Abstract
Bv8 is a pronociceptive peptide that binds to two G-protein coupled prokineticin receptors, PK-R1 and PK-R2. These receptors are localized in the dorsal horn of the spinal cord and dorsal root ganglia (DRG) of nociceptive neurons in rodents. Systemic administration of Bv8 elicits a biphasic reduction in nociceptive thresholds to thermal and mechanical stimuli. Here, the possibility that Bv8 might directly modulate the expression and release of excitatory transmitters within the early and late phases of hyperalgesia was evaluated. Administration of Bv8 to mouse lumbar spinal cord sections produced a direct, significant and concentration-related release of CGRP. Bv8- or capsaicin-stimulated CGRP release was markedly enhanced in tissues taken from Bv8-pretreated mice during the late, but not the early, phase of hyperalgesia. Pretreatment of rats with protein synthesis inhibitors blocked the expression of the late, but not early, phase of Bv8-induced hyperalgesia. Finally, during the late-phase of hyperalgesia, there was an upregulation of CGRP and substance P immunoreactivity in the rat lumbar dorsal horn and DRG. Such upregulation was prevented by pretreatment with protein synthesis inhibitors. These data suggest that Bv8 induces hyperalgesia by direct release of excitatory transmitters in the spinal cord, consistent with the first phase of hyperalgesia. Additionally, Bv8 elicits a subsequent, protein-synthesis dependent increase in expression and release of excitatory transmitters that may underlie the long-lasting second phase of hyperalgesia. Activation of prokineticin receptors may therefore contribute to persistent hyperalgesia occurring as a consequence of tissue injury further suggesting that these receptors are attractive targets for development of therapeutics for pain treatment.
Keywords: Prokineticins, Prokineticin receptors, CGRP, Sensitization
1. Introduction
Bv8, a protein belonging to the prokineticins family [5,6,10,17,21], is a specific ligand for two structurally related G-protein-coupled receptors that have been cloned and named prokineticin receptors 1 and 2 (PK-R1 and PK-R2)[7,9,14]. PK-R1 is expressed in a number of peripheral organs, while PK-R2 expression is mostly confined to the central nervous system [2,7]. Both receptors are expressed in small and medium cells of rat dorsal root ganglia (DRG) and in the spinal dorsal horn (DH), supporting a role for the PK-PKR system in sensory processing. In the dorsal horn of the spinal cord, PKR1 is more abundant than PKR2, whereas both receptors are expressed equally in DRG [12]. Binding studies using spinal cord tissue indicate the highest density of PKRs within the superficial laminae of the spinal cord, suggesting that these receptors may play a role in nociceptive signaling. In the rat DRG significant proportion (~70%) of TRPV1 expressing neurons co-express PKR1 whereas a smaller proportion (~9.5%) co-express PKR2 [13]. In vitro analysis of functional PKRs on DRG neurons show a remarkable overlap in neurons that respond both to Bv8 and capsaicin (~90%) indicating a high degree of co-localization of functional PKRs with TRPV1 channels [20] further supporting the possibility that these receptors contribute to nociceptive signaling. These data strongly suggest that PKRs, especially PKR1, are localized on nociceptive sensory fibers, and that PKR antagonists may, therefore, represent a class of compounds which may be useful for treating acute and chronic pain.
Systemic or spinal Bv8 administration results in a significant decrease in sensory thresholds to noxious thermal or mechanical stimuli applied to the tail and hindpaw of rats and mice [12]. An unusual feature of Bv8-induced changes in sensory thresholds is the emergence of a two-phased hyperalgesic response. The first phase occurs within 30 min following s.c. Bv8 administration to rats and mice [12]. Following return to baseline sensory thresholds, a second hyperalgesic phase emerges within 240 min and persists for approximately 90 min [12]. The mechanisms underlying these distinct phases of hyperalgesia are unknown.
Noxious mechanical, thermal or chemical (e.g., capsaicin) stimuli evoke the release of the nociceptive peptide calcitonin gene related peptide (CGRP) from the superficial dorsal horn of the spinal cord [11,18,19]. CGRP is primarily expressed by unmyelinated C and lightly myelinated Aδ primary afferent fibers [15] which take part in the transmission of pain and have been demonstrated to contain PKRs. Here, we attempted to uncover mechanisms of Bv8-induced hyperalgesia by evaluating whether Bv8 (a) can directly evoke the release of CGRP in tissues lumbar dorsal horn, (b) regulates the expression and release of pronociceptive transmitters in the dorsal root ganglion and spinal cord in a time course relevant to the two phases of Bv8-induced hyperalgesia, and (c) whether expression of Bv8-induced hyperalgesia is dependent upon protein synthesis. Our data suggest that the second phase of Bv8-induced hyperalgesia is dependent upon upregulation of excitatory transmitter resulting in a prolonged hyperalgesic state.
2. Materials and methods
2.1. Animals
Adult male C57BL/6 mice (35–40 g at the time of testing) and male Sprague-Dawley rats (200–300 g) were maintained in a climate-controlled room (temperature 20–24 °C and humidity 30–70%) on a 12 h light/dark cycle (7:00 AM–7:00 PM) with food and water ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals with approval received from the Institutional Animal Care and Use Committee of the University of Arizona. Groups of 6–12 animals were used in all experiments for a total of 132 mice and 60 rats.
2.2. Tissue extraction and preparation
Following deep anesthesia with ether, animals were decapitated and the spinal column was severed at the pelvic girdle. The spinal cord was expelled by hydraulic extrusion with ice-cold saline and immediately placed on ice in a glass Petri dish, and the dorsal half of the lumbar cord dissected, weighed and chopped into 0.2 mm cubes with a McIwain tissue chopper (Mickle Laboratory Engineering, Gomshall, UK).
2.3. CGRP release assays
Chopped mouse lumbar spinal cord tissue was placed in a 1 cc superfusion chamber and continuously superfused with oxygenated modified Krebs’ buffer (135 mM NaCl, 3.5 mM KCl, 20 mM NaHCO3, 1 mM NaHPO4, 2.5 mM CaCl2, 3.3 mM dextrose, 0.1 mM ascorbic acid, 10 mM thiorphan and 0.1% bovine serum albumine) maintained at 37 °C, pH 7.4, at a rate of 0.5 ml/min with a Brandel Superfusion Pump (Brandel Gaithersburg, MD). The tissues were equilibrated for 45 min prior to determination of basal release. Superfusate was collected in 3 min intervals into testing tubes using a fraction collector (Gilson, Middleton, WI). A total of 5 fractions were collected to establish baseline levels of CGRP before Bv8 or capsaicin were applied for 2 fractions. Superfusate was then collected for an additional 8 fractions.
2.4. Radioimmunoassay for CGRP
The superfusate obtained from the release assay was preincubated with 100 µl of a C-terminally directed anti-CGRP antibody (Peninsula Lab.) for 24 h at 4 °C. The samples were each mixed with 50 µl of goat anti-rabbit antiserum coupled to ferric beads and 100 µl of [125I-Tyr0]CGRP28–37 (at ~25,000 cpm per assay tube) and incubated for an additional 24 h. The [125I]CGRP bound to the CGRP antibody was separated from the free tracer through immunomagnetic separation (PerSeptive Diagnostics, Cambridge, MA). The immunoprecipitates were determined by gamma counting. Standard curves were generated and CGRP content was determined through logit–log analysis. This assay has a minimal detection limit of 1–3 fmol/tube. The CGRP antiserum used in these experiments binds near the C-terminal end of CGRP and does not cross react with cholecystokinin, neuropeptide Y, or other peptides with similar C-terminal residues. The CGRP concentrations were plotted against time in 3 min intervals. Evoked release was calculated as the total amount of CGRP released (i.e., CGRP-ir) during the Bv8 or capsaicin infusion above the basal release of CGRP.
2.5. In vivo treatments
Bv8 was injected intrathecally at 0.5 ng per rat and response latencies to noxious mechanical stimuli (i.e., Randall–Sellito test) were determined over the following 300 min. In some studies, rats were pretreated with puromycin (15 mg/kg, s.c. for 14 days) or anisomycin (50 mg/kg, s.c. −1 h) prior to Bv8 injection.
2.6. Immunofluorescence
For immunohistochemistry, rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine intraperitoneally and then perfused transcardially with 0.1 M PBS until the exudates run clear, then with 10% formalin in 0.1 M PBS, pH 7.4 for ~15 min. Lumbar spinal cords were harvested and postfixed in 10% formalin overnight and cryoprotected with 20% sucrose in 0.1 M PBS. The postfixed tissues were transferred to 30% sucrose in 0.1 M PBS until the tissues sank to the bottom of the container. Tissues were then embedded in O.C.T. (Tissue-Tek Optimal Cutting Temperature Compound: Sakura, Torrance, CA) compound. DRG and spinal cord sections were cut serially at 10 and 20 µm, respectively with a cryostat maintained at −20 °C. Sections of DRG and spinal cord were cut serially and placed onto slides such that each slide contained an ordered series of sections through the ganglion and spinal cord. Mounted DRG and spinal cord sections were washed in 0.1 M PBS for 30 min and then incubated with a rabbit anti-CGRP antiserum (1:20,0000 Peninsula) or anti-Substance P (SP) antiserum (1:10,000 Peninsula), in 0.1 M PBS with 2% normal goat serum overnight at 4 °C, followed by washing and secondary incubation with a secondary antiserum Cy3-conjugated goat anti-rabbit IgG (1:500 Jackson Immunoresearch, West Grove, PA) for 2 h. The sections were rinsed and mounted in Vectashield (Vector Laboratories). Fluorescence digital images were captured using a Nikon (Tokyo, Japan) E800 fluorescence microscope outfitted with a Hamamatsu (Hamamatsu, Bridgewater, NJ) CCD camera with output to a Pentium microcomputer.
2.7. Intrathecal injections
Under ketamine–xylazine anesthesia, the rats were implanted with catheters for i.th. administration of Bv8 into the region of the lumbar cord according to the method previously described by Yaksh and Rudy [22]. In brief, PE-10 polyethylene tubing (8 cm in length) was inserted through the atlanto-occipital membrane, fed down the i.th. space and secured to the musculature at the incision site. The rats received 4.4 mg/kg i.m. of gentamycin and were allowed to recover for 5 days. Bv8 or vehicle was administered, followed by 1 µl air bubble to assure movement through the i.th. catheter, followed by a 9 µl flush.
2.8. Mechanical nociception
The nociceptive threshold to mechanical stimuli was measured in rats with the Randall–Selitto test [16]. The rats were exposed to the procedure for several days preceding the experiment [12]. On the day of the experiment, nociceptive thresholds were measured 60 and 30 min before treatment. The mean of these measurements was taken as baseline nociceptive threshold (NTB). Nociception thresholds were then determined three times at 15, 30, 60, 90, 120, 150, 180, 240, 300 and 360 min after treatment. The mean of the three reading at each point was defined as the nociceptive threshold at that time in the presence of the test solution (NTts). The effect of the test drug was calculated as the percentage change in nociceptive threshold from baseline threshold (%ΔNT) according to the following equation:
2.9. Statistical analysis
Data are presented as mean ± s.e.m. values. Statistical analyses were performed using one-way ANOVA or 2-way ANOVA with drug and time as independent variables.
3. Results
3.1. Bv8 directly induces CGRP release in vitro
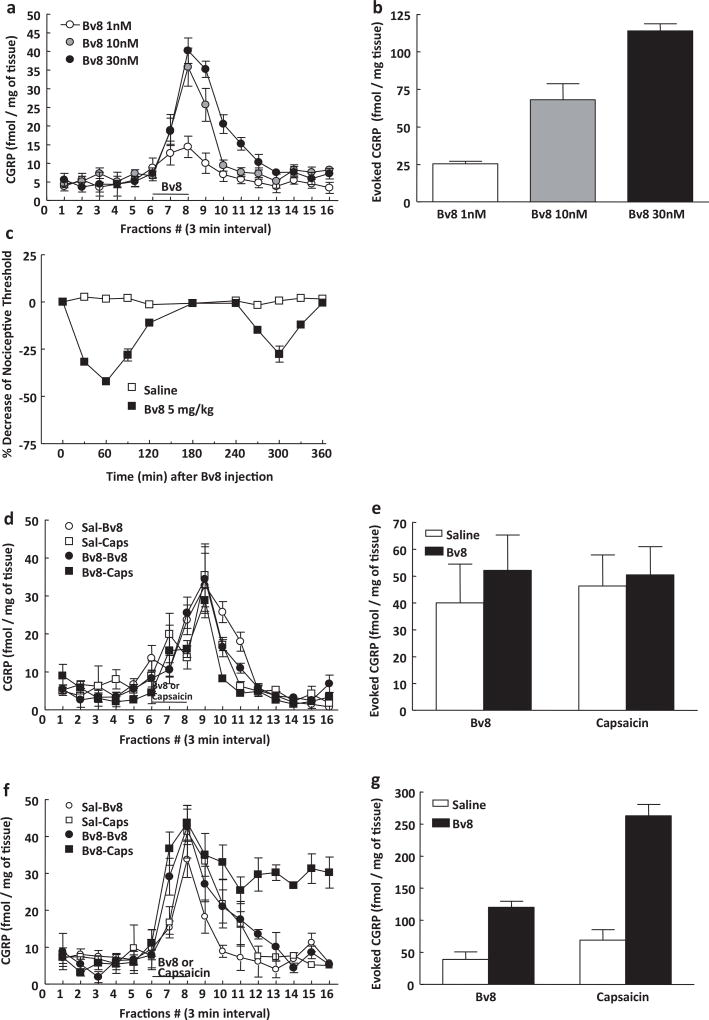
Basal CGRP release from naïve C57BL/6 mice was approximately 5 ± 0.8 fmol/mg of tissue (Fig. 1a and b). Addition of graded concentrations of Bv8 (1, 10, 30 nM) led to a significant, dose-dependent increase of CGRP release of approximately 2-, 6- and 8-fold, respectively (Fig. 1a and b). The area under the curve for Bv8-evoked CGRP release was plotted from the time of Bv8 application (fraction #6) until it returned to baseline (fraction #12) for a total of 18 min (Fig. 1a and b) demonstrating a concentration-dependent Bv8-evoked release. CGRP release evoked by Bv8 at 1 or 30 nM concentrations returned to basal levels within 9 or 18 min, respectively (Fig. 1a and b).
Fig. 1.
The dorsal lumbar spinal cord from male C57 mice was dissected, minced and placed in perfusion chambers. Basal and evoked release of CGRP into the perfusate was measured at 3 min interval (a), and Bv8-evoked CGRP release was indicated by the amount of CGRP present above the baseline release levels (b). The horizontal bar in (a) represents the period in which Bv8 (1–30 nM) was added to the perfusate. Application of Bv8 for 6 min following a 30-min equilibration period led to a concentration-dependent increase in CGRP release. Each treatment group consisted of 8 animals. (c) Treatment with Bv8 (5 mg/kg, s.c.) induced a biphasic hyperalgesic response. Mice tested for hot plate latency showed within 30 and 270 min, first and second phase of hyperalgesia after Bv8 injection. Each treatment group consisted of 6 animals. (d and e) Pretreatment with Bv8 (5 mg/kg, s.c.) enhanced Bv8 (10 nM) or capsaicin (1 µM)-evoked release in the lumbar dorsal horn of spinal cord of mice, only during the second phase of hyperalgesia. (f and g) In tissue from mice pretreated with Bv8 (−270 min), capsaicin-evoked CGRP release did not recover to baseline level. Each treatment group consisted of 12 animals.
3.2. Evoked CGRP release following Bv8 pretreatment in vivo
In accordance with previous studies, administration of Bv8 (5 mg/kg, s.c.) to mice elicited a typical biphasic hyperalgesia with a first and second phase (Fig. 1c). After assessment of thermal hyperalgesia tissues were taken at 45 and 270 min post-Bv8 treatment in order to evaluate possible effects of pretreatment on subsequent Bv8- or capsaicin-evoked CGRP release corresponding with the two behavioral phases. No significant difference was observed in basal or Bv8- or capsaicin-evoked CGRP release from tissues taken from Bv8 or saline-pretreated mice at the 45 min time point (Fig. 1d and e). Similarly, baseline CGRP release was not different in tissues taken 270 min following treatment of mice with Bv8 or saline. In contrast, the magnitude and duration of Bv8- or capsaicin-evoked CGRP release was significantly increased in tissues taken from mice 270 min following Bv8 treatment (Fig. 1f and g). Bv8-evoked CGRP release in tissues from mice pretreated 270 min previously with Bv8 was 120 ± 9 fmol/mg of tissue while that in tissue from saline pretreated (270 min prior) mice was 39 ± 11 fmol/mg of tissue, demonstrating a 3-fold enhancement of Bv8-evoked CGRP release. Likewise, capsaicin-evoked CGRP release was also increased in tissues taken 270 min following Bv8 treatment. The administration of capsaicin (1 µM) to tissues from Bv8-pretreated mice (270 min) resulted in CGRP release of 262 ± 18 fmol/mg of tissue compared to 68.7 ± 16.3 fmol/mg tissue in saline pretreated mice (Fig. 1f and g).
3.3. Bv8-induced upregulation of immunoreactivity for neurotransmitters
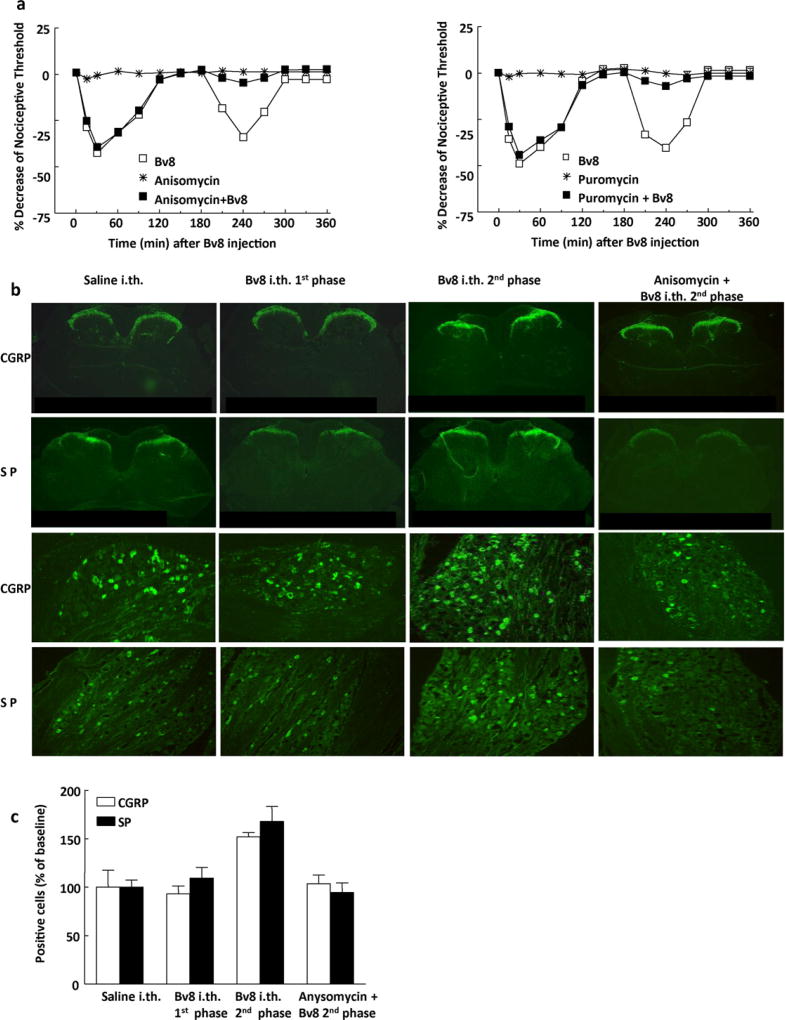
As previously reported, intrathecal (i.th.) treatment of rats with Bv8 (0.5 ng/rat) results in hyperalgesic responses that peaked at 30 and 240 min, corresponding to the typical two-phases of i.th. Bv8-induced hyperalgesia (Fig. 2a). Pretreatment of rats with either puromycin or anisomycin had no effect on baseline sensory threshold. While i.th. Bv8 to puromycin or anisomycin treated rats elicited the first phase of hyperalgesia, the second phase was significantly blocked (Fig. 2a). These results suggest that Bv8 increased synthesis of CGRP and substance P, which were blocked by the protein synthesis inhibitors puromycin and anisomycin.
Fig. 2.
(a) Pretreatment with anisomycin (50 mg/kg, s.c. 1 h prior) or with puromycin (15 mg/kg, s.c. for 14 days) significantly blocked the Bv8 (0.5 ng/rat, i.th.)-induced second phase of hyperalgesia. (b) CGRP and SP immunoreactivity in the rat spinal cord at approximately L5 and in the rat DRG L5. During the second phase of Bv8-induced hyperalgesia, the labeling was upregulated in lumbar dorsal horn of spinal cord of rats pretreated with Bv8 (0.5 ng/rat, i.th., 240 min prior to perfusion). No apparent difference in both CGRP and SP immunoreactivity was seen between the tissues from control rat, from tissues taken during the first phase of hyperalgesia, and from rat pretreated with anisomycin and treated with Bv8.
Spinal cord and DRG tissues were taken 30 or 240 min following Bv8 or saline treatment in order for immunohistochemical analysis and to correspond with the two phases of hyperalgesia. CGRP and SP were confined bilaterally to the outer laminae (I and II) of the spinal dorsal horn in control rats and rats pretreated with anisomycin 1 h prior (Fig. 2b). No changes in immunofluorescence labeling for either CGRP or SP were observed in tissue taken from animals treated with anisomycin, when compared to the saline treated group (data not shown). Enhanced fluorescence labeling for CGRP and SP was seen in the dorsal horn 240 min, but not 30 min, after Bv8 treatment, when compared with the saline treated group (Fig. 2b). The enhanced fluorescence following Bv8 was not observed in tissues taken from rats pretreated with anisomycin 1 h prior to Bv8 administration (i.e., tissue taken 240 min after Bv8 injection) (Fig. 2b).
No changes in the number of DRG (L4-L6) neuronal profiles labeled for either CGRP or SP were observed in tissue taken from animals pretreated with anisomycin, when compared to the saline pretreated group (data not shown). Pretreatment with anisomycin did not produce changes in CGRP or SP immunofluorescence in lumbar DRG of animals treated with i.th. saline, but prevented Bv8-induced enhanced CGRP and SP labeling (Fig. 2b). In the control group approximately 33% of the DRG neuronal profiles counted were positively labeled for CGRP and 21% for substance P. DRG neuronal profiles labeled for CGRP and SP in sections obtained from rats during the first phase of Bv8-induced hyperalgesia (−30 min) were 93.1 ± 8.2% and 109.5 ± 10.9% of the saline control group, respectively (Fig. 2c). DRG neuronal profiles labeled for CGRP and substance P in sections obtained from rats during the second phase of hyperalgesia (−270 min) were significantly increased (p < 0.05) to 152.0 ± 4.6% and 167.9 ± 15.4% of the saline control group, respectively (Fig. 2c). Examination of CGRP and SP labeling in DRG from rats pretreated with anisomycin (1 h prior to Bv8) in tissue taken during the second phase of Bv8-induced hyperalgesia (−270 min), resulted in labeling for CGRP and SP that was not different from that observed with i.th. saline; the values for CGRP and SP were 103.5 ± 9.2% and 94.4 ± 10.0% of the saline control group, respectively (Fig. 2c).
4. Discussion
The present study demonstrates that prokineticin receptors are likely to play an important role in activation and sensitization of nociceptors and in sustaining hyperalgesia. Systemic injection of Bv8 has been shown to induce a biphasic thermal and mechanical hyperalgesia [12]. Here we report that, within the isolated dorsal horn of the spinal cord, Bv8-induces a direct and concentration related release of CGRP suggesting a likely mechanism for the first phase of Bv8-induced hyperalgesia. This interpretation is supported by our previous observations that a local (intraplantar) injection of Bv8 elicits strong and localized hyperalgesia with a similar time course to that of the initial phase of hyperalgesia seen with systemic injections. However, intraplantar Bv8 does not result in the well-characterized second phase of hyperalgesia [12]. Additionally, however, our data provide a basis for the long lasting second phase of hyperalgesia which appears to depend on protein synthesis and enhanced expression of excitatory transmitters.
Administration of Bv8 has been shown to produce a biphasic hyperalgesic effect in mice and rats with the second phase peaking approximately within 300 min after administration [12]. To determine whether the Bv8 biphasic effect would result in enhanced spinal CGRP release within these two phases, we pretreated mice with a single systemic dose of Bv8 and measured Bv8-evoked release of CGRP from spinal cord tissue. Systemic Bv8 pretreatment for 45 min (representing the first phase) prior to performing CGRP release did not result in differences in release when compared with naïve spinal cord tissue. However, when animals were pretreated systemically with Bv8 for 270 min, representing a timepoint consistent with the second phase of hyperalgesia, a significantly enhanced CGRP release resulted from application of Bv8 or capsaicin was observed and the duration of release was increased when compared to saline pretreated tissues. Such results parallel behavioral biphasic hyperalgesic effects suggesting that, Bv8 can elicit long-lasting sensitization of nociceptors resulting in enhanced responses to evoking stimuli including Bv8 or capsaicin.
The basis for enhanced sensitivity to capsaicin in tissues taken to be consistent with late phase hyperalgesia is unknown but could be due to Bv8-induced neuronal plasticity and regulation of expression of TRPV1 channels as the second phase of hyperalgesia was dependent upon protein synthesis (see below). Additionally, several recent studies have demonstrated that G-protein coupled receptors may modulate the activity at the TRPV1 channel. Mice lacking the cannabinoid CB1 or P2Y nucleotide receptor have an impaired response to the algogenic compound capsaicin [4,8], while bradykinin, a Gq receptor agonist has been demonstrated to potentiate the TRPV1 function both in vitro and in vivo [1,3]. Similar to our findings, the systemic pretreatment (270 min) of Bv8 results in a significant and long lasting capsaicin-evoked CGRP release possibly due to an increased TRPV1 receptor sensitization. Previous behavioral and electrophysiological studies demonstrated that Bv8 sensitizes the TRPV1 channel [13,20] an effect that may account for the enhanced transmitter release seen here in tissues from Bv8 pretreated mice. Such Bv8 synergism of capsaicin was not directly at the TRPV1 channels since this effect was not observed in PKR1-knockout mice [13] supporting an indirect effect of Bv8 through the PKR1.
We tested whether the biphasic effect of Bv8 was related to alterations in protein synthesis. Our data indicate that administration of protein synthesis inhibitors did not alter the first phase of Bv8-induced hyperalgesia or the release of CGRP in tissues taken from animals to correspond with that time period. In contrast, protein synthesis inhibitors abolished expression of the second hyperalgesic phase of Bv8 indicating a requirement for synthesis of new protein for such evoked hyperalgesia. Consistent with this possibility, systemic Bv8 was found to produce an increase in the expression of CGRP and SP labeling in the DRG cells and in the dorsal horn of spinal cord at the 240 min time-point (during the second phase of hyperalgesia) and this effect was blocked in animals receiving anisomycin.
The present studies suggest that Bv8 likely induces the second phase of hyperalgesia through protein synthesis resulting in increased CGRP and SP expression and long-lasting enhanced evoked release. These observations provide a basis for long-lasting hypersensitivity that may result in conditions of tissue injury through modulation of the PK/PK-R system in which nociceptors can be activated and sensitized by activation of PKRs. It is likely, therefore, that blockade of PKRs may represent a novel strategy to provide therapeutic benefit in pain conditions.
highlights.
-
►
Bv8 produces a direct and concentration-related release of CGRP.
-
►
Bv8 pretreatment enhances Bv8- or capsaicin-stimulated CGRP release.
-
►
Pretreatment with protein synthesis inhibitors blocks the late phase of Bv8-induced hyperalgesia.
-
►
CGRP and SP are upregulated during the late phase of Bv8-induced hyperalgesia.
References
- 1.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. British Journal of Pharmacology. 2004;141(5):787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fioravanti B, De Felice M, Stucky CL, Medler KA, Luo MC, Gardell LR, Ibrahim M, Malan TP, Jr, Yamamura HI, Ossipov MH, King T, Lai J, Porreca F, Vanderah TW. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(45):11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412(6850):877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Molecular Pharmacology. 2001;59(4):692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 7.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. Journal of Biological Chemistry. 2002;277(22):19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 8.Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138(3):484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochemical and Biophysical Research Communications. 2002;293(1):396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 10.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil GF. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. European Journal of Pharmacology. 1999;374(2):189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 11.Morton CR, Hutchison WD. Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31(3):807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- 12.Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P. Nociceptive sensitization by the secretory protein Bv8. British Journal of Pharmacology. 2002;137(8):1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negri L, Lattanzi R, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, De Felice M, Porreca F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. Journal of Neuroscience. 2006;26(25):6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negri L, Lattanzi R, Giannini E, Melchiorri P. Bv8/Prokineticin proteins and their receptors. Life Sciences. 2007;81(14):1103–1116. doi: 10.1016/j.lfs.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Pohl M, Benoliel JJ, Bourgoin S, Lombard MC, Mauborgne A, Taquet H, Carayon A, Besson JM, Cesselin F, Hamon M. Regional distribution of calcitonin gene-related peptide-, substance P-, cholecystokinin-, Met5-enkephalin-, and dynorphin A (1–8)-like materials in the spinal cord and dorsal root ganglia of adult rats: effects of dorsal rhizotomy and neonatal capsaicin. Journal of Neurochemistry. 1990;55(4):1122–1130. doi: 10.1111/j.1471-4159.1990.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 16.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Archives Internationales de Pharmacodynamie et de Therapie. 1957;111(4):409–419. [PubMed] [Google Scholar]
- 17.Schweitz H, Bidard JN, Lazdunski M, Schweitz H, Bidard JN, Lazdunski M. Purification and pharmacological characterization of peptide toxins from the black mamba (Dendroaspis polylepis) venom. Toxicon. 1990;28(7):847–856. doi: 10.1016/s0041-0101(09)80007-x. [DOI] [PubMed] [Google Scholar]
- 18.van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neuroscience and Biobehavioral Reviews. 1997;21(5):649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 19.Vasko MR. Prostaglandin-induced neuropeptide release from spinal cord. Progress in Brain Research. 1995;104:367–380. doi: 10.1016/s0079-6123(08)61801-4. [DOI] [PubMed] [Google Scholar]
- 20.Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. Journal of Neuroscience. 2006;26(19):5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Letters. 1999;462(1–2):177–181. doi: 10.1016/s0014-5793(99)01473-8. [DOI] [PubMed] [Google Scholar]
- 22.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiology and Behavior. 1976;17(2):1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]