the various PA categories differs between weight loss Maintainers and Non-maintainers. * indicates that the percentage of Maintain participants falling within a particular MVPA category is significantly different from the percentage of Non-maintain participants falling into that category. a. Aim 3: Percentage of participants maintaining or not maintaining weight loss from years 1-4, stratified by MVPA levels at year 1, b. Aim 3: Percentage of participants maintaining or not maintaining weight loss from years 1-4, stratified by MVPA levels at year 4
Discussion
In this cohort of older adults with type 2 diabetes, higher levels of objectively-assessed MVPA were associated with improved WL maintenance. Weight loss maintainers averaged approximately 250 minutes/week of bout-related MVPA at year 1 and 150 minutes/week at year 4. While WL maintainers were more likely to engage in ≥250 minutes/week of MVPA compared to non-maintainers, there was large variability, with 40-60% of individuals engaging in <150 minutes/week at year 1 or year 4. This suggests that there may be more than one pathway to successful WL maintenance in this population and that ≥250 minutes/week of MVPA may not be necessary.
In addition to WL maintenance, this study also assessed the relationship between PA and initial WL. The current findings confirm and extend previous reports indicating that higher bout-related MVPA at Year 1 was associated with greater 1-year WL (18, 19). Approximately one-third of study participants engaged in ≥150 min/week of MVPA at Year 1. These individuals lost an additional 4% of initial body weight compared to those engaging in <150 min/week. This 4% difference in observed WL is similar to that of a previous study in which behavioral WL participants engaging in ≥150 min/week of self-reported PA lost approximately 9% of initial body weight at Year 1, while those engaging in <150 min/week only achieved a 4% WL (20). However, the latter study (20) reported that those engaging in ≥200 min/week at Year 1 had even greater weight losses (approximately 14% of initial body weight) compared to those engaging in ≥150 min/week. In the current study, this was not the case - engagement in ≥250 min/week had no additional effect on WL, compared to 150-250 min/week. Thus in older adults with type 2 diabetes, meeting the national public health recommendation of ≥150 min/week of bout-related MVPA within the context of a comprehensive weight management program may be sufficient for initial WL success.
A second aim of this study was to examine the relationship between PA and long-term WL. It is generally accepted that high PA is important for long-term weight control (1); however few behavioral WL interventions have been >24 months in duration, making it difficult to assess this long-term relationship. Previously, Tate et al (21) reported that participants engaging in approximately 300 minutes/week of MVPA at 30 months lost 12 kg in comparison to a 1 kg WL observed among those engaging in <300 min/week. In the Diabetes Prevention Program, the odds of achieving a 7% WL goal at the end of the intervention (∼3.2 years) was 4.11 times as great in those who achieved the PA goal of ≥150 min/week compared to those not achieving the goal (22). While these data suggest the potential importance of PA for long-term weight control, these studies utilized self-report PA measures, had shorter study durations and smaller sample sizes than the Look AHEAD Trial. In the current study, we report that 4-year PA (not 1-year PA) was the strongest predictor of 4-year weight change and participants achieving ≥250 min/week of MVPA at Year 4 lost 8.2% of initial body weight which was significantly greater than those engaging in any of the lower levels of activity, all of whom lost about lost 5.7%. However, while these data speak to the importance of ≥250 minutes, it is important to note that only 13% of all participants achieved ≥250 min/week at year 4 and fewer than 25% of study participants achieved ≥150 min/week; on average 4-year PA levels were relatively low (110 min/week).
The final aim of this study was to assess the relationship between PA and WL maintenance. Approximately half of the participants who lost ≥10% at Year 1 maintained this magnitude of WL through Year 4. Among WL maintainers, 1-year MVPA levels (∼250 min/week) were consistent with ACSM's guidelines for preventing weight regain following WL (1); however 4-year MVPA (∼150 min/wk) was well below this threshold. When these findings were compared to another behavioral WL trial in a younger cohort (mean age: 43 years), objectively-assessed MVPA at Year 1 (236 min/week) was similar to the current study, but unlike the decrease in MVPA that we observed between 1 and 4 years, MVPA levels in the later study remained unchanged between 12 and 18 months among WL maintainers (6). Analysis of the individual level data in the current study revealed that only about 40% of WL maintainers achieved ≥250 min/week at year 1 and this was further reduced to 22% at Year 4. Further, over half of the WL maintainers engaged in <150 min/week at Year 4. While variability in MVPA among WL maintainers has also been reported in the National Weight Control Registry (NWCR), fewer NWCR participants were engaging in little PA (∼25% of participants reported <1000 kcals/wk which is approximately 150 min/wk), as compared to WL maintainers in the current study (23). Together these data suggest that many individuals can maintain clinically significant weight losses with lower than recommended levels of PA.
Given previous reports indicating that ≥250 minutes/week may be necessary for WL maintenance, it is unclear why WL maintainers in the current study maintained large weight losses despite engaging in only 150 minutes/week of MVPA at Year 4. There are several potential explanations for this finding. First, the national PA guidelines were largely based upon self-reported PA and the current study assessed PA objectively. Given that self-reported PA is often higher than objective PA estimates it is possible that the magnitude of objectively-assessed PA that is optimal for WL maintenance may actually be lower than what has been recommended based upon self-report estimates. Second, it is possible that dietary intake, not PA was the primary driver of weight. While there is usually a strong correlation between adherence to physical activity and diet recommendations, it is likely that that those individuals engaging in lower than recommended levels of PA were maintaining their WL through greater adherence to the calorie restricted diet. However, dietary intake was assessed in a subset of participants in Look AHEAD that differed from the subset with accelerometry, making it difficult to examine this. Finally, given that this was an older population with more comorbidities, it is possible that using a 3 MET threshold may have failed to capture all activity done for the purpose of exercise or that some individuals lost weight or decreased PA over time as result of illness; thus reducing the association between MVPA and WL maintenance. Therefore, the generalizability of these findings may be limited to older adults with type 2 diabetes.
This study had numerous strengths including the objective measurement of PA, long-term follow-up data, and a large sample size. However, it is not without limitations. All analyses were post-hoc comparisons, examining the association between PA and weight change. Further, the direction of this relationship could not be determined – it is unclear whether WL led to increased PA or whether increased PA resulted in additional WL. Finally, although individuals included in these analyses were demographically similar to those without valid accelerometer data at all 3 time points, it is possible that these subgroups of individuals differed on other unmeasured parameters thereby limiting the generalizability of these findings to other populations.
In summary, these findings confirm previous reports regarding the importance of PA for weight control, with higher levels of PA associated with greater initial WL, long-term WL, and weight maintenance. However, within an older population with Type 2 diabetes, our data suggest that many individuals are able to achieve and maintain clinically significant long-term WL while engaging in less than the recommended level of MVPA for WL maintenance. Future studies should examine how those who maintain WL with high PA differ in other weight control behaviors (e.g., dietary intake) or cardiometabolic risk profiles compared to those maintaining clinically significant WL with lower PA.
What is already known about this subject.
Several studies, which have relied on self-reported physical activity (PA) data, suggest that high levels of PA are important for long-term weight control.
For weight loss maintenance, it is recommended that individuals engage in ≥250 min/week of moderate-to-vigorous intensity physical activity (MVPA); however long-term follow-up data using objective PA monitors is lacking.
What does this study add.
Using objective PA monitors, this study confirms that weight loss maintainers engage in more MVPA compared to non-maintainers (i.e., those who regain weight between years 1 and 4); however average MVPA levels at year 4 are well below the recommended guideline of ≥250 min/week.
This study highlights the variability in MVPA among WL maintainers and suggests that many individuals can maintain a clinically significant weight loss while engaging in less than the recommended amount of MVPA.
This study suggests that the MVPA threshold for WL maintenance for older individuals with type 2 diabetes may be lower than what is recommended for the general population; however additional studies are warranted.
Acknowledgments
This study would not have been possible without the contributions of the entire Look AHEAD Research Group (See online S1).
Funding and Support: This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women's Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). Further, Dr. Kitzman is supported by R01AG18915 and P30AG21332.
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00017953
Disclosure: Dr. Hill reports having stock options in Retrofit, a company providing weight management to the public and is also a member of LLC-Shakabuku, which also provides weight management to the public. Dr Peters has served as a consultant and speaker for Boehringer Ingelheim, Eli Lilly and Company, Janssen, Merck, NovoNordisk, and Sanofi. Dr. Jakicic received an honorarium for serving on the Scientific Advisory Board for Weight Watchers International, was the Principal Investigator on a grant to examine the validity of activity monitors awarded to the University of Pittsburgh by Jawbone, Inc., a co-investigator on a grant award to the University of Pittsburgh by HumanScale, a co-investigator on a grant awarded to the University of Pittsburgh by Weight Watchers International. The remaining authors declare no potential conflict of interest.
References
- 1.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 2.Wadden TA, Vogt RA, Foster GD, Anderson DA. Exercise and the maintenance of weight loss: 1-year follow-up of a controlled clinical trial. J Consult Clin Psychol. 1998;66(2):429–33. doi: 10.1037//0022-006x.66.2.429. [DOI] [PubMed] [Google Scholar]
- 3.Leser MS, Yanovski SZ, Yanovski JA. A low-fat intake and greater activity level are associated with lower weight regain 3 years after completing a very-low-calorie diet. J Am Diet Assoc. 2002;102(9):1252–6. doi: 10.1016/s0002-8223(02)90277-4. [DOI] [PubMed] [Google Scholar]
- 4.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550–9. doi: 10.1001/archinte.168.14.1550. discussion 9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37(3):197–206. doi: 10.1136/bjsm.37.3.197. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakicic JM, Tate DF, Lang W, Davis KK, Polzien K, Neiberg RH, et al. Objective physical activity and weight loss in adults: the step-up randomized clinical trial. Obesity (Silver Spring) 2014;22(11):2284–92. doi: 10.1002/oby.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atienza AA, Moser RP, Perna F, Dodd K, Ballard-Barbash R, Troiano RP, et al. Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc. 2011;43(5):815–21. doi: 10.1249/MSS.0b013e3181fdfc32. [DOI] [PubMed] [Google Scholar]
- 8.Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–5S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 10.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond) 2006;30(3):391–9. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 11.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 12.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40(4):454–61. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19(10):1987–98. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unick JL, Gaussoin SA, Hill JO, Jakicic JM, Bond DS, Hellgren M, et al. Four-Year Physical Activity Levels among Intervention Participants with Type 2 Diabetes. Med Sci Sports Exerc. 2016;48(12):2437–45. doi: 10.1249/MSS.0000000000001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller GD, Jakicic JM, Rejeski WJ, Whit-Glover MC, Lang W, Walkup MP, et al. Effect of varying accelerometry criteria on physical activity: the look ahead study. Obesity (Silver Spring) 2013;21(1):32–44. doi: 10.1038/oby.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Physical activity guidelines advisory committee. Physical activity guidelines advisory committee report, 2008. Washington DC: US Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 17.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. Jama. 1999;282(16):1554–60. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78(4):684–9. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 20.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290(10):1323–30. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 21.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85(4):954–9. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 22.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catenacci VA, Ogden LG, Stuht J, Phelan S, Wing RR, Hill JO, et al. Physical activity patterns in the National Weight Control Registry. Obesity (Silver Spring) 2008;16(1):153–61. doi: 10.1038/oby.2007.6. [DOI] [PMC free article] [PubMed] [Google Scholar]